Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening disorder of immune regulation leading to widespread lymphocytic and hemophagocytic infiltration of vital organs. Apparent cure has only been achieved with allogeneic bone marrow transplantation (BMT). This report describes 20 consecutive patients, who underwent either matched sibling donor (n = 4) or unrelated donor (URD; n = 16) BMT. Age at the time of BMT was 0.4 to 5.3 years (median, 0.8 years). Central nervous system disease was present at diagnosis in 13 patients. At BMT, 14 patients were in a clinical remission, whereas 6 patients had active HLH. All patients were engrafted after cytoreduction with busulfan, cyclophosphamide, and etoposide. The probability of grade II-III acute graft-versus-host disease (GVHD) for all patients was 57% (95% confidence limit [CL], 0.28, 0.86), and 73% (95% CL, 0.44, 1.0) in URD patients. The overall probability of survival at 3 years was 45% (95% CL, 0.23, 0.67) and 44% (95% CL, 0.19, 0.68) when URD BMT was evaluated separately. Favorable BMT outcome was associated with clinical remission status at the time of BMT. The preparative regimen was well tolerated, and in the 9 surviving patients it provided durable engraftment and was effective at eradicating the underlying disease.
HEMOPHAGOCYTIC lymphohistiocytosis (HLH) is a life-threatening immunoregulatory disorder resulting in infiltration of lymphocytes and macrophages, with extensive hemophagocytosis particularly involving the liver, spleen, bone marrow, and central nervous system.1 It is characterized by fever, massive hepatosplenomegaly, pancytopenia, hypertriglyceridemia, hypofibrinogenemia, decreased natural killer cell function, and, frequently, seizures.2 The etiology of this disorder remains obscure. Symptomatic disease can be unremitting or may follow a relapsing course leading to death from infection and/or bleeding with a median survival after onset of only a few months.3 HLH frequently occurs in children less than 2 years of age as a familial autosomal recessive disorder.4 It also has been reported in association with infections, usually viral, in patients with underlying hematopoietic malignancies, in children with congenital or acquired immunodeficiencies, or as a sporadic and isolated event without other identified conditions.
Attempts to control the hemophagocytosis with steroids, epipodophyllotoxins, and intrathecal methotrexate with or without cranial radiation have, in general, offered patients only temporary remissions. Relapses occur an average of 5.4 months after initial chemotherapy.5 More recently, chronic combination immunosuppressive therapy including cyclosporine, steroids, and antithymocyte globulin (ATG) has been shown to be effective in inducing clinical remissions in patients with HLH.6 However, relapse of clinical HLH eventually occurs. Apparent cure of HLH has only been reproducibly achieved in patients undergoing allogeneic bone marrow transplantation (BMT). Because 20% or less of patients with HLH have HLA-matched sibling donors (MSD) available, unrelated donors (URD) have been increasingly used for BMT in patients with HLH. We report here our experience with 20 consecutive patients diagnosed with HLH who underwent BMT with either matched sibling (n = 4) or unrelated (n = 16) donors.
PATIENTS AND METHODS
Patients.From October 1988 through June 1995, 20 patients were referred for BMT to the University of Minnesota (Minneapolis, MN; n = 16) or the Children's Hospital Medical Center (Cincinnati, OH; n = 4) with a diagnosis of HLH based on the diagnostic criteria set forth by the HLH subcommittee of the Histiocyte Society7 (Table 1). Patient characteristics are detailed in Table 2. There were 13 male and 7 female patients. Age at the time of BMT ranged from 0.4 to 5.3 years, with a mean of 1.5 years and a median of 0.8 years. Family history of a previously affected sibling was present in 6 of 20 patients (30%).
Diagnostic Guidelines for HLH
| Fever: duration ≥7 days with peaks ≥38.5°C |
| Splenomegaly: 3 cm or more below the costal margin |
| Two of the following hematological abnormalities: |
| Anemia: hemoglobin <90 g/L |
| Thrombocytopenia: platelets <100 × 109/L |
| Neutropenia: neutrophils <1.0 × 109/L |
| Hypertriglyceridemia: ≥2.0 mmol/L or 3 SD over the normal value for the patients age and/or Hypofibrinogenemia: fibrinogen ≤1.5 g/L or 3 SD below the normal value for the patients age |
| Hemophagocytosis in bone marrow, spleen, or lymph node. No evidence of malignancy. |
| HLH: all of the above criteria are required for the diagnosis of HLH. |
| Familial HLH: Diagnosis of FHL is justified in the presence of a family history of HLH and all criteria listed above. |
| Fever: duration ≥7 days with peaks ≥38.5°C |
| Splenomegaly: 3 cm or more below the costal margin |
| Two of the following hematological abnormalities: |
| Anemia: hemoglobin <90 g/L |
| Thrombocytopenia: platelets <100 × 109/L |
| Neutropenia: neutrophils <1.0 × 109/L |
| Hypertriglyceridemia: ≥2.0 mmol/L or 3 SD over the normal value for the patients age and/or Hypofibrinogenemia: fibrinogen ≤1.5 g/L or 3 SD below the normal value for the patients age |
| Hemophagocytosis in bone marrow, spleen, or lymph node. No evidence of malignancy. |
| HLH: all of the above criteria are required for the diagnosis of HLH. |
| Familial HLH: Diagnosis of FHL is justified in the presence of a family history of HLH and all criteria listed above. |
Abbreviation: FHL, familial hemophagocytic lymphohistiocytosis.
Patient Characteristics
| UPN . | Family . | CNS . | HLH Stage . | Age at . | Sex . | Donor . | Engrafted . | Days to . | Maximum . | Maximum . | HLH Stage . | Status/Autopsy . | Survival . | Cause of Death . | Karnofsky . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | History . | Disease . | at BMT . | BMT (yr) . | . | Match . | . | ANC >500 . | aGVHD . | cGVHD . | Post-BMT . | (Y/N) . | (d) . | . | Score . |
| . | . | at DX . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 9905 | − | + | Quiescent | 0.4 | M | MSD 6/6 | + | 16 | 0 | 0 | NED | Died/(N) | 41 | Aspiration pneumonia | − |
| ø972 | − | + | Active | 0.6 | M | MSD 6/6 | + | 25 | 0 | 0 | NED | Died/(Y) | 43 | MOF | − |
| 1925 | − | − | Quiescent | 4 | M | MSD 6/6 | + | 29 | 0 | 0 | NED | Alive* | 1,029 | — | 100% |
| 2153 | + | + | Quiescent | 0.7 | M | MSD 6/6 | + | 19 | 0 | 0 | NED | Alive | 645 | — | 100% |
| 9901 | − | + | Active | 0.8 | M | URD 6/6 | + | 40 | 0 | 0 | Progressive | Died/(N) | 99 | Progressive HLH | — |
| 9902 | + | + | Quiescent | 1.5 | M | URD 6/6 | + | 25 | 0 | 0 | NED | Alive | 1,337 | — | 90% |
| 9904 | − | + | Quiescent | 3 | M | URD 6/6 | + | 19 | III | Extensive | NED | Alive | 1,116 | — | 60% |
| 1187 | − | + | Active | 0.6 | F | URD 5/6 | NE | NE | 0 | 0 | Progressive | Died/(Y) | 1 | Aspergillosis | — |
| 1221 | + | + | Active | 0.6 | F | URD 6/6 | NE | NE | 0 | 0 | Progressive | Died/(Y) | 180 | Progressive HLH | — |
| 1527 | − | + | Quiescent | 0.65 | F | URD 6/6 | + | 34 | II | Extensive | NED | Died/(N) | 463 | Sepsis | — |
| 1541 | − | − | Quiescent | 0.9 | F | URD 5/6 | + | 25 | II | 0 | NED | Died/(Y) | 46 | Hemorrhage | — |
| 1602 | − | − | Quiescent | 0.5 | M | URD 6/6 | + | 24 | I | 0 | NED | Died/(N) | 39 | ARDS | — |
| 1660 | + | − | Quiescent | 0.7 | M | URD 6/6 | + | 24 | 0 | Extensive | NED | Alive | 1,532 | — | 100% |
| 1769 | − | − | Quiescent | 0.6 | F | URD 5/6 | + | 16 | 0 | Extensive | NED | Alive | 1,278 | — | 100% |
| 1884 | − | − | Active | 1.7 | F | URD 6/6 | + | 25 | II | Limited | NED | Alive | 1,112 | — | 100% |
| 2034 | − | + | Quiescent | 1.3 | M | URD 5/6 | + | 23 | II | 0 | NED | Died/(Y) | 134 | ARDS | — |
| 2056 | + | + | Quiescent | 3.7 | F | URD 5/6 | + | 21 | II | 0 | NED | Died/(N) | 86 | Fungal infection | — |
| 2177 | − | − | Quiescent | 1.9 | M | URD 5/6 | + | 50 | I | 0 | NED | Alive | 609 | — | 100% |
| 2186 | + | + | Active | 5.3 | M | URD 5/6 | + | 19 | III | 0 | NED | Died/(N) | 85 | MOF/GVHD | — |
| 2229 | − | + | Quiescent | 0.56 | M | URD 6/6 | + | 29 | III | 0 | NED | Alive | 503 | — | 100% |
| UPN . | Family . | CNS . | HLH Stage . | Age at . | Sex . | Donor . | Engrafted . | Days to . | Maximum . | Maximum . | HLH Stage . | Status/Autopsy . | Survival . | Cause of Death . | Karnofsky . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | History . | Disease . | at BMT . | BMT (yr) . | . | Match . | . | ANC >500 . | aGVHD . | cGVHD . | Post-BMT . | (Y/N) . | (d) . | . | Score . |
| . | . | at DX . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 9905 | − | + | Quiescent | 0.4 | M | MSD 6/6 | + | 16 | 0 | 0 | NED | Died/(N) | 41 | Aspiration pneumonia | − |
| ø972 | − | + | Active | 0.6 | M | MSD 6/6 | + | 25 | 0 | 0 | NED | Died/(Y) | 43 | MOF | − |
| 1925 | − | − | Quiescent | 4 | M | MSD 6/6 | + | 29 | 0 | 0 | NED | Alive* | 1,029 | — | 100% |
| 2153 | + | + | Quiescent | 0.7 | M | MSD 6/6 | + | 19 | 0 | 0 | NED | Alive | 645 | — | 100% |
| 9901 | − | + | Active | 0.8 | M | URD 6/6 | + | 40 | 0 | 0 | Progressive | Died/(N) | 99 | Progressive HLH | — |
| 9902 | + | + | Quiescent | 1.5 | M | URD 6/6 | + | 25 | 0 | 0 | NED | Alive | 1,337 | — | 90% |
| 9904 | − | + | Quiescent | 3 | M | URD 6/6 | + | 19 | III | Extensive | NED | Alive | 1,116 | — | 60% |
| 1187 | − | + | Active | 0.6 | F | URD 5/6 | NE | NE | 0 | 0 | Progressive | Died/(Y) | 1 | Aspergillosis | — |
| 1221 | + | + | Active | 0.6 | F | URD 6/6 | NE | NE | 0 | 0 | Progressive | Died/(Y) | 180 | Progressive HLH | — |
| 1527 | − | + | Quiescent | 0.65 | F | URD 6/6 | + | 34 | II | Extensive | NED | Died/(N) | 463 | Sepsis | — |
| 1541 | − | − | Quiescent | 0.9 | F | URD 5/6 | + | 25 | II | 0 | NED | Died/(Y) | 46 | Hemorrhage | — |
| 1602 | − | − | Quiescent | 0.5 | M | URD 6/6 | + | 24 | I | 0 | NED | Died/(N) | 39 | ARDS | — |
| 1660 | + | − | Quiescent | 0.7 | M | URD 6/6 | + | 24 | 0 | Extensive | NED | Alive | 1,532 | — | 100% |
| 1769 | − | − | Quiescent | 0.6 | F | URD 5/6 | + | 16 | 0 | Extensive | NED | Alive | 1,278 | — | 100% |
| 1884 | − | − | Active | 1.7 | F | URD 6/6 | + | 25 | II | Limited | NED | Alive | 1,112 | — | 100% |
| 2034 | − | + | Quiescent | 1.3 | M | URD 5/6 | + | 23 | II | 0 | NED | Died/(Y) | 134 | ARDS | — |
| 2056 | + | + | Quiescent | 3.7 | F | URD 5/6 | + | 21 | II | 0 | NED | Died/(N) | 86 | Fungal infection | — |
| 2177 | − | − | Quiescent | 1.9 | M | URD 5/6 | + | 50 | I | 0 | NED | Alive | 609 | — | 100% |
| 2186 | + | + | Active | 5.3 | M | URD 5/6 | + | 19 | III | 0 | NED | Died/(N) | 85 | MOF/GVHD | — |
| 2229 | − | + | Quiescent | 0.56 | M | URD 6/6 | + | 29 | III | 0 | NED | Alive | 503 | — | 100% |
Abbreviations: ARDS, adult respiratory distress syndrome; cGVHD, chronic graft-versus-host disease; Dx, diagnosis; NE, not evaluable; NED, no evidence of disease; MOF, multiorgan failure.
Alive also indicates the patient to be free of HLH.
Central nervous system (CNS) involvement (as defined by cerebrospinal fluid [CSF] pleocytosis [white blood cell count (WBC) > upper limit of normal from referring institution] and elevated levels of protein, with or without evidence of hemophagocytosis in the cerebrospinal fluid) was found in 13 of 20 patients (65%) at the time of diagnosis. All patients were deemed eligible for high-dose chemotherapy and BMT based on standard evaluation criteria followed at each institution. Informed consent was obtained.
Pre-BMT therapy and disease status at time of BMT.All patients initially received chemotherapy and immunosuppressive therapy, after the diagnosis was made, consisting of etoposide (VP-16) and steroids with or without cyclosporine. With this therapy, 14 patients achieved a clinical remission defined by normalization of blood counts, triglyceride and fibrinogen levels, liver enzymes, and improvement or resolution of hepatosplenomegaly. Eight of 13 patients with CNS disease had resolution after treatment with intrathecal methotrexate, although 2 of those (unique patient no. [UPN] 9902 and 9904) also received cranial radiation (1,800 cGy) as part of their CNS therapy. Six patients who did not achieve a clinical remission underwent transplantion while showing evidence of active disease, such as persistent cytopenias, and liver dysfunction as well as massive hepatosplenomegaly (liver >3 cm below right costal margin; spleen >5 cm below left costal margin). Additionally 5 of those 6 had persistent cerebrospinal fluid abnormalities. Patients with only mild persistent hepatosplenomegaly (liver ≤3 cm below right costal margin; spleen ≤5 cm below left costal margin) were not considered to have active disease.
Natural killer (NK) cell function assays.Assays of NK cell function were performed on 18 of 20 patients pre-BMT and on all patients surviving ≥28 days posttransplantation at the University of Minnesota using a standard method. The function of NK cells was tested by quantitating the ability of peripheral blood lymphocytes from patients and normal controls to damage K562 leukemia target cells labeled with 51Cr. NK-mediated lysis was measured by the amount of 51Cr released into the culture supernatant after 4 hours of contact between the lymphocytes (effectors) and targets. Various concentrations of effectors were incubated together with a single concentration of targets at E:T ratios of 50:1, 25:1, 12.5:1, and 6.25:1 in quadruplicate in 96-well round-bottom plates at 37°C and 5% CO2 for 4 hours. Target cells incubated without effectors were used to generate background counts of spontaneous 51Cr release into the supernatant, whereas maximum uptake of 51Cr was determined by detergent lysis of effector cells, resulting in release of all of the radioisotope from target cells into the supernatant. After incubation, the plates were spun to pellet the cells and 10 μL of supernatant was collected from each well and counted in a gamma counter. The percentage of cytotoxicity for each E:T ratio was determined according to the following formula: % Cytotoxicity = (Mean Counts Per Million [cpm] of Dilution − Mean Spontaneous cpm/Mean cpm of Maximum − Mean Spontaneous cpm) × 100, and then expressed as lytic units per 106 cells.
Donors.HLA-matched sibling donors (MSD) were available for only 4 patients (20%). All donors were healthy without any abnormal physical findings. One sibling donor was found to have decreased NK function. The remaining 16 patients had unrelated donors identified through the National Marrow Donor Program; 8 were matched at HLA-A, B, DR (listed as 6 of 6 match in Table 2), 7 were mismatched at one HLA-A or -B locus, and 1 was a DR B1 minor mismatch (listed as 5 of 6 match in Table 2).
Transplant conditioning regimen graft-versus-host disease (GVHD) prophylaxis.Patients were admitted to the BMT units at each institution and were kept in high efficiency particular outflow (HEPA) filtered rooms. The preparative regimen consisted of oral busulfan at 4 mg/kg/d on days −9, −8, −7, and −6 (total dose, 16 mg/kg); intravenous cyclophosphamide at 50 mg/kg/d on days −5, −4, −3, and −2 (total dose, 200 mg/kg); and intravenous etoposide on days −4, −3, and −2 at a dose of 500 mg/m2/d (1,500 mg/m2 total dose), except for the last 4 patients transplanted at the University of Minnesota (UPNs 2153, 2177, 2186, and 2229) in whom the VP-16 dose was reduced to 300 mg/m2/d (900 mg/m2 total dose). Sodium-2-mercaptoethane sulfonate was administered on days of cyclophosphamide infusions for uroprophylaxis. All marrows were unmanipulated, and patients received a median cell dose of 3 × 108 nucleated cells/kg recipient body weight (range, 3.0 to 5.0 × 108 nucleated cells/kg). For patients undergoing BMT at the University of Minnesota, the 13 receiving marrow from URD received additional immunosuppression with intravenous ATG at 15 mg/kg twice daily on days −2, and −1 and 15 mg/kg once daily on days +1 and +2. For those patients, as well as 3 patients grafted with an MSD, GVHD prophylaxis was provided with short-course methotrexate (15 mg/m2 on day +1 and 10 mg/m2 days +3, +5, and +11) and cyclosporine. The remaining 3 patients transplanted at the Children's Hospital Medical Center in Cincinnati with URD (UPNs 9901, 9902, and 9904) and 1 with a sibling donor (UPN 9905) received combination immunosuppression/GVHD prophylaxis with ATG (40 mg/kg on days −6, −4, and −2 and 20 mg/kg on days +2, +4, +6, +8, +10, and +12), prednisone (days −2 through +14 and then tapered to day +28), and cyclosporine. All patients received intravenous gamma globulin (500 mg/kg weekly) to day 100.
Staging for GVHD.Acute GVHD (aGVHD) was assigned a grade and stage based on standard clinical criteria described previously,8 and the maximum overall clinical grade of GVHD that occurred was used for the analysis. Similarly, chronic GVHD was classified as either limited or extensive based on the Seattle clinicopathologic classification system.9
Regimen-related toxicity and engraftment.Regimen-related toxicity was evaluated based on the Seattle scale of regimen-related toxicity according to organ systems that has been reported previously.10 Day of engraftment was defined as the first day to an absolute neutrophil count greater than 500 cell/μL and documented to be of donor origin by either restriction fragment length polymorphisms (RFLP) analysis or standard cytogenetics performed between days 28 and 35.
Statistical methods.Overall survival and cumulative probabilities of developing GVHD were calculated using the product-limit method.11 Surviving patients were censored as of the date of analysis, October 25, 1996. Deceased patients without GVHD were censored at the date of death. Cox regression techniques12 were used to estimate the relative risk and corresponding 95% confidence interval. Variables evaluated in the regression model included recipient sex, age at the time of BMT, family history of HLH, CNS disease at diagnosis, and HLH stage (active v quiescent) at BMT.
RESULTS
The BMT preparative regimen was well tolerated and no deaths occurred secondary to regimen-related toxicity. There were 2 patients who experienced grade 3 mucositis and required elective intubation for airway protection; both survived. All patients surviving past 20 days had full recovery of hematopoiesis. In 16 patients engraftment was documented between days 28 and 35 by RFLP analysis and by standard cytogenetics on 1 patient (UPN 9904) whose donor was of opposite sex. There were 3 patients with same-sex donors in whom neither RFLP or standard cytogenetics were performed (UPNs 9901, 9902, and 9905). The average length of time for the absolute neutrophil count to reach 500/μL was 24 days (range, 16 to 43 days). The graft was durable, with no graft failures, although 1 patient (UPN 9901) after initial engraftment experienced progression of HLH and subsequently became pancytopenic. His bone marrow remained completely hypocellular with no hematopoietic recovery, and no cytogenetic or RFLP analysis was performed before his death.
Additional evidence to support engraftment, initial immunologic recovery and reversal of HLH was provided by assays of NK function. NK function was evaluated on 18 patients pre-BMT and on all patients surviving greater than 28 days post-BMT (Table 3). Before BMT, NK function was found to be either absent (n = 15) or markedly decreased (n = 2), except in 1 patient who had normal pre-BMT NK function. NK function was then followed post-BMT, and by 6 months, 6 surviving patients were found to have normal NK function, 1 was decreased after previously being normal at 3 months, and 2 patients did not have NK function repeated, although they had been normal (UPN 1925) or nearly normal (UPN 9902) at 3 months. In 2 additional patients (UPNs 9904 and 1769) who developed chronic GVHD and required long-term immunosuppression, NK function was normal at 3 months and at 6 months was present but decreased.
NK Cell Function
| UPN . | Pre-BMT . | Post-BMT . | Post-BMT . |
|---|---|---|---|
| . | NK . | NK . | NK . |
| . | Function3-150 . | Function3-150 . | Function3-150 . |
| . | . | (3 mo) . | (6 mo) . |
| 9905 | 0 | NE | — |
| ø972 | ND | NE | — |
| 1925 | 0 | 7.40 | ND |
| 2153 | 0 | 2.26 | 5.75 |
| 9901 | 0 | NE | — |
| 9902 | 0 | 3.82 | ND |
| 9904 | 0 | 25.60 | 0.40 |
| 1187 | 13.90 | NE | — |
| 1221 | 0 | NE | — |
| 1527 | 0 | 3.60 | 7.00 |
| 1541 | 0 | NE | — |
| 1602 | 0.06 | NE | — |
| 1660 | 0 | 2.75 | 15.06 |
| 1769 | 0 | 8.70 | 0.87 |
| 1884 | ND | 1.28 | 19.90 |
| 2034 | 0 | 0.01 | 7.00 |
| 2056 | 0 | NE | — |
| 2177 | 0 | 8.26 | 16.50 |
| 2186 | 0.58 | 0 | NE |
| 2229 | 0 | 5.18 | 0.63 |
| UPN . | Pre-BMT . | Post-BMT . | Post-BMT . |
|---|---|---|---|
| . | NK . | NK . | NK . |
| . | Function3-150 . | Function3-150 . | Function3-150 . |
| . | . | (3 mo) . | (6 mo) . |
| 9905 | 0 | NE | — |
| ø972 | ND | NE | — |
| 1925 | 0 | 7.40 | ND |
| 2153 | 0 | 2.26 | 5.75 |
| 9901 | 0 | NE | — |
| 9902 | 0 | 3.82 | ND |
| 9904 | 0 | 25.60 | 0.40 |
| 1187 | 13.90 | NE | — |
| 1221 | 0 | NE | — |
| 1527 | 0 | 3.60 | 7.00 |
| 1541 | 0 | NE | — |
| 1602 | 0.06 | NE | — |
| 1660 | 0 | 2.75 | 15.06 |
| 1769 | 0 | 8.70 | 0.87 |
| 1884 | ND | 1.28 | 19.90 |
| 2034 | 0 | 0.01 | 7.00 |
| 2056 | 0 | NE | — |
| 2177 | 0 | 8.26 | 16.50 |
| 2186 | 0.58 | 0 | NE |
| 2229 | 0 | 5.18 | 0.63 |
Abbreviations: ND, not done; NE, died before evaluation.
NK function expressed as lytic units, normal range >4 lytic units.
The probability of developing clinical grade II-III aGVHD was 57% (95% confidence limit [CL], 0.28, 0.86). For the URD patients, the probability of grade II-III aGVHD was somewhat higher at 73% (95% CL, 0.44, 1.0). No cases of grade IV GVHD were diagnosed. In only 1 patient was GVHD felt to be a significant factor contributing to death (UPN 2186). This patient died from acute GVHD of the gastrointestinal tract, complicated by disseminated intravascular coagulation and multiorgan failure. An autopsy was not performed. Five patients developed chronic GVHD (4 extensive and 1 limited) and 4 are surviving. UPN 1527 died 463 days after BMT of sepsis with extensive chronic GVHD.
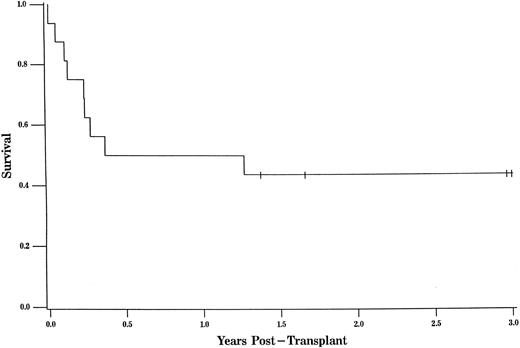
The overall survival was 45% at 3 years of follow-up (95% CL, 0.23, 0.67; Fig 1). The overall survival for URD recipients at 3 years was nearly identical at 44% (95% CL, 0.19, 0.68; Fig 2).
The probability of survival for all patients was 45% at 3 years of follow-up (95% CL, 0.23, 0.67). Tic marks represent surviving patients censored as of the date of analysis, with a single tic mark at 3 years representing 4 patients who survive more than 3 years.
The probability of survival for all patients was 45% at 3 years of follow-up (95% CL, 0.23, 0.67). Tic marks represent surviving patients censored as of the date of analysis, with a single tic mark at 3 years representing 4 patients who survive more than 3 years.
The probability of survival for patients undergoing URD transplant was 44% at 3 years of follow-up (95% CL, 0.19, 0.68). Tic marks represent surviving patients censored as of the date of analysis, with a single tic mark at 3 years representing 4 patients who survive more than 3 years.
The probability of survival for patients undergoing URD transplant was 44% at 3 years of follow-up (95% CL, 0.19, 0.68). Tic marks represent surviving patients censored as of the date of analysis, with a single tic mark at 3 years representing 4 patients who survive more than 3 years.
Survival for patients transplanted while showing evidence of active HLH was significantly worse than for those patients who were transplanted in clinical remission (17% compared with 54% at 3 years; P = .04; 95% CL, 0.25, 0.82). There were 6 patients who underwent BMT with active HLH as indicated by the presence of cytopenias, elevated liver enzymes, and significant hepato-splenomegaly. Five of those patients also had CNS manifestations of HLH at the time of BMT. All 5 of those died, including the 3 patients who had evidence of progressive HLH after transplant. Only 1 patient with active disease at the time of BMT survived (UPN 1884). She did not have CNS involvement at diagnosis.
CNS involvement was present in 65% (13 of 20) of the patients at the time of diagnosis. Of the 13 patients with CNS disease at diagnosis, 8 were transplanted when the disease was in remission (including CNS) and, of those, 4 were long-term survivors.
Several other factors were analyzed to determine their impact on overall survival. These factors included recipient sex, age at the time of BMT, and a family history of HLH; however, none were found to have any statistically significant effect on survival. Age at the time of diagnosis was also evaluated and patients greater than 1 year of age when diagnosed had an overall survival of 63%, compared with those patients less than 1 year old when diagnosed who had an overall survival of 28%. However, due to the small sample size, this difference was not statistically significant (P = .13).
All surviving patients had Karnofsky performance scores of 90% to 100%, with the exception of 1 patient (UPN 9904) who had a score of 60%. That patient had significant CNS involvement pre-BMT and developed acute and extensive chronic GVHD post-BMT. Of the 11 deaths, only 3 were associated with relapse of HLH and all had active disease at the time of BMT. Death in the other 8 patients was attributed to infection (2), adult respiratory distress syndrome (2), hemorrhage after liver biopsy (1), multiorgan failure (1), GVHD/multiorgan failure (1), and aspiration pneumonia (1). Of these 8 patients, none had clinical evidence of active HLH at the time of death and 5 had autopsies performed that found no evidence of hemophagocytosis.
DISCUSSION
Recently reported data on 122 patients from the HLH registry13 found the estimated 5-year survival in patients with HLH treated with chemotherapy alone to be 10.1%. It is also well known that, despite chemotherapy responses, late relapses will occur.14 Additionally, from the registry, a 66% 5-year survival for allogeneic BMT was reported, the majority of those being MSD transplants. Others have reported that BMT can correct the immunologic defect in patients with HLH and provide long-term, durable remissions of the disease.5,15-20 Although it would be preferable to offer a matched sibling donor transplant to these patients, in our experience this donor type has been available in less than 20% of patients. There also exists the concern that siblings being used as donors may also be affected with HLH but have yet to demonstrate symptoms. Such a case has been reported previously.16 One patient in this series was transplanted with marrow from a sibling who had decreased NK function (UPN 9905). This patient died early from aspiration pneumonia, and the sibling has remained healthy with no signs of HLH. Even though the age of symptomatic onset has been similar in most affected siblings, differences of up to 3 years have been noted.13 Therefore, unrelated donor BMT may have a theoretical advantage over MSD, and at this point in time may provide the only chance of long-term survival for a majority of children with HLH. Our analysis represents the largest experience to date with unrelated BMT for this disorder, demonstrating a disease-free survival of 44% at 3 years.
There has been concern that the time required to identify an unrelated donor may adversely affect outcome for patients with HLH and other highly lethal immune deficiencies. However, during the course of this study no patient died while waiting for an unrelated donor to be identified. The majority of delay for patients coming to BMT was not from the unrelated search process, but secondary to delays in diagnosis, induction of clinical remission, subsequent referral to a BMT center, and approval of insurance funding.
The regimen-related toxicity observed in this group of patients consisted primarily of oral mucositis, which prompted elective intubation (for airway protection) in two infants. However, severe mucositis was reduced by decreasing the total dose of VP-16 from 1,500 mg/m2 to 900 mg/m2 (300 mg/m2/d for 3 days). Additionally, no excessive incidence of transplant-related mortality or of severe aGVHD was observed even in the unrelated donor setting. The cytoreductive regimen consisting of busulfan, cyclophosphamide, and VP-16 was adequate for eradication of the underlying disease and allowed persistent engraftment of both matched-sibling and unrelated donor marrows.
Patient age was not identified as a statistically significant prognostic factor for survival. However, 4 of the 6 patients who underwent BMT with active disease were less than 1 year of age at the time of diagnosis and subsequent BMT. It has been observed that patients who present in the first few weeks of life tend to have a more aggressive phenotype of the disease that is inherently more difficult to control. Once the diagnosis of HLH is made, it is important to proceed to BMT as quickly as possible because the outcome with chemotherapy alone is dismal and relapses are often difficult to treat.13 14
It has been previously reported that abnormalities of NK cell function may be present in patients with HLH.21 Data from the HLH registry found 36 of 37 patients evaluated to have impaired NK activity.13 Likewise, in our patients, NK function was absent or decreased in all but 1 who was tested. After BMT, all patients were monitored for the return of NK function. It was shown that in all surviving patients NK function was normal by 3 or 6 months after BMT. Therefore, NK function appears to be a good indicator of disease status, and correlates well with donor cell engraftment, and posttransplantation immunologic recovery.
CNS involvement is a common finding at diagnosis in patients with HLH.2 22 Sixty-five percent (13 of 20) of this group of patients had CNS disease at the time of diagnosis. Five of 6 patients transplanted with evidence of active systemic disease also had CNS involvement at the time of BMT. All 5 of those patients died, including the 3 patients in this series who had progressive HLH post-BMT. However, 50% of patients (4 of 8) with prior CNS involvement at diagnosis that was quiescent at the time of BMT had a favorable outcome. Therefore, CNS disease at diagnosis did not signify a worse prognosis if clinical CNS remission was achieved before BMT.
One patient in this series (UPN 9904) who was judged to have quiescent CNS disease at the time of BMT experienced neurologic deterioration (with blindness, loss of developmental milestones, and partial paresis) after BMT with an associated CSF pleocytosis. It is unclear whether this was due to the underlying disease, to drug toxicity secondary to the BMT preparative regimen and cyclosporine, or to intensive pre-BMT treatment with intrathecal methotrexate and cranial radiation. In his case, CNS deterioration was temporally associated with the development of aGVHD. Although the CSF findings normalized in this patient, the neurologic abnormalities persisted. It is possible that it may take longer after BMT for the normalization of immunologic regulation to occur in the CNS and that the consequences of histiocytic infiltration may continue until this has occurred. Alternatively, the inflammatory component of aGVHD may in some way accelerate tissue damage already initiated by the underlying HLH involvement in the CNS. In summary, our experience suggests that CNS involvement does not preclude successful BMT, but may adversely effect prognosis when present in a patient who does not achieve a complete clinical remission before BMT.
Persistent unexplained fevers, cytopenias, elevated liver enzymes and hypofibrinogenemia with hepatosplenomegaly, CSF abnormalities, and/or increased triglyceride levels all suggest the presence of continued active HLH. Of the 6 patients in whom a clinical remission could not be secured before BMT, only one is a long-term survivor. However, the number of patients in this circumstance reported here is small, and there is no identified alternative treatment at this time. Furthermore, clinical assessment of HLH disease activity is subjective and imprecise. Given the rarity of this condition, few transplant centers and investigators have much experience with the variability of this disease. Previous efforts at prolonged chemotherapy administration with VP-16 have been unsuccessful due to eventual relapse of HLH. Furthermore, the development of myelodysplastic syndrome has been reported in an HLH patient treated with VP-1623 as well as in patients receiving this drug for malignancies. Further efforts towards developing improved therapies to induce and maintain clinical remissions of HLH are needed. Hopefully these will emerge from a deeper understanding of the imbalances in the immunoregulatory molecules responsible for disease progression.
Despite published19 reports of failures using unrelated donor BMT for patients with HLH, the experience reported here shows that the use of this donor source can be justified. BMT after cytoreductive therapy with busulfan, cyclophosphamide, and VP-16 was well tolerated, effective at eradicating the underlying disease, and provided durable engraftment in the related and unrelated donor setting. Further improvements in the prevention and/or treatment of acute and chronic GVHD should reduce transplant-related morbidity and perhaps mortality. The difficult question remains as to whether patients with evidence of active disease should be offered the opportunity to undergo BMT. The poor survival of patients transplanted while showing evidence of active disease reported here is no better than that achieved historically with chemotherapy and immunosuppression. Hopefully, international collaboration and participation in standardized protocols of pre-BMT therapy such as that advised by the HLH Subcommittee of the Histiocyte Society in the HLH-94 protocol (with VP-16, steroids, and cyclosporine)24 will ultimately improve the outlook for this difficult group of patients.
ACKNOWLEDGMENT
The authors thank Dr Bruce Gordon for critical review of this manuscript as well as the other members of the bone marrow transplant teams at the University of Minnesota (Minneapolis, MN) and the Children's Hospital Medical Center (Cincinnati, OH), who helped care for these patients, and the National Marrow Donor Program for identification of the unrelated donors.
Address reprint requests to K. Scott Baker, MD, University of Nebraska Medical Center, Department of Pediatrics, Section of Pediatric Hematology/Oncology and Bone Marrow Transplant, 600 S 42nd St, Omaha, NE 68198-2168


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal