Abstract
Immunohistochemical analysis of the apoptosis-effector protease CPP32 (Caspase-3) in normal lymph nodes, tonsils, and nodes affected with reactive hyperplasia (n = 22) showed strong immunoreactivity in the apoptosis-prone germinal center B-lymphocytes of secondary follicles, but little or no reactivity in the surrounding long-lived mantle zone lymphocytes. Immunoblot analysis of fluorescence-activated cell sorted germinal center and mantle zone B cells supported the immunohistochemical results. In 22 of 27 (81%) follicular small cleaved cell non-Hodgkin's B-cell lymphomas, the CPP32-immunopositive germinal center lymphocytes were replaced by CPP32-negative tumor cells. In contrast, the large cell component of follicular mixed cells (FMs) and follicular large cell lymphomas (FLCLs) was strongly CPP32 immunopositive in 12 of 17 (71%) and in 8 of 14 (57%) cases, respectively, whereas the residual small-cleaved cells were poorly stained for CPP32 in all FLCLs and in 12 of 17 (71%) FMs, suggesting that an upregulation of CPP32 immunoreactivity occurred during progression. Similarly, cytosolic immunostaining for CPP32 was present in 10 of 12 (83%) diffuse large cell lymphomas (DLCLs) and 2 of 3 diffuse mixed B-cell lymphomas (DMs). Immunopositivity for CPP32 was also found in the majority of other types of non-Hodgkin's lymphomas studied. Plasmacytomas were CPP32 immunonegative in 4 of 12 (33%) cases, in contrast to normal plasma cells, which uniformly contained intense CPP32 immunoreactivity, implying downregulation of CPP32 in a subset of these malignancies. All 12 peripheral blood B-cell chronic lymphocyte leukemia specimens examined were CPP32 immunopositive, whereas 3 of 3 small lymphocytic lymphomas were CPP32 negative, suggesting that CPP32 expression may vary depending on the tissue compartment in which these neoplastic B cells reside. The results show dynamic regulation of CPP32 expression in normal and malignant lymphocytes.
PROGRAMMED CELL DEATH plays a critical role in normal tissue homeostasis, where it ensures that new cell production as a result of cell division is offset by a commensurate amount of cell eradication.1 One of the first cell death-regulating genes to be identified was Bcl-2, an anti-apoptotic gene that appears to block a distal step in an evolutionarily conserved pathway involved in apoptosis and programmed cell death.2-4 The Bcl-2 gene becomes overexpressed in germinal center B cells as a result of t(14; 18) chromosomal translocations that occur in greater than 85% of follicular non-Hodgkin's lymphomas (NHLs).5 Subsequent studies led to the isolation of a homolog of Bcl-2 in the nematode Caenorhibitis elegans, called Ced-9, which was necessary for the survival of essentially all cells in this organism.6 Ced-9 opposes the actions of two cell death effector genes, Ced-3 and Ced-4, which are absolutely required for developmental cell death in C elegans.7
The Ced-3 gene encodes a cysteine protease with homology to the interleukin-1β converting enzyme (ICE).8,9 These proteases have specificity for aspartic acid in the P1 position and exist as inactive zymogens in cells, becoming proteolytically activated by cleavage at particular aspartic acid residues, thereby generating active subunits with molecular masses typically of 17 to 20 kD and 10 to 12 kD. These larger and smaller subunits assemble into an enzymatically active heterotetramer.10-12 To date, at least 10 members of the ICE/Ced-3 family of cysteine proteases have been identified in humans (now known as “Caspases”).13 Among these, CPP32 (Caspase-3; also known as Yama and Apopain) is probably the best correlated with apoptosis.14-18 Proteolytic processing and activation of CPP32 has been described in a number of settings in which apoptosis occurs, including in T cells stimulated through Fas (APO1/CD95) and in B cells on which surface Ig has been cross-linked.14,18-20 Moreover, viral proteins, such as crmA from cow pox and p35 from baculovirus, which can inhibit CPP32 activity in vitro, as well as tetrapeptide inhibitors of CPP32, can prevent the induction of cell death under several circumstances.21-27 Recently, it has been shown that the anti-apoptotic protein Bcl-2 and its close homolog Bcl-XL can prevent processing and activation of CPP32,19,28,29 thus establishing a biochemical connection between Bcl-2 family proteins and ICE/Ced-3 family proteases, which is consistent with genetic arguments from C elegans suggesting that Ced-9 is an upstream inhibitor of Ced-3.7 Moreover, of potential relevance to cell death regulation in B cells, stimulation of germinal center B cells through CD40 has been shown to induce expression of Bcl-2 and Bcl-XL , prevent processing of CPP32, and provide protection from anti-Ig–induced apoptosis.20,30 31
As a first attempt to gain insights into the role of CPP32 in the pathogenesis of NHLs and B-cell chronic lymphocyte leukemias (B-CLLs), we prepared a highly specific antiserum against human CPP32 protein and analyzed by immunohistochemistry the patterns of CPP32 immunoreactivity in normal and reactive lymph nodes and various types of NHLs. The findings suggest that the expression of CPP32 is dynamically regulated in normal and neoplastic lymphocytes, and provide the first insights into the in vivo regulation of CPP32 expression in these cells.
MATERIALS AND METHODS
Patient specimens.All biopsy materials originated from the British Columbia Cancer Agency and represented archival paraffin-embedded specimens. The histological diagnosis was established by an expert hematopathologist (R.D. Gascoyne), and the specimens were classified according to the National Cancer Institute working formulation.32 Distinct clinicopathological entities, such as mantle cell lymphoma, monocytoid B-cell lymphoma, MALT lymphomas, and anaplastic large-cell lymphomas, as recently recognized in the revised European-American lymphoma (REAL) proposal, are also included.33 The majority of the cases were immunophenotyped by flow cytometry using mononuclear cell suspensions prepared from tissue biopsies according to established methods. A direct antibody labeling technique was employed using mouse monoclonal antibodies CD2, 3, 4, 5, 7, 8, 10, 11c, 14, 19, 20, 23, and 45 (Becton Dickinson, Mountain View, CA); goat polyclonal anti-κ and anti-λ (Tago Inc, Burlingame, CA); and FMC-7 (Silenus Laboratories) labeled with either phycoerythrin or fluorescein isothiocyanate. Specimens were analyzed on a Coulter Epics Profile II flow cytometer. Those cases not analyzed using flow cytometry were immunophenotyped as B- versus T-cell origin based on routine paraffin section immunohistochemistry using a panel of antibodies including anti-CD20 (L26), anti-CD79, anti-CD45RO (A6 and UCHL-1), and polyclonal anti-CD3. Cytogenetic studies were performed by established methods. Metaphases were stained by GTG banding methods. Descriptions of chromosomal rearrangements follow the International Society of Cytogenetic Nomenclature (ISCN) (1991) guidelines for cancer cytogenetics.34
Expression of human CPP32 and generation of antiserum.A cDNA encoding human CPP32 was generated by polymerase chain reaction (PCR) using the plasmid pKSII-CPP32 (gift from Guy Salvesen, Burnham Institute, La Jolla, CA) as a template and the primers 5′-GGAATTCCATATGGAGAACACTGAAAACTCAGTG3′ (forward), and 5′-CCGCTCGAGGTGATAAAAATAGAGTTCTTTTG-3′ (reverse). After digestion with Xho I and Nde I, this PCR-generated cDNA was subcloned into pET21b (Novagen) at the Nde I/Xho I sites to produce CPP32 protein with six histidine residues at its carboxyl-terminus. pET21b-CPP32 plasmid DNA was transformed into BL21 (DE3) (Novagen, Madison, WI) strain Escherichia coli. Cells were grown in LB medium containing 50 μg/mL ampicillin at 37°C to an OD600 of ∼1.0, and expression was induced with 1 mmol/L isopropyl β-D-Thiogalactopyranoside (IPTG) at 37°C for 5 hours. The cells were collected by centrifugation, frozen on dry ice, and then thawed into Buffer A (6 mol/L guanidinium-HCl, 0.1 mol/L Na-phosphate, 0.01 mol/L Tris/HCl [pH 8.0]) at 5 mL per gram wet weight. After stirring for 1 hour at room temperature and centrifugation at 10,000g for 15 minutes at 4°C, the supernatant (8 mL) was added to a 50% slurry of Ni-NTA resin, mixed at room temperature for 45 minutes, and the resin was loaded into a 1.6-cm diameter glass column. The resin was washed with 10 column volumes of Buffer A, followed by 5 column volumes of 8 mol/L urea, 0.1 mol/L Na-phosphate, 0.01 mol/L Tris/HCl [pH 8.0] until the OD280 of the flow-through was less than 0.01. Next, the column was washed with 8 mol/L urea, 0.1 mol/L Na-phosphate, 0.01 mol/L Tris/HCl [pH 6.3] until the OD280 of the flow-through was less than 0.01. Finally, CPP32/His6 protein was eluted with 8 mol/L urea, 0.1 mol/L Na-phosphate, 0.01 mol/L Tris/HCl [pH 4.5]. The fractions containing recombinant protein were pooled and dialyzed against 2 L of 50 mmol/L HEPES [pH 7.5], 0.1 mol/L NaCl, 0.1% 3-[(3cholamidopropyl) dimethylammonio]-1-propane-sulfonate (CHAPS), 10% Sucrose at 4°C.
New Zealand white female rabbits were injected subcutaneously with 200 μg of purified CPP32-His6 fusion protein mixed (1:1 vol/vol) with Freund's complete adjuvant and then boosted six times with 200 μg of protein in Freund's incomplete adjuvant before collecting blood and obtaining immune serum.
Cell isolation.B cells were isolated from human tonsils.35 Briefly, tonsils were finely minced and the resulting cell suspension was subjected to two rounds of T-cell depletion using first rosetting with sheep red blood cells and then depletion with anti-CD3, anti-CD14, and anti-CD16 magnetic beads. The purity of resulting B cells was analyzed by flow cytometry with anti-CD19–fluorescein isothiocyanate (FITC), anti-CD14–FITC, and anti-CD3–FITC antibody conjugates. The resulting cells contained more than 97% CD19+ B cells and less than 1% CD3+ or CD14+ cells. High- and low-density B cells were then prepared by centrifugation of total B cells on discontinuous Percoll gradients, as described.35 For isolation of IgD-CD38+ GC B cells, low-density B cells were incubated with biotinylated goat antihuman IgD and biotinylated mouse antihuman CD39 for 30 minutes. After washing twice, cells were incubated with Dynabeads M-280 coated with streptavidin (Dynal A.S., Oslo, Norway) for 30 minutes. The Dynabeads were prewashed and used at a ratio of 4 beads/cell. Finally, positive and negative cells were separated by a magnetic particle contractor, Dynal-MPC-1. For isolation of IgD+CD38− naive B cells, high-density B cells were incubated with mouse antibodies aginst CD38 (OKT10) in phosphate-buffered saline with 1% bovine serum albumin (PBS-BSA) for 30 minutes. After washing twice, cells were incubated with Dynabeads coated with sheep antimouse IgG and the positive cells were depleted. The CD38− B cells were incubated with biotinylated goat antihuman IgD, followed by positive selection with streptavidin-coated magnetic beads.
B-CLLs were derived from the peripheral blood of previously untreated patients who presented to the Scripps Clinic (La Jolla, CA; provided by L. Piro), after informed consent was obtained. Lymphocytes (>95% CD20+) were obtained by Ficol-Hypaque density centrifugation,36 resuspended at ∼106 per mL in phosphate-buffered saline (PBS), spread on Superfrost-Plus (Fisher Scientific Inc, Pittsburgh, PA) glass slides, air-dried, and fixed in 4% formaldehyde/PBS [pH 7.1] before immunostaining as described above.
Immunoblotting.Cell lysates were prepared, normalized for total protein content (either 25 or 50 μg per lane), and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)/immunoblotting as described previously37 using the anti-CPP32 antiserum at 0.1% (vol/vol) and an enhanced chemiluminescence (ECL) detection system (Amersham Inc, Arlington Heights, IL).
Immunohistochemistry.Tissue sections (4 μm) were immunostained using 0.1% (vol/vol) anti-CPP32 antiserum and a diaminobenzidine (DAB)-based colorimetric method, as described.38-40 Nuclei were lightly counterstained with hematoxylin. The intensity of CPP32 immunostaining was scored as undetectable (negative), weak, or moderate/strong. In all but one specimen of follicular small cleaved cells (FSC) (which was omitted from the analysis), strongly CPP32 immunopositive normal lymphoid cells were present, which served as an internal control for assessing relative intensity and verifying adequate preservation of the samples. The specificity of the CPP32 immunostaining results was confirmed by using preimmune serum for immunostaining and by preadsorbing the anti-CPP32 antiserum with recombinant CPP32 protein before performing immunostaining, for at least several of the examples of each type of lymphoma or normal tissue analyzed.
RESULTS
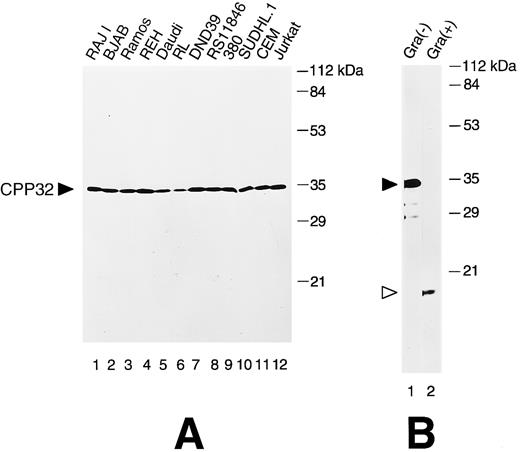
Immunoblot analysis of anti-CPP32 antiserum.Cell lysates derived from a variety of human lymphoma and leukemia cell lines were analyzed by immunoblotting to determine the specificity of the anti-CPP32 antibody. In all cases, a prominent band of ∼32 kD was detected, consistent with the unprocessed form of CPP32 (Fig 1A). This antiserum was also capable of reacting with the 17-kD subunit of the proteolytically processed, active CPP32 protease, as shown by immunoblot analysis of recombinant human CPP32-His6 protein that had been activated in vitro using Granzyme B41 (Fig 1B). The failure to detect the p12 subunit of processed CPP32 presumably is attributed either to a failure of our antibody to react with epitopes in this subunit or poor retention of this smaller band on nitrocellulose filters during electrotransfer.
Immunoblot analysis of CPP32 in lymphoid cell lines. (A) Cell lysates were prepared, normalized for total protein content (50 μg per lane), and subjected to SDS-PAGE/immunoblot assay using 0.1% (vol/vol) anti-CPP32 antiserum, followed by HRPase conjugated goat antirabbit secondary antibody and an ECL substrate.47 The position of the unprocessed 32-kD CPP32 protein is indicated. Reprobing the blot with an antibody against tubulin confirmed loading of essentially equivalent amount of total protein for all samples (not shown). (B) Recombinant CPP32 was treated (+) or not treated (−) with granzyme B and subjected to SDS-PAGE immunoblot analysis. The positions of the 32-kD pro-enzyme and 17-kD subunit of the processed CPP32 protease are indicated.
Immunoblot analysis of CPP32 in lymphoid cell lines. (A) Cell lysates were prepared, normalized for total protein content (50 μg per lane), and subjected to SDS-PAGE/immunoblot assay using 0.1% (vol/vol) anti-CPP32 antiserum, followed by HRPase conjugated goat antirabbit secondary antibody and an ECL substrate.47 The position of the unprocessed 32-kD CPP32 protein is indicated. Reprobing the blot with an antibody against tubulin confirmed loading of essentially equivalent amount of total protein for all samples (not shown). (B) Recombinant CPP32 was treated (+) or not treated (−) with granzyme B and subjected to SDS-PAGE immunoblot analysis. The positions of the 32-kD pro-enzyme and 17-kD subunit of the processed CPP32 protease are indicated.
Immunohistochemical analysis of normal and reactive lymph nodes.A few examples of normal lymph nodes and tonsils, as well as 22 cases of reactive follicular hyperplasia (RFH) were analyzed for CPP32 by immunohistochemical methods using a DAB colorimetric method. Figure 2 shows some representative results. In normal and reactive nodes, intense CPP32 immunostaining was present in the germinal center (GC) lymphocytes of secondary follicles, the only difference being that the size of the follicles was markedly enlarged in RFH compared with normal nodes (compare Fig 1A and B). The surrounding cuff of small round mantle zone (MZ) lymphocytes contained little or no CPP32 immunostaining, with rare exception (Fig 1C). Primary B-cell follicles were also CPP32 immunonegative (not shown). In the GCs of secondary follicles, CPP32 immunoreactivity appeared to be located in the cytosol of cells or in both the cytosol and nucleus (Fig 1D). The vast majority of the GC lymphocytes with nuclear CPP32 immunopositivity did not have apoptotic morphology. Moreover, the number of GC lymphocytes that contained fragmented DNA typical of apoptosis, as determined by DNA end-labeling using terminal deoxynucleotidyl transferase and biotinylated dUTP (TUNEL method),42 was at least 10 times less than the approximate number of cells with nuclear CPP32 immunopositivity (not shown). Occasional small round lymphocytes within the GCs of follicles were often but not always CPP32 immunonegative, whereas small cleaved cells were typically CPP32 immunopositive.
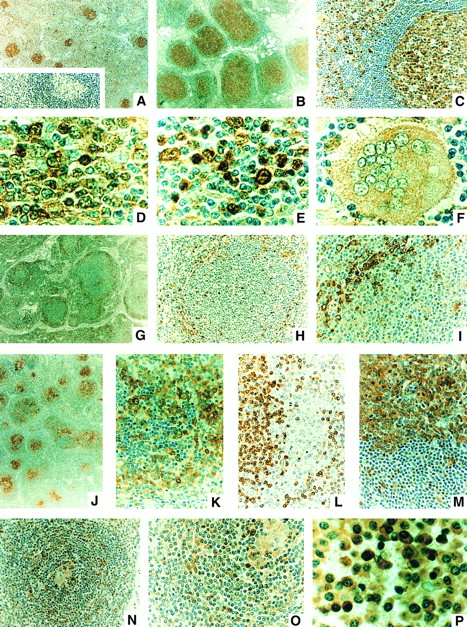
CPP32 immunostaining of normal and reactive lymph nodes and in B-cell malignancies. Some representative examples of the CPP32 immunostaining results are presented. (A) Normal node showing small secondary follicles with strong CPP32 immunostaining in germinal centers (GC) surrounded by a cuff of mantle zone (MZ) cells lacking CPP32 immunoreactivity and scattered cells in the interfollicular regions (IF ) with CPP32 immunopositivity (original magnification ×40). Inset shows same sample in which the CPP32 antiserum was first preadsorbed with CPP32 protein. (B and C) RFH (original magnification ×40 and ×200, respectively), showing enlarged follicles with strongly CPP32 immunopositive GCs, surrounding cuff of CPP32-negative MZ lymphocytes, and scattered cells in IF region with strong CPP32 immunoreactivity. In (C), the GC is located towards the right and the IF zone to the left. (D and E) Higher-power (original magnification ×1,000) views of CPP32-immunostaining in GC and IF zone, respectively, from a node with RFH. CPP32 immunostaining is found in cytosol or both cytosol and nucleus of some lymphocytes, particularly large noncleaved, transformed (“activated”) lymphocytes in the IF zone as well as in the plasma cells. Small round lymphocytes in the IF region are typically CPP32 negative or only weakly immunostained. In (F ), a multinucleated giant cell with moderate intensity cytosolic CPP32 immunostaining is shown, surrounded by mostly CPP32-negative small lymphocytes, in a case of RFH (original magnification ×1,000). (G through I) A case of FSC lymphoma at original magnification ×40, ×200, and ×400, respectively, showing CPP32-negative small cleaved cells filling centers of follicles, with residual strongly CPP32-positive normal transformed (activated) lymphocytes and plasma cells surrounding the follicles. In (I), the center of the follicle is oriented towards the right/bottom corner and a band of CPP32-positive normal cells located at the periphery of the follicle is shown in upper-left corner. (J through L) A case of FLCL immunostained for CPP32 (J and K) (original magnification ×40 and ×400) or Bcl-2 (L) (original magnification ×400) showing mixtures of large cells with strong cytosolic CPP32 immunostaining and small cleaved cells with little or no CPP32 immunoreactivity. The small cleaved cells, however, stain strongly for Bcl-2 in their cytosol (L). (M) DLCL showing large neoplastic cells with strong, mostly cytosolic CPP32 immunoreactivity (top) at the border with residual normal small, round lymphocytes (bottom) still present in the node (original magnification ×400). (N and O) MCL showing remnant of normal GC (located at center in N and in upper-right corner in O) with moderately CPP32-positive histiocytes and occasional adjacent large noncleaved cells with strong CPP32 immunoreactivity, surrounded by MCL cells that mostly contain no or only weak CPP32 immunoreactivity (original magnification ×200 and ×400). (P) Plasmacytoma, showing plasmacytoid cells with moderate to strong intensity CPP32 immunoreactivity, mostly in the cytosol (original magnification ×1,000). Red cells are immunonegative.
CPP32 immunostaining of normal and reactive lymph nodes and in B-cell malignancies. Some representative examples of the CPP32 immunostaining results are presented. (A) Normal node showing small secondary follicles with strong CPP32 immunostaining in germinal centers (GC) surrounded by a cuff of mantle zone (MZ) cells lacking CPP32 immunoreactivity and scattered cells in the interfollicular regions (IF ) with CPP32 immunopositivity (original magnification ×40). Inset shows same sample in which the CPP32 antiserum was first preadsorbed with CPP32 protein. (B and C) RFH (original magnification ×40 and ×200, respectively), showing enlarged follicles with strongly CPP32 immunopositive GCs, surrounding cuff of CPP32-negative MZ lymphocytes, and scattered cells in IF region with strong CPP32 immunoreactivity. In (C), the GC is located towards the right and the IF zone to the left. (D and E) Higher-power (original magnification ×1,000) views of CPP32-immunostaining in GC and IF zone, respectively, from a node with RFH. CPP32 immunostaining is found in cytosol or both cytosol and nucleus of some lymphocytes, particularly large noncleaved, transformed (“activated”) lymphocytes in the IF zone as well as in the plasma cells. Small round lymphocytes in the IF region are typically CPP32 negative or only weakly immunostained. In (F ), a multinucleated giant cell with moderate intensity cytosolic CPP32 immunostaining is shown, surrounded by mostly CPP32-negative small lymphocytes, in a case of RFH (original magnification ×1,000). (G through I) A case of FSC lymphoma at original magnification ×40, ×200, and ×400, respectively, showing CPP32-negative small cleaved cells filling centers of follicles, with residual strongly CPP32-positive normal transformed (activated) lymphocytes and plasma cells surrounding the follicles. In (I), the center of the follicle is oriented towards the right/bottom corner and a band of CPP32-positive normal cells located at the periphery of the follicle is shown in upper-left corner. (J through L) A case of FLCL immunostained for CPP32 (J and K) (original magnification ×40 and ×400) or Bcl-2 (L) (original magnification ×400) showing mixtures of large cells with strong cytosolic CPP32 immunostaining and small cleaved cells with little or no CPP32 immunoreactivity. The small cleaved cells, however, stain strongly for Bcl-2 in their cytosol (L). (M) DLCL showing large neoplastic cells with strong, mostly cytosolic CPP32 immunoreactivity (top) at the border with residual normal small, round lymphocytes (bottom) still present in the node (original magnification ×400). (N and O) MCL showing remnant of normal GC (located at center in N and in upper-right corner in O) with moderately CPP32-positive histiocytes and occasional adjacent large noncleaved cells with strong CPP32 immunoreactivity, surrounded by MCL cells that mostly contain no or only weak CPP32 immunoreactivity (original magnification ×200 and ×400). (P) Plasmacytoma, showing plasmacytoid cells with moderate to strong intensity CPP32 immunoreactivity, mostly in the cytosol (original magnification ×1,000). Red cells are immunonegative.
In the interfollicular areas of the paracortex of nodes, small round lymphocytes were mostly immunonegative. In contrast, the large transformed (“activated”) lymphocytes almost uniformly contained intense CPP32 immunoreactivity that was typically located in both the cytosol and nucleus (Fig 1E). Plasma cells also were strongly immunostained for CPP32, but the immunoreactivity was localized usually to the cytosol and did not involve the nucleus. Histiocytes and multinucleated giant cells contained weak to moderate intensity cytosolic CPP32 immunostaining (Fig 1F ). The specificity of all immunostaining results was confirmed by preadsorption of the antiserum with recombinant CPP32 protein (see Fig 1A for example) and by comparisons with tissue sections immunostained using preimmune serum (not shown).
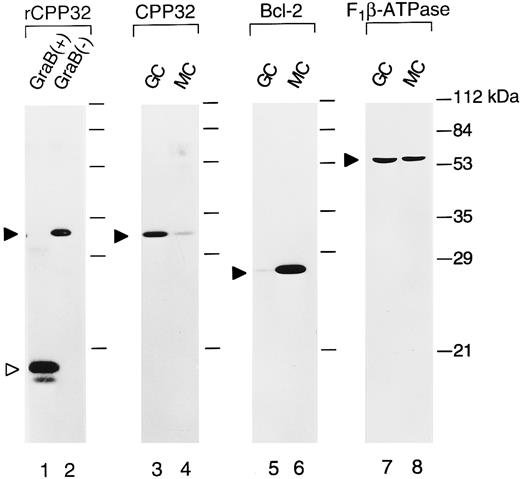
Immunoblot analysis of CPP32 protein in GC and MZ lymphocytes.To confirm the immunostaining results that showed strong intensity CPP32 immunoreactivity in GC cells but not in MZ B-cell lymphocytes, immunoblot analysis was performed on isolated GC and MZ B cells. sIgD-CD38+ GC B cells (>96% pure) and sIgD+/CD38+ naive B cells (>98% pure) were purified from human tonsillar B cells using immunomagnetic beads. As shown in Fig 3, GC B cells contained much higher levels of the 32-kD CPP32 protein than MZ B cells (lanes 3 and 4). Essentially no processed 17-kD CPP32 was detected in GC B cells, despite the high rate of apoptosis in this compartment of the lymph node. However, it should be noted that the fluorescence-activated cell sorter (FACS) procedure used here selected only for nonapoptotic cells. In contrast to the higher levels of CPP32 seen in GC compared with MZ B cells, Bcl-2 protein levels were markedly higher in MZ and GC B cells (lanes 5 and 6) — consistent with immunostaining results derived by use of anti–Bcl-2 antibodies, which showed intense Bcl-2 immunoreactivity in MZ but not GC B cells (not shown).38,43 44 The levels of F1 β-ATPase (used as a control) were similar in GC and MZ B cells, confirming loading of approximately equivalent amounts of total protein (lanes 7 and 8). Taken together, these immunoblot data therefore confirm the CPP32 immunostaining results obtained for normal and reactive nodes.
Immunoblot comparisons of CPP32 and Bcl-2 in normal GC and MZ B cells. GC and MZ B cells were obtained by FACS of human tonsilar B cells. Lysates were prepared and normalized for total protein content (25 μg per lane), and a Western blot was prepared. The blot was sequentially probed with antisera specific for CPP32 (lanes 1 through 4), Bcl-2 (lanes 5 and 6), and F1 -β-ATPase (lanes 7 and 8). Lanes 1 and 2 represent recombinant CPP32 protein (50 ng) that was either treated (+; lane 1) or untreated (−; lane 2) with granzyme B (included as a positive control).
Immunoblot comparisons of CPP32 and Bcl-2 in normal GC and MZ B cells. GC and MZ B cells were obtained by FACS of human tonsilar B cells. Lysates were prepared and normalized for total protein content (25 μg per lane), and a Western blot was prepared. The blot was sequentially probed with antisera specific for CPP32 (lanes 1 through 4), Bcl-2 (lanes 5 and 6), and F1 -β-ATPase (lanes 7 and 8). Lanes 1 and 2 represent recombinant CPP32 protein (50 ng) that was either treated (+; lane 1) or untreated (−; lane 2) with granzyme B (included as a positive control).
CPP32 immunostaining of follicular lymphomas.A total of 58 follicular lymphoma (Fls; 27 follicular small-cell [FSC] lymphomas, 17 follicular mixed cells [FMs], and 14 follicular large-cell lymphomas [FLCLs]) were immunostained for CPP32, including 42 with and 16 without (14; 18) translocations as determined by cytogenetics (Table 1). In 22 of 27 of the FSC lymphomas, the CPP32-immunopositive GC lymphocytes were replaced by malignant lymphoma cells that contained little or no CPP32 immunostaining (Fig 2G through I and Table 1). In many cases, normal transformed (“activated”) lymphocytes and plasma cells containing intense CPP32 immunoreactivity were seen surrounding the neoplastic follicles, giving the impression that these residual normal GC cells had been pushed to the periphery of the follicles (Fig 2G through I). In 12 of 17 (71%) FM lymphomas, strong immunostaining for CPP32 was observed. This CPP32 immunoreactivity resided in the large cell component of the tumors in 10 of 17 (59%) cases (Fig 2J) and in the small cleaved cells in 4 of 17 (24%) specimens. In all but 9 of these FSC and FM lymphomas, residual normal germinal centers were present that were not involved with tumor, thus allowing for direct side-by-side comparisons of the strong CPP32 immunostaining of normal germinal center B cells with the weak or negative immunostaining of the lymphoma cells (not shown). Eight of the 14 FLCLs (57%) were CPP32 immunopositive, with most of the malignant large cells uniformly containing strong cytosolic CPP32 immunostaining in those cases that were immunopositive. In none of the 14 cases of FLCL were the residual small cleaved cells strongly immunostained for CPP32. No clear association between CPP32 immunostaining and the presence or absence of a t(14:18) translocation was evident (Table 1).
Summary of CPP32 Immunostaining Results
| Diagnosis . | Total . | Positive . | Negative . |
|---|---|---|---|
| Reactive | 22 | 22 | 0* |
| FSC | 27 | 5 | 22 |
| t(14:18) | 22 | 5 | 17 |
| Non t(14:18) | 5 | 0 | 5 |
| FM | 17 | 12 | 5† |
| t(14:18) | 11 | 8 | 3 |
| Non t(14:18) | 6 | 4 | 2 |
| FLCL | 14 | 8 | 6 |
| t(14:18) | 9 | 5 | 4 |
| Non t(14:18) | 5 | 3 | 2 |
| DM-B | 3 | 2 | 1 |
| DLCL-B | 12 | 10 (1)‡ | 2 |
| SLL | 3 | 0 | 3 |
| SLL-plasmacytoid | 2 | 2 (LC) | 1 SC |
| 1 (SC) | |||
| PC | 12 | 8 (1)‡ | 4 |
| B-CLL | 12 | 12 (1)‡ | 0 |
| MBCL | 5 | 0 | 5 |
| MCL | 11 | 8 (8)‡ | 3 |
| MALTomas | 9 | 2 | 7 |
| SC | 4 | 0 | 4 |
| SC and LC | 1 | 0 | 1 |
| LC | 4 | 2 | 2 |
| SNC-B | 3 | 3 (1)‡ | 0 |
| SNC-NB | 5 | 5 (1)‡ | 0 |
| IBL | 5 | 5 | 0 |
| LBL | 1 | 0 | 1 |
| ALCL | 9 | 7 | 2 |
| B-cell | 3 | 2 | 1 |
| T-cell | 6 | 5 | 1 |
| DLCL/DM-T | 14 | 13 (2)‡ | 1 |
| Diagnosis . | Total . | Positive . | Negative . |
|---|---|---|---|
| Reactive | 22 | 22 | 0* |
| FSC | 27 | 5 | 22 |
| t(14:18) | 22 | 5 | 17 |
| Non t(14:18) | 5 | 0 | 5 |
| FM | 17 | 12 | 5† |
| t(14:18) | 11 | 8 | 3 |
| Non t(14:18) | 6 | 4 | 2 |
| FLCL | 14 | 8 | 6 |
| t(14:18) | 9 | 5 | 4 |
| Non t(14:18) | 5 | 3 | 2 |
| DM-B | 3 | 2 | 1 |
| DLCL-B | 12 | 10 (1)‡ | 2 |
| SLL | 3 | 0 | 3 |
| SLL-plasmacytoid | 2 | 2 (LC) | 1 SC |
| 1 (SC) | |||
| PC | 12 | 8 (1)‡ | 4 |
| B-CLL | 12 | 12 (1)‡ | 0 |
| MBCL | 5 | 0 | 5 |
| MCL | 11 | 8 (8)‡ | 3 |
| MALTomas | 9 | 2 | 7 |
| SC | 4 | 0 | 4 |
| SC and LC | 1 | 0 | 1 |
| LC | 4 | 2 | 2 |
| SNC-B | 3 | 3 (1)‡ | 0 |
| SNC-NB | 5 | 5 (1)‡ | 0 |
| IBL | 5 | 5 | 0 |
| LBL | 1 | 0 | 1 |
| ALCL | 9 | 7 | 2 |
| B-cell | 3 | 2 | 1 |
| T-cell | 6 | 5 | 1 |
| DLCL/DM-T | 14 | 13 (2)‡ | 1 |
Abbreviations: FSC, follicular small-cleaved cell; FM, follicular mixed cell; FLCL, follicular large cell lymphomas; DM-B, diffuse mixed B-cell lymphoma; DLCL-B, diffuse large B-cell lymphoma; SLL, small lymphocytic lymphoma; PC, plasmacytomas; B-CLL, B-cell chronic lymphocytic leukemia; MALToma, mucosal associated lymphomatous tumor; MBCL, monocytoid B-cell lymphoma; MCL, mantle cell lymphoma; SC, small cell; LC, large cell; SNC-B, small noncleaved Burkitt; SNC-NB, small noncleaved non-Burkitt; IBL, immunoblastic lymphoma; LBL, lymphoblastic lymphoma; ALCL, anaplastic large B cell; DLCL, diffuse large cell lymphoma; DM-T, diffuse mixed T cell.
GC strongly CPP32 stained. MZ B cells negative.
LC were CPP32 positive. In 4 cases, the small cells were also CPP32 positive.
Designates number of cases with weak-intensity CPP32 immunostaining. All others have moderate-strong intensity CPP32 immunoreactivity in majority of the cells.
CPP32 immunostaining of diffuse mixed (DM) and diffuse large B-cell lymphomas (DLCL).Moderate to strong intensity CPP32 immunostaining was present in the cytosol of 9 of 12 (75%) B-cell DLCLs (Fig 2M). In one case, weak intensity CPP32 immunostaining was found, whereas 2 cases were entirely CPP32 negative (Table 1). Two of 3 B-cell DM lymphomas were CPP32 immunopositive, with the large- but not the small-cell component exhibiting positive immunostaining.
Small lymphocytic lymphomas (SLLs) and plasmacytomas (PCs).All three of the SLLs studies here were CPP32 immunonegative (Table 1). In contrast, both cases of SLLs with plasmacytoid differentiation were CPP32 immunopositive. Both the large- and small-cell components exhibited positive immunostaining in one case, but only the large cells were immunopositive in the other specimen. Although normal plasma cells were uniformly strongly immunostained for CPP32, 4 of 12 (33%) of PCs were completely CPP32 immunonegative, and 1 was only weakly immunostained (Table 1).
B-CLL.Twelve cases of B-CLL were evaluated by immunohistochemistry for CPP32 immunostaining, using smears prepared from peripheral blood lymphocytes (>95% CD20+). All 12 B-CLLs were CPP32 immunopositive, with the percentage of immunopositive cells ranging from 30% to 95% (median, 50% to 60%; mean, 57%). In 11 of 12 (92%) cases, the preponderance of the immunopositive cells contained moderate-strong intensity CPP32 immunostaining, but in 1 specimen only weak intensity CPP32 immunostaining was present. The CPP32 immunoreactivity resided in the cytosol in all 12 B-CLLs, but in two specimens scattered cells with normal morphology (ie, nonapoptotic) and nuclear immunostaining were also present. The presence of unprocessed 32-kD CPP32 protein was confirmed by immunoblotting for all 12 of these B-CLLs (not shown).
Monocytoid B-cell lymphomas (MBCL) and mantle cell lymphomas (MCLs).None of the 5 MBCLs evaluated here exhibited CPP32 immunostaining. In contrast, 8 of 11 (73%) MCLs were CPP32 immunopositive. However, it should be noted that except for the blastic transformed cells, which were strongly CPP32 immunostained, the intensity of the CPP32 immunostaining in these tumors was clearly less than in the residual normal GC lymphocytes often found in the centers of tumor follicles. Thus, the overall impression when viewing the neoplastic follicles was that the intensely immunostained normal GC lymphocytes had been replaced by a population of weakly positive tumor cells, somewhat similar to the FSC lymphomas (see Fig 1N through O for examples).
MALTomas.Among nine MALTomas, only two (22%) contained 10% or more tumor cells with strong intensity CPP32 immunoreactivity. Both of these CPP32-immunopositive MALTomas comprised mostly large cells (Table 1). One of the cases of MALToma with mostly small cells contained occasional (<1%) large cells that were strongly CPP32 immunopositive.
Small noncleaved Burkitt (SNC-B) and non-Burkitt (SNC-NB), immunoblastic lymphomas (IBL), and lymphoblastic lymphomas (LBL).Similar to normal germinal center B cells, all five SNC-B and all three SNC-NB lymphomas studied here comprised tumor cells with CPP32 immunopositivity. However, in one case each of SNC-B and SNC-NB, the intensity of CPP32 immunostaining was weaker than normal, based on side-by-side comparisons with normal cells in the same node biopsies (Table 1). All five IBLs evaluated here were also strongly CPP32 immunopositive, whereas the one case of LBL studied was CPP32 immunonegative.
Anaplastic large B-cell (ALCL-B) and T-cell (ALCL-T) lymphomas.Two of three (67%) ALCL-B and five of six (83%) ALCL-T lymphomas comprised mostly tumor cells with moderate to strong intensity CPP32 immunostaining (Table 1). However, the single case of CPP32-immunonegative ALCL-B did contain a small percent (<2%) of cells with strong cytosolic CPP32 immunoreactivity.
Diffuse large-cell lymphomas (DLCLs) and diffuse mixed (DM) T-cell lymphomas.Among the 14 T-cell DLCL and DM lymphomas evaluated here, 13 were CPP32-immunopositive with 11 displaying moderate to strong intensity cytosolic CPP32 immunoreactivity and 2 containing only weak immunostaining (Table 1).
DISCUSSION
In this report, we describe for the first time the patterns of CPP32 protein production in normal and neoplastic lymphocytes, using a highly specific anti-CPP32 antiserum and an immunohistochemical approach. Interestingly, several instances were identified in which the CPP32 immunostaining results were inversely correlated with Bcl-2, based on comparisons with previous reports of Bcl-2 expression in normal and malignant lymphoid tissues. For example, Bcl-2 is highly expressed in the MZ B cells that surround the germinal centers, but is only scarcely present or entirely absent from most GC B cells.38,43 44
Conversely, we observed that CPP32 immunoreactivity was strong within most of the GC lymphocytes, but absent or just barely detectable in most MZ B cells. These patterns of Bcl-2 and CPP32 immunostaining are in agreement with evidence that GC B cells are highly apoptosis-prone, short-lived cells, whereas MZ B cells are generally long-lived cells. In addition, an inverse correlation between Bcl-2 expression and CPP32 immunostaining was also noted for most of the FSC lymphomas and most of the FM lymphomas examined, in that normal GC B cells contain high CPP32 immunoreactivity and low Bcl-2, whereas these neoplastic follicular B cells contained high Bcl-2 and low CPP32 immunostaining. Although not as impressive, MCLs, which contain high levels of Bcl-2,38 43 generally contained weaker CPP32 immunostaining than normal GC B cells, like their non-neoplastic counterparts, the normal MZ B cells.
This inverse correlation between Bcl-2 and CPP32 immunostaining, however, was not uniformly observed, because the intensity of CPP32 immunostaining was also markedly reduced in all FSC lymphomas that lacked t(14; 18) translocations involving the Bcl-2 gene. Immunostaining for Bcl-2 confirmed that most of these t(14; 18)-negative tumors do not over-express Bcl-2 in the malignant follicular center cells (not shown). In addition, strong intensity CPP32 was observed in some FLCLs and DLCLs that were strongly Bcl-2 immunopositive (unpublished data), as well as in the small cleaved cells in 9 of 44 (20%) of FSC/FM lymphomas. Thus, the inverse relations seen between Bcl-2 and CPP32 in the follicles of normal/reactive nodes and in FSC lymphomas may be more circumstantial than of a direct cause-and-effect nature.
To date, little information has been available concerning the intracellular locations of Caspase family proteases.45 Our immunostaining results suggest that CPP32 resides primarily in the cytoplasm of most types of cells but also can be found in the nucleus. Although the in vivo substrates of CPP32 have yet to be confirmed, this protease can directly cleave the nuclear protein poly-ADPribosyl-polymerase in vitro. Several other nuclear proteins that are also cleaved by Aspases during apoptosis, including lamin, U1-RNP-70 kD, and the catalytic subunit of DNA-dependent protein kinase.12 Thus, CPP32 or other Caspase family proteases must gain access to the nucleus at some point during apoptotic cell death. The finding of CPP32 immunostaining in the nuclei of GC B cells, in large transformed (activated) lymphocytes in the interfollicular zone of normal nodes, and in some types of malignant B cells shows for the first time that CPP32 can indeed enter the nucleus. However, because ∼10 times fewer cells were TUNEL-positive compared with nuclear CPP32 immunopositive in normal nodes (data not shown), it remains to be determined whether the presence of CPP32 protein in the nucleus is an indication of impending apoptosis. Conceivably, many of the lymphocytes with nuclear CPP32 may not yet have experienced the DNA degradation typical of the later stages of apoptosis but were nevertheless “committed” to cell death or, alternatively, that the TUNEL method employed here was simply incapable of detecting cells with only small amounts of DNA fragmentation in the very early stages of genomic digestion and apoptosis.
Of interest was the observation that three of three SLLs were CPP32 immunonegative, whereas all 12 peripheral blood B-CLLs were CPP32 positive (Table 1). Because SLL is thought to represent a lymphomatous version of B-CLL, this finding suggests that the expression of CPP32 may vary within these malignant B-cells depending on the tissue compartments in which they reside in the body. Moreover, given the association between CPP32 and apoptosis, it would be of interest to contrast the apoptotic responses to chemotherapeutic drugs of CPP32-positive peripheral blood B-CLLs with CPP32-negative SLLs.
Although the diagnostic and prognostic potentials of CPP32 immunostaining remain to be established, the striking difference in the intensity of CPP32 immunostaining in normal and reactive GCs compared with the neoplastic follicles seen in most FSC and FM lymphomas raises the possibility that CPP32 immunostaining might be helpful for the differential diagnosis of RFH versus FSC/FM lymphomas. Moreover, because CPP32 immunointensity was reduced in most FSC and FM lymphomas, regardless of whether or not these tumors contained a t(14; 18) translocation, immunostaining for CPP32 could potentially provide an adjunct to Bcl-2 immunostaining for distinguishing reactive and lymphomatous follicles. In addition, because the determination of the approximate relative proportions of small versus large tumor cells may be of prognostic significance for patients with FLs,46-48 immunostaining for CPP32 could possibly be of assistance inasmuch as CPP32 immunostaining was significantly associated with larger cell size in FLs (P < .001 based on Pearson χ2 analysis; n = 58). Correlations of CPP32 immunostaining with clinical outcome might also be informative in plasma cell dyscrasias, because CPP32 expression was reduced in 5 of 12 (42%) of plasmacytomas compared with normal plasma cells that uniformly contained intense CPP32 immunostaining. It may be of relevance, in this regard, that normal plasma cells are generally short-lived cells, and thus, downregulation of CPP32 could potentially provide a mechanism of prolonging the lifespan of malignant plasma cells. Further studies are currently under way to determine the prognostic significance of CPP32 immunostaining in FL, DLCL, and myeloma.
ACKNOWLEDGMENT
We thank Guy Salvesen for providing the CPP32 cDNA and granzyme B-treated CPP32 protein and R. Lemoine, C. Herndon, and H. Gallant for manuscript preparation.
Supported in part by National Cancer Institute Grant No. CA-60421. J.M.Z. is the recipient of the Fullbright/Spanish Ministry of Education and Science Fellowship.
Address reprint requests to John C. Reed, MD, PhD, The Burnham Institute, Cancer Research Center, 10901 N Torrey Pines Rd, La Jolla, CA 92037.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal