Abstract
In this study we investigated the possibility that an alternative pathway exists for neutrophil recruitment, namely an α4β1 -dependent pathway. A parallel plate chamber was used to investigate whether neutrophils could tether, roll, and adhere to tumor necrosis factor α (TNFα)-stimulated endothelium via α4β1 . α4β1 -integrin was induced on neutrophils using dihydrocytochalasin B and either an endogenous (endothelial-derived) chemotactic agent or an exogenous chemotactic molecule. α4β1 -expressing neutrophils could stably adhere under shear force (2 dyne/cm2) to TNFα-stimulated endothelium independent of the β2 -integrin. The firm adhesion was entirely abolished by antibodies directed against either the α4 or β1 -integrin subunits. However, the rolling interaction was not dependent on α4β1 but was abolished by antiselectin therapy. Neutrophils expressing α4β1 could also tether to the endothelium in the presence of antiselectin therapy, but at shear stresses less than 2 dyne/cm2. α4β1 -expressing neutrophils also tethered to and stably adhered (no rolling) to VCAM-1– but not to ICAM-1–transfected L cells. The interaction only occurred at shear stress less than 2 dyne/cm2. A cell line (Ramos) known to express high quantities of α4β1 -integrin interacted with VCAM-1–transfected L cells at very similar shear conditions. α4β1 -expressing neutrophils were also able to adhere to a second α4 -integrin ligand, fibronectin; however, this interaction only occurred under static conditions. These data suggest that, under certain conditions, neutrophils can adhere independently of the β2 -integrin pathway and adhere via the α4β1 -integrin. This study refutes the concept that α4β1 -integrin adhesion is restricted to mononuclear leukocytes and is not functional on human neutrophils.
INAPPROPRIATE activation or recruitment of neutrophils has been implicated in the pathogenesis of various inflammatory diseases.1 The recruitment process is characterized by a cascade of events, starting with the neutrophil initially tethering to activated endothelium and then rolling along the endothelial surface. The tethering and rolling event is thought to be dependent on a number of adhesion molecules on the surface of neutrophils, including L-selectin2,3 and P-selectin glycoprotein ligand-1 (PSGL-1).4 There is also a requirement for the expression of counterligands on endothelium, including P-selectin, a rapidly mobilized molecule,5-8 and E-selectin, which is expressed after cytokine stimulation.9 The identity and expression profiles of other selectin ligands remain unclear. The rolling velocity of neutrophils is then reduced and the cells come to a complete stop. This firm adhesion is known to be mediated by β2 integrins (CD11/CD18) on the neutrophil surface.10 Although this cascade of events has been proposed for neutrophils as well as other leukocytes, it is becoming clear that non-neutrophilic leukocytes can use a second integrin, α4β1 , to firmly adhere and in some but not all instances to tether and roll.11 12 The importance of this observation is that α4β1 -integrin could conceivably replace the selectin and β2 -integrin pathway and constitute an alternative recruitment pathway.
Based on the fact that neutrophils are generally thought not to express α4β1 -integrin, this pathway should be unimportant in neutrophil recruitment. However, we have recently reported that human neutrophils stimulated with chemotactic factors in the presence of dihydrocytochalasin B (DHCB) to optimize neutrophil effector responses expressed α4β1 -integrin.13 This expression occurred quickly (within 10 minutes), indicating that α4β1 was stored and available for rapid mobilization. Although DHCB was not a physiologic stimulus, transmigration of neutrophils across endothelium also induced the expression of α4β1 -integrin on neutrophils, suggesting, albeit indirectly, that nonpharmacologic stimuli can also induce expression of α4β1 -integrin on the surface of neutrophils. Interestingly, a recent publication reported that circulating rat neutrophils express α4 -integrin and that blockade of α4 -integrin improved the ability of a β2 -integrin antibody to inhibit neutrophil infiltration into arthritic joints.14 Although intriguing, the inhibition of neutrophil infiltration by an antibody that immunoneutralizes α4 -integrin may be related to indirect effects such as inhibition of mononuclear cell infiltration that could affect subsequent neutrophil recruitment.
Therefore, the objective of this study was to determine whether α4β1 -integrin could directly contribute to neutrophil interactions with biologic substrata under shear conditions. To do this, we used a flow chamber apparatus to mimic the shear conditions found in blood vessels and examined whether DHCB-primed neutrophils were able to use α4β1 for rolling and adhesion on tumor necrosis factor α (TNFα)-stimulated endothelium. Next, we examined whether neutrophils could use α4β1 to interact with VCAM-1–transfected L cells at a variety of shear forces and we examined how these interactions compared with interactions of Ramos cells that are known to express α4β1 .15 Finally, we examined whether α4β1 -expressing neutrophils could interact with another α4β1 -integrin ligand, fibronectin, under both static and shear conditions.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs) and reagents.The MoAb IB4 (anti-CD18) was a gift from Dr Karl E. Arfors (Experimental Medicine Inc, Princeton, NJ). MoAb EL-246 (anti–E/L-selectin) was a gift from Dr Mark Jutila (Montana State University, Bozeman, MT). MoAb HP2/1 (anti-α4 ) was purchased from Serotec (Kidlington, Oxford, UK) and MoAb HP1/2 (anti-α4 ) was a gift from Dr Roy Lobb (Biogen, Cambridge, MA). MoAbs K20 and LIA 1/2 (anti–β1 -integrin antibodies) were purchased from AMAC Inc (Westbrook, ME). TNFα was a gift from Knoll Pharmaceutical (Markum, Ontario, Canada). Purified human fibronectin fragment 40K was purchased from Chemicon International Inc (Temecula, CA). Unless otherwise stated, all other reagents were purchased from Sigma Chemical Co (St Louis, MO).
Preparation of substrates.Human umbilical vein endothelial cells (HUVEC) were harvested from freshly collected umbilical cords. Briefly, umbilical cord veins were rinsed of formed blood products with phosphate-buffered saline (PBS), after which the vein was filled with a collagenase solution (320 U/mL in PBS). After 20 minutes of incubation at 37°C, the cords were gently massaged to ensure detachment of endothelial cells from the vessel wall. The digest was collected into centrifuge tubes and the collagenase was inactivated with fetal calf serum (FCS), after which the tube was centrifuged (400g for 10 minutes at 37°C). The pellet was resuspended in medium 199 containing 10% FCS, endothelial mitogen (Biomedical Technologies, Inc, Stoughton, MA), and antibiotics. The endothelial cells were seeded onto 75-cm2 culture flasks and maintained in 5% CO2 at 37°C and 96% humidity. Occasionally, the identity of endothelial cultures was confirmed by staining with fluorescein isothiocyanate-labeled factor VIII antibody. HUVEC were expanded by trypsinization and used for experimentation before the fifth passage.
The construction of mouse L-cell transfectants was accomplished as follows. cDNAs encoding VCAM-1 and ICAM-1 proteins were generated by polymerase chain reaction and ligated into the plasmid pJFE14 using the Xba I and Not I sites. pMSD4-HPRT, which expresses murine HPRT under the control of a Moloney murine leukemia virus promoter, was a gift from Dr D. Denney (Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, Alberta, Canada). Mouse LA9 cells (American Type Culture Collection [ATCC], Rockville, MD) that permanently express VCAM-1 or ICAM-1 were constructed by cotransfecting Sal I-cut pJFE14 containing the appropriate cDNA (200 μg/electrophoration) and Sal I-cut pMSD4-HPRT DNA (10 μg/electrophoration). After electrophoration (BioRad Gene Pulser apparatus; BioRad, Mississauga, Ontario, Canada), cells were cultured in selection media (Dulbecco's modified Eagle's medium [DMEM; GIBCO, Grand Island, NY] with 10% fetal calf serum [Hyclone, Logan, UT] and hypoxanthine 100 μmol/L/azaserine 5.8 μmol/L). After 10 to 14 days, single colonies of HPRT+ LA9 cells were picked using cloning cylinders and expanded. Clones were incubated for an additional 6 to 10 days in selection media, after which they were cultured in DMEM containing 10% FCS. VCAM-1 expression on TNFα-stimulated HUVEC was 60% of that found on VCAM-1 transfectants as determined by enzyme-linked immunosorbent assay.
Neutrophil isolation, labeling, and treatment.Human neutrophils were harvested from citrate anticoagulated venous blood collected from healthy donors as previously described,9 with minor modifications. All isolation steps were performed at room temperature. Neutrophils were purified by dextran sedimentation (Dextran 250,000; Spectrum Chemicals, Gardena, CA) followed by centrifugation through a density gradient (6.07% Ficoll Type 400 [Sigma] with 10% Hypaque Sodium [Markham, Ontario, Canada]). Isolated neutrophils were resuspended in Hanks' balanced salt solution (HBSS) at a density of 1 × 106 cells/mL. This yielded neutrophils that were 97% pure and 95% viable.
α4β1 -dependent interactions of neutrophils with various substrata were compared directly with those of Ramos cells (ATCC) that use this integrin to tether, roll, and adhere. Ramos cells were cultured in RPMI medium 1640 with 10% FCS. For experimental procedures, Ramos cells were pelleted and resuspended in HBSS at a density of 1 × 106 cells/mL.
For static adhesion assays, neutrophils were radiolabeled by incubating purified neutrophils with Na51CrO4 (30 μCi/mL) at 37°C for 30 minutes. The cells were washed three times and resuspended in HBSS at 2 × 107 cells/mL.
Flow chamber assay.To study neutrophil behavior under shear conditions, a flow chamber assay was established as previously described.16 HUVEC, VCAM-1 transfectants, or ICAM-1 transfectants were seeded onto glass coverslips (Fisher Scientific, Ottawa, Ontario, Canada) at a density of 1 × 106 cells per coverslip. The cells were allowed to grow to form confluent monolayers. Coverslips were mounted into a polycarbonate chamber with parallel plate geometry. The flow chamber was placed onto the stage of an inverted microscope (Zeiss, Don Mills, Ontario, Canada) and monolayers were visualized at 200× magnification using phase contrast imagery. The stage area was enclosed in a warm air cabinet and maintained at 37°C. Neutrophil or Ramos cell suspensions were warmed to 37°C using a water bath. A syringe pump (Harvard Apparatus, St. Laurent, Quebec, Canada) was used to draw the cell suspensions through the flow chamber at defined wall shear stresses. Experiments were video recorded for later analysis via a CCD camera (Hatachi Denshi, Ltd, San Jose, CA) and a video cassette recorder (Panasonic, Secaucus, NJ) that was attached to the microscope.
α4β1 -dependent neutrophil interactions with TNFα-stimulated endothelium (300 U/mL) were performed in the presence of DHCB at 2.5 μg/mL. This concentration of DHCB in the presence of an endothelial-derived chemotactic agent (as a result of TNFα stimulation) has previously been shown to induce significant α4β1 -integrin expression on neutrophils.13 DHCB was present in the perfusion medium throughout the tethering, rolling, and adhesion assays. Neutrophils were pretreated with DHCB at 2.5 μg/mL for 10 minutes. MoAb IB4 (20 μg/mL) directed against the β2 -integrin was used alone or in combination with anti-α4 MoAbs (HP2/1 [2.0 μg/mL], HP1/2 [2.0 μg/mL]) or anti–β1 -integrin (MoAbs LIA 1/2 [2.0 μg/mL] or K20 [2.0 μg/mL]) to elucidate the adhesive mechanism of DHCB-pretreated neutrophils with TNFα-stimulated HUVEC under shear conditions (2 dyne/cm2 ). A role for selectins on α4β1 -expressing neutrophils was determined using an anti–E/L-selectin antibody MoAb EL-246 (50 μg/mL) or the selectin-binding carbohydrate fucoidan (75 μg/mL). In additional experiments, MoAb EL-246 was added and neutrophils were perfused under conditions of decreasing shears (0.5 dyne/cm2 at 2-minute intervals) in the presence and absence of HP2/1. This was performed to establish whether α4β1 could tether neutrophils to endothelium independent of selectins.
To determine whether α4β1 -expressing neutrophils could support interaction with VCAM-1–transfected L cells, neutrophils expressing α4β1 were perfused over either VCAM-1 or ICAM-1 transfectants at a starting shear of 2 dyne/cm2. Because neither Ramos cells nor α4β1 -expressing neutrophils would tether to VCAM-1 transfectants, shear was decreased by 0.5 dyne/cm2 at 2-minute intervals. In this series of experiments, TNFα could not be used (as endothelium was not used), so neutrophils were pretreated with an exogenous chemotactic agent FMLP (20 μmol/L) + DHCB (2.5 μg/mL) for 10 minutes, which also expresses α4β1 .13 In additional experiments, Ramos cells were also pretreated with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL for 10 minutes) to increase α4β1 avidity. Detachment assays were performed on α4β1 -expressing neutrophils or Ramos cells by allowing the leukocytes to settle for 2 minutes on VCAM-1 transfectants under static conditions and then re-establishing flow through the chamber to 2 dyne/cm2.
In addition to α4β1 -integrin interacting with VCAM-1, α4β1 also interacts with the CS-1 region of fibronectin.17 In a final series of flow experiments, α4β1 -expressing neutrophils were perfused over fibronectin-treated coverslips (25 μg/mL for 30 minutes) under conditions of decreasing shear. Neither Ramos nor α4β1 -expressing neutrophils interacted with this surface at any shear stress tested; therefore, static adhesion assays were performed.
Static adhesion assay.Forty-eight–well plates (Costar, Cambridge, MA) were coated with human fibronectin (25 μg/mL in PBS) and radiolabeled neutrophils were added to the wells (1 × 106/well in 500 μL HBSS) and the plate was incubated for 30 minutes at 37°C. At the end of the incubation, the supernatant of each well was collected and the wells were gently washed with 500 μL of HBSS. The cells that remained adherent were lysed by an overnight incubation with 500 μL of NaOH (2 N). Supernatant, wash, and lysate were analyzed for 51Cr activity and the neutrophil adherence was calculated as the ratio of radioactivity in the cell lysate versus that in supernatant, wash, and lysate combined. α4β1 -expressing neutrophils were exposed to fibronectin in the presence of antibody IB4 (20 μg/mL) in combination with a fibronectin fragment (fragment 40K, 0.2 μg/mL) that contains the CS-1 binding region for α4β1 or with antibody HP2/1 (2.0 μg/mL).
Experimental analysis and statistics.Rolling and adhesive interactions of perfused cells with cell monolayers was determined for each minute of perfusion. Cell counts were made for the field of microscopic view and represented as cells per millimeter squared of the monolayer surface. A rolling cell was defined as a cell that was in contact with the monolayer but was not stationary. These cells could be observed to roll end over end across the monolayer surface. An adherent cell was defined as one that was in contact with the monolayer and remained stationary for at least 10 seconds. In experiments in which flow in the chamber was stopped, rolling and adherent leukocyte number was determined for the first minute of flow reperfusion. All experiments were repeated between 3 and 8 times. All values are expressed as mean ± SEM, and means were compared by a Student's t-test with Bonferroni correction for multiple comparisons. Statistical significance was set at P < .05.
RESULTS
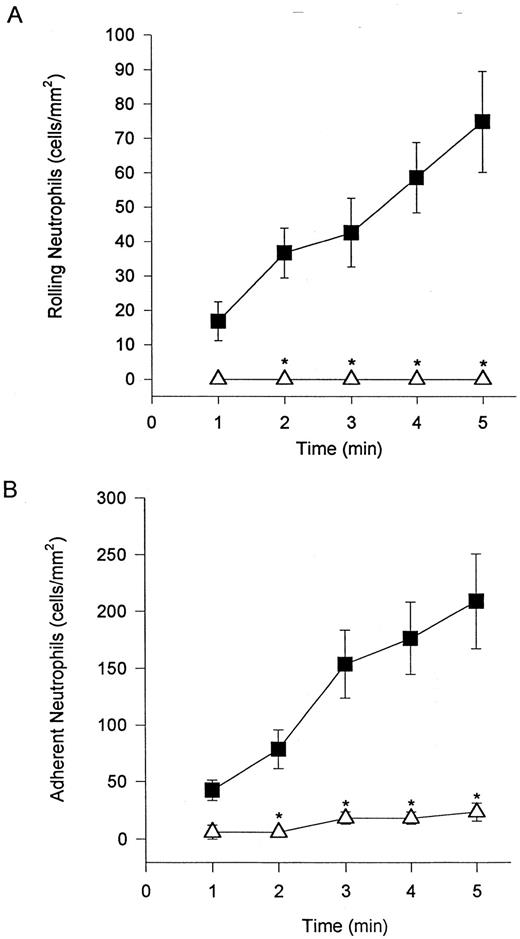
Unstimulated neutrophils interact with cytokine-stimulated endothelium under shear conditions via selectins and β2 -integrins.Freshly isolated neutrophils were observed to tether and roll on TNFα-stimulated endothelium (HUVEC) at a wall shear stress of 2 dyne/cm2 (Fig 1A). Also, rolling cells were observed to stop and firmly adhere to the endothelium (Fig 1B). Tethering, rolling, and adhesive interactions increased during the time of perfusion. The addition of an MoAb (EL-246) that inhibits the actions of both E- and L-selectin completely blocked tethering and rolling (Fig 1A), indicating that these interactions are selectin-dependent. Identical results were observed when fucoidan, a selectin-binding polysaccharide, was administered (data not shown). These regimens also completely inhibited neutrophil adhesion, consistent with the view that tethering and rolling are a prerequisite for firm adhesion. The addition of an MoAb (IB4 ) that immunoneutralizes β2 -integrins blocked neutrophil adhesion to TNFα-stimulated endothelium (Fig 1B). IB4 did not inhibit this adhesion by affecting the ability of neutrophils to tether and roll on endothelium. In fact, IB4 pretreatment resulted in an increase in the number of rolling cells (data not shown) most likely as a result of the reduced propensity of these cells to adhere.
Neutrophils tether to TNFα-treated endothelium, roll (A), and adhere (B). The tethering and rolling was entirely inhibited by an E-selectin/L-selectin antibody (EL-246; A). The anti-CD18 antibody (IB4 ) inhibited neutrophil adhesion (B) without inhibiting rolling (data not shown). (▪) Without antibody; (▵) with antibody. *P < .05 relative to untreated neutrophils.
Neutrophils tether to TNFα-treated endothelium, roll (A), and adhere (B). The tethering and rolling was entirely inhibited by an E-selectin/L-selectin antibody (EL-246; A). The anti-CD18 antibody (IB4 ) inhibited neutrophil adhesion (B) without inhibiting rolling (data not shown). (▪) Without antibody; (▵) with antibody. *P < .05 relative to untreated neutrophils.
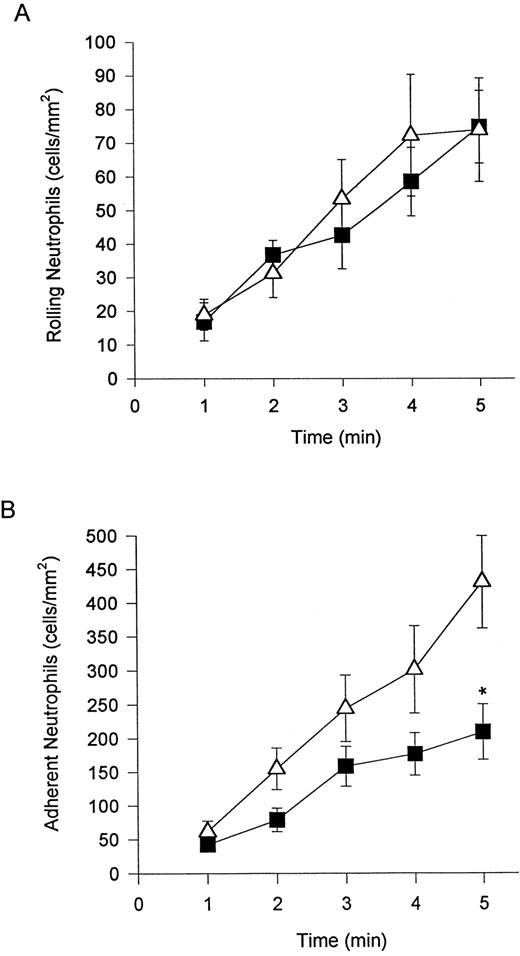
α4β1 -expressing neutrophils adhere to TNFα-stimulated endothelium independent of β2 -integrin.We have previously shown that neutrophils can be induced to express α4β1 when stimulated with DHCB in combination with an exogenous chemotactic stimulus or in the presence of an endogenous chemotactic stimulus (derived from TNFα-stimulated endothelium).13 The addition of DHCB did not increase the number of rolling cells (Fig 2A) or affect rolling velocity (data not shown) on TNFα-stimulated endothelium. DHCB treatment did result in a greater propensity of rolling cells to adhere (a 2-fold increase) relative to their untreated neutrophil counterparts (Fig 2B). The rolling of DHCB-treated neutrophils was still entirely selectin-mediated, because pretreatment with EL-246 (Fig 3A) or fucoidan (data not shown) completely blocked any rolling interaction. DHCB-treated neutrophil adhesion was reduced by the β2 -integrin antibody (IB4 ), but a significant degree of CD18-independent adhesion remained (Fig 3B). It should be noted that neither untreated nor DHCB-treated neutrophils interacted with unstimulated endothelium under shear conditions of 2 dyne/cm2.
Addition of DHCB (causes α4β1 expression in the presence of chemotactic stimuli) had no further effect on the number of rolling neutrophils on TNFα-treated endothelium (A). Neutrophil adhesion (B) was further increased (doubled) relative to neutrophils not treated with DHCB. (▪) Untreated; (▵) DHCB-treated. *P < .05 relative to treated cells.
Addition of DHCB (causes α4β1 expression in the presence of chemotactic stimuli) had no further effect on the number of rolling neutrophils on TNFα-treated endothelium (A). Neutrophil adhesion (B) was further increased (doubled) relative to neutrophils not treated with DHCB. (▪) Untreated; (▵) DHCB-treated. *P < .05 relative to treated cells.
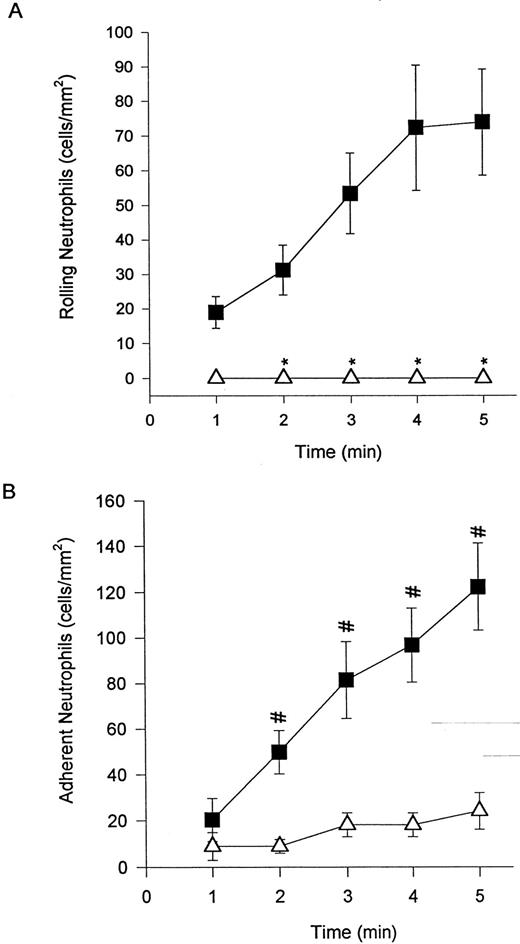
Addition of EL-246 eliminated neutrophil rolling (A) and adhesion (data not shown) of DHCB-treated cells. The addition of IB4 (B) at a concentration that entirely inhibited untreated neutrophil adhesion to TNFα-treated endothelium (Fig 1B) was only 60% effective at reducing adhesion of DHCB-treated neutrophils. However, it should be noted that the number of adherent cells was reduced by 30% from untreated cells (Fig 1B) and 60% from DHCB-treated cells (Fig 2B). For (A), (▪) DHCB-treated or (▵) DHCB-treated + EL-246; for (B), (▪) DHCB-treated + IB4 or (▵) untreated + IB4 . *P < .05 relative to DHCB-treated cells. #P < .05 relative to untreated neutrophils + IB4 .
Addition of EL-246 eliminated neutrophil rolling (A) and adhesion (data not shown) of DHCB-treated cells. The addition of IB4 (B) at a concentration that entirely inhibited untreated neutrophil adhesion to TNFα-treated endothelium (Fig 1B) was only 60% effective at reducing adhesion of DHCB-treated neutrophils. However, it should be noted that the number of adherent cells was reduced by 30% from untreated cells (Fig 1B) and 60% from DHCB-treated cells (Fig 2B). For (A), (▪) DHCB-treated or (▵) DHCB-treated + EL-246; for (B), (▪) DHCB-treated + IB4 or (▵) untreated + IB4 . *P < .05 relative to DHCB-treated cells. #P < .05 relative to untreated neutrophils + IB4 .
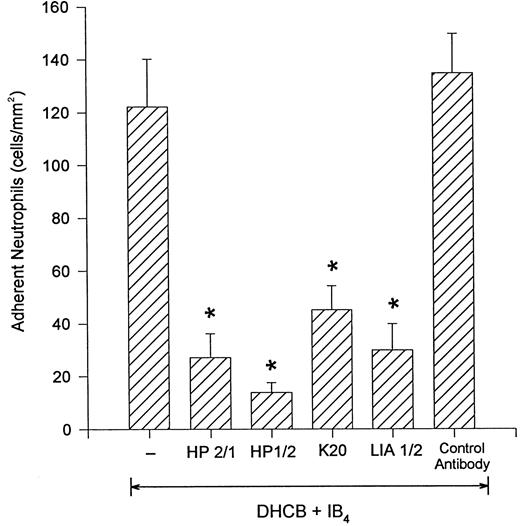
DHCB-treated neutrophils adhere to cytokine-stimulated endothelium via the integrin, α4β1 .Figure 4 shows that the β2 -integrin–independent adhesion of DHCB-pretreated neutrophils was abrogated if, in addition to IB4 , the neutrophils were pretreated with antibodies that inhibit the α4 portion of the α4β1 integrin (HP2/1 or HP1/2). To determine whether the β1 subunit of α4β1 -integrin was involved, we pretreated some cells with an inhibitory antibody that binds β1 -integrin (K20).13 18 This antibody was as effective as the α4 -integrin antibodies in preventing DHCB-treated neutrophil adhesion to TNFα-stimulated HUVEC. A second anti–β1 -integrin antibody (LIA1/2) also elicited identical inhibitory results. An isotype control antibody had no effect on the number of adherent cells. Addition of the anti-α4 antibody, HP2/1, by itself (in the absence of an anti–β2 -integrin antibody) did not inhibit α4β1 -expressing neutrophil adhesion to TNFα-stimulated endothelium (DHCB-treated [430.8 ± 68.8]; DHCB-treated + HP2/1 [400.6 ± 53.7]; 5-minute values).
Addition of the anti–α4 -integrin antibodies HP2/1 or HP1/2 inhibited the remaining firm adhesion in the presence of IB4. Similar results were seen with the addition of the anti-β1 antibodies K20 or LIA1/2. A control antibody was not inhibitory. *P < .05 relative to DHCB-treated neutrophils + IB4 .
Addition of the anti–α4 -integrin antibodies HP2/1 or HP1/2 inhibited the remaining firm adhesion in the presence of IB4. Similar results were seen with the addition of the anti-β1 antibodies K20 or LIA1/2. A control antibody was not inhibitory. *P < .05 relative to DHCB-treated neutrophils + IB4 .
Table 1 summarizes the rolling data for α4β1 -expressing neutrophils. Each of the antibodies directed against either the α4 or β1 subunit did not decrease the number of rolling neutrophils. In fact, the inhibition of adhesion with α4 - or β1 -integrin antibodies resulted in increased numbers of rolling neutrophils, an observation previously reported for monocytes.12 Addition of the anti-α4 antibody, HP2/1, by itself (in the absence of an anti–β2 -integrin antibody) did not effect neutrophil rolling. As shown in Fig 3A, selectins mediated all of the tethering, rolling, and, as a result, subsequent adhesion at 2 dyne/cm2. When shear was reduced by 0.5 dyne/cm2 at 2-minute intervals in the presence of EL-246, DHCB-treated neutrophils were observed to tether and immediately adhere to the endothelium (Fig 5), with adhesion increasing as shear was decreased. This interaction was inhibited by anti-α4 antibody (HP2/1).
α4β1-Integrin Does Not Support Rolling of DHCB-Treated Neutrophils on TNFα-Stimulated Endothelium
| Neutrophil Treatment . | Rolling Cells (no./mm2 ) . |
|---|---|
| DHCB + IB4 | 84.5 ± 12.3 |
| DHCB + IB4 + HP2/1 (anti-α4) | 149.9 ± 22.7* |
| DHCB + IB4 + HP1/2 (anti-α4) | 114.5 ± 2.5* |
| DHCB + IB4 + K20 (anti-β1) | 122.7 ± 4.5* |
| DHCB + IB4 + LIA1/2 (anti-β1) | 150.8 ± 55.7* |
| DHCB + IB4 + isotype control | 73.1 ± 15.6 |
| DHCB + HP2/1 | 96.2 ± 9.2 |
| Neutrophil Treatment . | Rolling Cells (no./mm2 ) . |
|---|---|
| DHCB + IB4 | 84.5 ± 12.3 |
| DHCB + IB4 + HP2/1 (anti-α4) | 149.9 ± 22.7* |
| DHCB + IB4 + HP1/2 (anti-α4) | 114.5 ± 2.5* |
| DHCB + IB4 + K20 (anti-β1) | 122.7 ± 4.5* |
| DHCB + IB4 + LIA1/2 (anti-β1) | 150.8 ± 55.7* |
| DHCB + IB4 + isotype control | 73.1 ± 15.6 |
| DHCB + HP2/1 | 96.2 ± 9.2 |
In each case, the addition of anti-α4 (HP2/1 or HP1/2) or anti-β1 (K20 or LIA1/2) antibodies in the presence of IB4 blocked the adhesion, which translated into increased rolling (5-minute values).
P < .05 relative to DHCB + IB4 condition.
In the presence of antiselectin antibody (EL-246), DHCB-treated neutrophils did not roll or adhere to TNFα-treated endothelium at 2 dyne/cm2. Shear was decreased at 2-minute intervals and neutrophil tethering and adhesion was examined in the (▵) presence and (▪) absence of the anti–α4 -integrin antibody, HP2/1. It should be noted that rolling was not observed in this series of experiments. *P < .05 relative to the no antibody condition.
In the presence of antiselectin antibody (EL-246), DHCB-treated neutrophils did not roll or adhere to TNFα-treated endothelium at 2 dyne/cm2. Shear was decreased at 2-minute intervals and neutrophil tethering and adhesion was examined in the (▵) presence and (▪) absence of the anti–α4 -integrin antibody, HP2/1. It should be noted that rolling was not observed in this series of experiments. *P < .05 relative to the no antibody condition.
α4β1 -expressing neutrophils can tether to VCAM-1.For experiments on L cells expressing either VCAM-1 or ICAM-1, neutrophils were stimulated with DHCB and a chemoattractant (FMLP), a combination known to directly induce α4β1 expression.13 FMLP + DHCB-stimulated neutrophils express the α4 - and β1 -subunit but do not express L-selectin. These data have previously been published and are not presented herein. Nevertheless, this observation was reproduced on a number of the days when the flow chamber assays were performed.
When these neutrophils were perfused at wall shear stress of 2 dyne/cm2, they did not interact with monolayers of VCAM-1–transfected L cells, consistent with the importance of selectins in the initial adhesive interaction (Fig 3A). When the shear stress was decreased by decrements of 0.5 dyne/cm2 at 2-minute intervals, α4β1 -expressing neutrophils began to adhere at a shear stress of 1.5 dyne/cm2, with adherence increasing as the shear stress was decreased (Fig 6A). Unstimulated neutrophils or neutrophils stimulated with either FMLP alone or DHCB alone expressed no α4 -integrin and, expectedly, did not roll on or adhere to VCAM-1 transfectants at any shear stress (data not shown). Ramos cells behaved similarly to the α4β1 -expressing neutrophils; Ramos cells also did not adhere at 2 dyne/cm2, but adhered to monolayers of VCAM-1 transfectants at shears of 1.0 dyne/cm2 or less (Fig 6A). Interestingly, α4β1 -expressing neutrophils did not exhibit significant rolling behavior (Fig 6B) on VCAM-1 but rather tethered to the monolayer and adhered immediately at that position. In contrast to α4β1 -expressing neutrophils, Ramos cells exhibited significant rolling behavior (Fig 6B). However, when Ramos cells were activated with PMA, an agent shown to upregulate α4β1 adhesivity,11 they also tethered and immediately adhered (52 ± 9.6 Ramos cells adherent at 5 minutes).
To induce α4β1 expression on neutrophils, the cells were treated with DHCB and an exogenous chemotactic factor, FMLP. Perfusion of (▪) these cells or (▵) Ramos cells over VCAM-1–transfected L cells showed no interactions at 2 dyne/cm2. Reducing shear to less than 2 dyne/cm2 at 2-minute intervals caused neutrophils to tether to VCAM-1 transfectants (A) with very limited rolling (B). Untreated Ramos cells (known to express α4β1 ) interacted with VCAM-1 transfectants at very similar shears as the neutrophils but displayed primarily rolling (B), not adhesion (A).
To induce α4β1 expression on neutrophils, the cells were treated with DHCB and an exogenous chemotactic factor, FMLP. Perfusion of (▪) these cells or (▵) Ramos cells over VCAM-1–transfected L cells showed no interactions at 2 dyne/cm2. Reducing shear to less than 2 dyne/cm2 at 2-minute intervals caused neutrophils to tether to VCAM-1 transfectants (A) with very limited rolling (B). Untreated Ramos cells (known to express α4β1 ) interacted with VCAM-1 transfectants at very similar shears as the neutrophils but displayed primarily rolling (B), not adhesion (A).
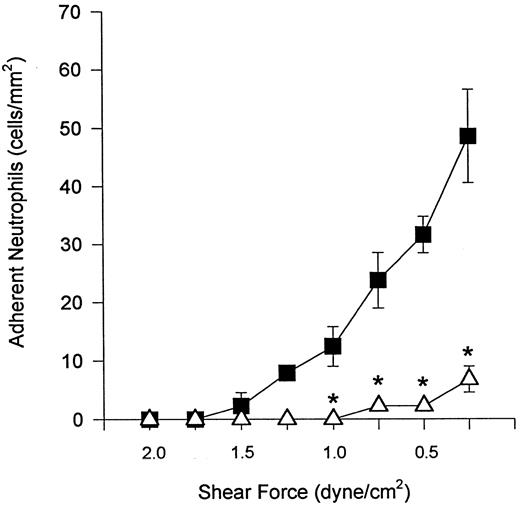
The interaction of α4β1 -expressing neutrophils with VCAM-1 could have occurred in a nonspecific fashion in light of the fact that FMLP + DHCB is known to maximally activate neutrophils, resulting in increased avidity and expression of CD18. However, when α4β1 -expressing neutrophils were perfused over monolayers of ICAM-1–transfected L cells (ligand for CD18) under conditions of decreasing shear, tethering interactions did not occur until shear stress was reduced to 0.5 dyne/cm2, at which point minimal tethering interactions were observed (Fig 7A). Thus, α4β1 -expressing neutrophils adhered preferentially to VCAM-1 when compared directly with the ICAM-1 substratum. This interaction was α4β1 -integrin–dependent because HP2/1 blocked adhesion of α4β1 -expressing neutrophils to VCAM-1 transfectants at various shear forces (Fig 7B). In this series of experiments, it was not necessary to add an anti-CD18 antibody, suggesting that CD18 could not tether to VCAM-1 or other surface molecules on L cells. Therefore, the neutrophil tethering and adhesion was entirely dependent on α4β1 -integrin and independent of the β2 -integrin. HP2/1 also blocked Ramos cell interactions with VCAM-1 transfectants (data not shown). Similar results were seen for the anti-β1 antibody LIA1/2 for both cell types (data not shown). Thus, α4β1 expressed on neutrophils can be used by these cells to tether and adhere under lower shear conditions, an event that is not generalizable to all integrins in vitro.
α4β1 -expressing neutrophils tethered to VCAM-1–transfected L cells at shears of 1.5, 1.0, and 0.5 dyne/cm2, whereas these same cells did not interact with ICAM-1–transfected L cells until very low shear stresses (A). There were approximately the same number of ICAM-1 and VCAM-1 adhesion molecules per cell (data not shown). When used alone, anti-α4 antibody (HP2/1) blocked neutrophil adhesion to VCAM-1 (B). For (A), (▪) VCAM-1 transfectants or (▵) ICAM-1 transfectants; for (B), (▪) without HP2/1 or (▵) with HP2/1. *P < .05 relative to ICAM-1 value. #P < .05 relative to neutrophils + HP2/1.
α4β1 -expressing neutrophils tethered to VCAM-1–transfected L cells at shears of 1.5, 1.0, and 0.5 dyne/cm2, whereas these same cells did not interact with ICAM-1–transfected L cells until very low shear stresses (A). There were approximately the same number of ICAM-1 and VCAM-1 adhesion molecules per cell (data not shown). When used alone, anti-α4 antibody (HP2/1) blocked neutrophil adhesion to VCAM-1 (B). For (A), (▪) VCAM-1 transfectants or (▵) ICAM-1 transfectants; for (B), (▪) without HP2/1 or (▵) with HP2/1. *P < .05 relative to ICAM-1 value. #P < .05 relative to neutrophils + HP2/1.
To determine whether the neutrophils adherent to VCAM-1 transfectants could be detached, α4β1 -expressing neutrophils were allowed to settle on VCAM-1 transfectants for 2 minutes, after which flow through the chamber was reestablished to 2 dyne/cm2. Stimulated neutrophils were observed to remain adherent to the VCAM-1 transfectants under shear, an event that was not inhibited by anti-CD18 antibody (IB4 ) but was significantly inhibited by further addition of anti-β1 antibody (K20) or anti-α4 antibody (HP2/1; Fig 8A). Unstimulated Ramos cells also interacted with VCAM-1 transfectants and, upon reintroduction of flow (2 dyne/cm2 ), some cells detached, but the majority of the cells rolled along the VCAM-1 transfectants. The same concentrations of K20 or HP2/1 also inhibited Ramos cell rolling on VCAM-1 transfectants when flow conditions were reestablished (Fig 8B).
α4β1 -expressing neutrophils were allowed to settle onto VCAM-1–transfected L cells and flow was initiated to 2 dyne/cm2. This force did not displace the neutrophils in the presence or absence of the anti-CD18 antibody, IB4 (A). Pretreatment of neutrophils with either the anti-β1 antibody (K20) or the anti-α4 integrin antibody (HP2/1) significantly reduced the number of adhering cells (A). These concentrations of HP2/1 or K20 were sufficient to displace Ramos cells from the VCAM-1–transfected L cells (B). For (A), (□) fMLP + DHCB, (▨) fMLP + DHCB + IB4 , (▧) fMLP + DHCB + IB4 + K20, or () fMLP + DHCB + IB4 + HP2/1. For (B), (□) no antibody, (▨) K20, or (▧) HP2/1. *P < .05 relative to FMLP + DHCB value. #P < .05 relative to the no antibody condition.
α4β1 -expressing neutrophils were allowed to settle onto VCAM-1–transfected L cells and flow was initiated to 2 dyne/cm2. This force did not displace the neutrophils in the presence or absence of the anti-CD18 antibody, IB4 (A). Pretreatment of neutrophils with either the anti-β1 antibody (K20) or the anti-α4 integrin antibody (HP2/1) significantly reduced the number of adhering cells (A). These concentrations of HP2/1 or K20 were sufficient to displace Ramos cells from the VCAM-1–transfected L cells (B). For (A), (□) fMLP + DHCB, (▨) fMLP + DHCB + IB4 , (▧) fMLP + DHCB + IB4 + K20, or () fMLP + DHCB + IB4 + HP2/1. For (B), (□) no antibody, (▨) K20, or (▧) HP2/1. *P < .05 relative to FMLP + DHCB value. #P < .05 relative to the no antibody condition.
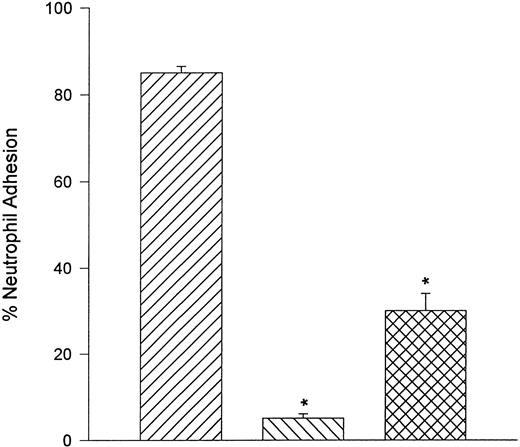
Neutrophils adhere to fibronectin under static but not flow conditions.Fibronectin is also a ligand for α4β1 . When α4β1 -expressing neutrophils were perfused over glass coverslips that were coated with human fibronectin, no interactions were observed between shears of 0.5 and 2.0 dyne/cm2. However, α4β1 -expressing neutrophils adhered to fibronectin in a static assay system. Moreover, the adhesion was almost entirely a CD18-independent mechanism (Fig 9). However, the adhesion could be attenuated by the addition of anti-CD18 antibody (IB4 ) in combination with human fibronectin fragment 40K (FN40) or the anti-α4 antibody (HP2/1). FN40 competes with α4β1 -integrin for the fibronectin binding region (CS-1).17
Under static conditions, fibronectin served as ligand for α4 -integrin. Neutrophils adhered avidly to fibronectin and this interaction was not reduced with pretreatment of neutrophils with the anti-CD18 antibody, IB4 . Addition of a fibronectin fragment (FN-40) that contains the CS-1 binding motif for VLA-4 or HP2/1 (anti–α4 -integrin antibody) significantly reduced the adhesive interaction. Under flow conditions, neutrophils were unable to tether to fibronectin (data not shown). (▨) FMLP + DHCB + IB4 ; (▧) FMLP + DHCB + IB4 + FN40; () FMLP + DHCB + IB4 + HP2/1. *P < .05 relative to FMLP + DHCB + IB4 value.
Under static conditions, fibronectin served as ligand for α4 -integrin. Neutrophils adhered avidly to fibronectin and this interaction was not reduced with pretreatment of neutrophils with the anti-CD18 antibody, IB4 . Addition of a fibronectin fragment (FN-40) that contains the CS-1 binding motif for VLA-4 or HP2/1 (anti–α4 -integrin antibody) significantly reduced the adhesive interaction. Under flow conditions, neutrophils were unable to tether to fibronectin (data not shown). (▨) FMLP + DHCB + IB4 ; (▧) FMLP + DHCB + IB4 + FN40; () FMLP + DHCB + IB4 + HP2/1. *P < .05 relative to FMLP + DHCB + IB4 value.
DISCUSSION
Until recently, it was generally accepted that α4β1 -integrin was restricted to leukocytes other than neutrophils (the latter use β2 -integrin), and so the potential role of α4β1 -integrin as a recruiter of neutrophils has not been addressed. However, there is a growing body of evidence to suggest that neutrophils can infiltrate numerous tissues independent of β2 -integrin.19-22 Moreover, certain stimuli in vitro can induce the expression of α4β1 -integrin on the surface of human neutrophils13 and, at least in adjuvant-induced arthritic rats, circulating neutrophils express some α4β1 -integrin.14 Based on these findings and the view that α4β1 -integrin has a multifaceted capacity to support tethering, rolling, and adhesion, in this study we investigated the possibility that neutrophils could be recruited to the surface of endothelium independent of the conventional selectin and β2 -integrin pathway.
Our results show that activated endothelium (TNFα for 4 hours) could recruit DHCB-treated neutrophils via the α4β1 -integrin pathway even if an anti–β2 -integrin antibody was present. However, neutrophils treated with antibodies against β1 or α4 could not block neutrophil adhesion unless β2 -integrin was simultaneously immunoneutralized. Clearly, the β1 - and β2 -integrin can work in unison or independently to recruit neutrophils to endothelial surfaces. Interestingly, the antibodies directed against either β1 or α4 that prevented neutrophil adhesion did not reduce neutrophil rolling. That particular interaction was dependent on selectins inasmuch as addition of an E/L-selectin antibody or a selectin binding carbohydrate prevented the rolling and the associated adhesion. These data clearly differ from the results with T cells11 and eosinophils,23 which have been reported to roll via α4β1 in vitro. Additionally, we have observed a population of leukocytes that roll via α4 -integrin in chronically inflamed microvessels in vivo.24 The role of α4β1 on neutrophils is reminiscent of the α4β1 -integrin on monocytes, wherein the cells adhered firmly but did not roll via this integrin.12 The differential roles for α4 on different cell types may reflect differences in number and/or avidity of the α4 -integrin.
The role of α4β1 -integrin on neutrophils was further elucidated by perfusing these cells over a single adhesion molecule (VCAM-1). Under the shear conditions (2 dyne/cm2 ) used for endothelium, the neutrophils were not able to interact with the VCAM-1, an observation previously reported for monocytes,12 T cells, and a K562 erythroleukemia cell line transfected with α4β1 .11 However, even a subtle reduction in shear to 1.5 dyne/cm2 was sufficient to permit neutrophil interactions with the VCAM-1–transfected L cells. This interaction was different from the endothelial cell interactions in that the α4β1 -expressing neutrophils tethered and immediately adhered to the VCAM-1 transfectants with essentially no discernible rolling. The difference may be related to the fact that high concentrations of FMLP in the presence of DHCB may maximally activate α4β1 , which translates into immediate adhesion after the tethering interaction. There is a precedent for this behavior; when the avidity of α4β1 on T cells11 or, in this study, on Ramos cells was increased with PMA, these cells also tethered and stably adhered without notable rolling.
This is the first study to demonstrate a role for a β2 -integrin–independent, α4β1 -integrin–dependent mechanism of neutrophil recruitment under shear conditions in the human system. There is a growing body of evidence to invoke a β2 -integrin–independent pathway by which neutrophils can infiltrate inflamed tissues in animal models. Doerschuk et al19 showed that an antibody directed against CD18 entirely inhibited PMA-induced neutrophil movement out of the pulmonary microcirculation; however, the same antibody had no effect on neutrophil emigration associated with Streptococcus pneumoniae or hydrochloric acid. Moreover, a CD18-independent neutrophil infiltration to delayed-type hypersensitivity reactions in joints was also reported.20 Although the role of α4β1 -integrin was not examined in either of these studies, other investigations have shown that antibodies directed against α4 -integrin partly decreased accumulation of neutrophils into skin in a contact hypersensitivity model.25 Because neutrophils were thought not to express α4 -integrin, the investigators suggested that the neutrophil infiltration was dependent in part on α4 -integrin expressing cells that were responsible for recruiting the neutrophils. However, an alternative explanation may be that, following certain inflammatory conditions, the expression and/or activation of α4 -integrin on the surface of neutrophils may contribute to neutrophil recruitment. The data in the present study support this view; human neutrophils can be induced to express α4β1 -integrin and use this adhesion pathway to interact effectively with endothelium even in flow conditions. Interestingly, circulating neutrophils from rats with adjuvant arthritis were positive for the α4 -integrin,14 suggesting that, under at least certain specific inflammatory conditions, this adhesion molecule may be upregulated and may underlie the CD18-independent mechanism of neutrophil infiltration.
In this study, DHCB was used in concert with either endogenous or exogenous chemotactic stimuli to maximally activate neutrophils. It is conceivable that, in systemic inflammatory conditions associated with bacteremia, sepsis, and/or multiple organ failure, accumulation of numerous inflammatory mediators could function like DHCB as a maximal stimulus to induce α4β1 -integrin expression on neutrophils, thereby activating a secondary pathway for neutrophil infiltration. Although increased circulating levels of inflammatory mediators may shed L-selectin and selectin ligands from the surface of neutrophils, because α4β1 -integrin on neutrophils can tether to VCAM-1 at a higher shear than CD18 does to ICAM-1, this may become an important alternative pathway for the recruitment of α4β1 -expressing circulating neutrophils under shear conditions. This was further supported by the observation that α4β1 -expressing neutrophils treated with antiselectin therapy could tether to activated endothelium although shear forces had to be reduced. Although α4β1 -integrin was less efficient at tethering to endothelium and VCAM-1 transfectants than are the selectins, in inflammatory disorders such as ischemia/reperfusion 50% reductions in shear forces are often observed.26 Moreover, in liver and lung, in which neutrophils adhere to the endothelium of sinusoids and capillaries, respectively, the shear forces are greatly reduced and α4β1 -integrin may also be sufficient to support tethering and adhesion.
Although in this study we used a nonphysiologic or artificial stimulus (DHCB) to induce the α4 -integrin–dependent interaction, there are some data to argue that this event may occur in the human system independently of DHCB. First, transmigration of neutrophils across endothelial monolayers in response to chemotactic agents (no DHCB) is sufficient to induce α4β1 -integrin expression.13 This experiment suggests that as yet unidentified physiologic stimuli can indeed induce α4 -integrin expression. Secondarily, Bohnsack et al27 showed that neutrophils lacking β2 -integrin could still adhere to laminin via β1 -integrin. It is presently unclear how DHCB functions to induce the expression of α4β1 -integrin; however, some possibilities presently being investigated include a priming function, a maximal stimulus as might occur with a cocktail of proinflammatory molecules, or simply to maintain the α4 -integrin on the surface of neutrophils, which might rapidly be reinternalized in the absence of appropriate ligands. Indeed, there is evidence that removal of DHCB rapidly reversed the CD18-independent component (unpublished observations).
It remains to be seen whether the CD18-independent component of neutrophil adhesion could mediate significant neutrophil recruitment in vivo. In this study, over the first 5 minutes, the CD18-independent component accounted for approximately 30% to 40% of maximal neutrophil adhesion. It is possible that, over time, the number of cells adhering would reach a plateau that could be similar in magnitude but delayed for CD18-independent adhesion. Indeed, CD18-deficient mice appear to have no impairment in neutrophil recruitment into the inflamed peritoneum at a 24-hour time point, suggesting an efficient CD18-independent mechanism of neutrophil recruitment (Dr C.M. Doerschuk, personal communication, December 1996). Experiments were performed for only 5 minutes because there was no effect of DHCB on the endothelium during that time. Longer periods of time resulted in some endothelial retraction and exposure to substratum (fibronectin) could cause difficulty in interpretation (despite the inability of fibronectin to support adhesion under flow). Nevertheless, over the first 5 minutes, our results clearly show a role for the α4β1 -integrin on neutrophil recruitment under shear conditions and raise the possibility that this mechanism may underlie the CD18-independent recruitment of neutrophils previously described in vivo.
Supported by a grant from the Canadian Medical Research Council (MRC). P.K. is an AHFMR and MRC scholar. P.H.R. is funded by an MRC studentship.
Address reprint requests to Paul Kubes, Immunology Research Group, Department of Medical Physiology, Faculty of Medicine, University of Calgary, Calgary, Alberta, T2N 4N1 Canada.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal