Abstract
Although the cellular origin of Reed-Sternberg (RS) cells of classical Hodgkin's disease (HD) has been a controversial issue for many years, recent immunophenotypic and molecular studies have suggested that RS cells of a subset of classical HD cases may be related to B cells. To further define the immunophenotypic features and the differentiation stage of RS cells, a series of 56 HD samples, including both nodular lymphocyte predominance (LP) (eight cases) and classical HD (nodular sclerosis [NS], 32 cases; mixed cellularity [MC], 16 cases) with a non-T–cell phenotype, were evaluated for the immunohistochemical expression of the B-B4 antigen, a specific marker for terminally differentiated B cells. Because the cDNA of the B-B4 antigen encodes syndecan-1, a member of a family of transmembrane heparan sulfate proteoglycans thought to be involved in binding cells of the B lineage to the interstitial matrix, the B-B4 immunoreactivity was correlated with the expression of syndecan-1 in HD-derived cell lines (L428, KM-H2), as detected by both reverse transcriptase polymerase chain reaction (RT-PCR) studies and Western blotting. Our results show that B-B4 reacts with RS cells and their morphological variants of all cases of classical HD, irrespective of their antigenic phenotype (B, undetermined), albeit at a varying degree of cellular expression. Notably, a high reactivity and staining intensity for the B-B4 monoclonal antibody (MoAb) was restricted to tumor cells from NS HD. In cases of the latter subtype, B-B4 positivity was also found in sclerosis-trapped spindle cells (fibrocytes/fibroblasts). Conversely, the putative tumor cells of nodular LP HD were consistently unreactive with the B-B4 MoAb. Finally, we have demonstrated by RT-PCR, flow cytometry, and Western blotting that cultured RS cells, of B and undetermined phenotype, express syndecan-1 mRNA and produce a form of syndecan-1, recognized by the B-B4 MoAb, which is predominantly associated with glycosaminoglycans and is present at the cell surface. Our detection of the plasma cell-specific antigen B-B4 (syndecan-1) on tumor cells of classical HD further supports that RS cell progenitors may be related to germinal/postgerminal center mature B cells and suggests that expression of syndecan-1 may contribute to some of the typical biologic and histopathologic features of classical HD, with a special regard to the NS subtype.
OVER THE LAST 25 YEARS, it has become evident that the nodular lymphocyte predominance (LP) subtype of Hodgkin's disease (HD) may represent a germinal center-related B-cell clinicopathological entity.1 It can be distinguished from classical HD by clinicopathological, phenotypic, and molecular features.1 2
In contrast to nodular LP HD, the lineage of Reed-Sternberg (RS) cells in cases of classical HD, including nodular sclerosis (NS) and mixed cellularity (MC) subtypes, continues to be debated, as most studies analyzing conventional B-and T-cell markers on these HD subtypes have shown heterogeneous phenotypes (reviewed in Harris et al2 and Drexler3 ). Recently, several observations have suggested that RS cells of classical HD may be related to B cells, at least in a fraction of cases. First, extensive phenotypic studies have demonstrated that RS cells express the CD40 and CD79a B-cell associated antigens.4,5 Second, single cell polymerase chain reaction (PCR) analysis has shown the presence of clonal immunoglobulin (Ig) gene rearrangements in B-cell antigen positive RS cells of classical HD, as well as RS cells of a subset of classical HD cases lacking B- and T-cell markers.6-8 Finally, RS cells have been shown to harbor somatic mutations of Ig variable region heavy chain (VH ) genes. Notably, somatic mutations of Ig VH genes are detectable in most, but not all, cases of classical HD indicating that RS cells may differ in their differentiation stage, even within the same histologic subtype of HD.7-9 Taken together, the immunophenotypic and molecular characteristics of classical HD suggest that RS cells may represent a B-cell–derived monoclonal population, which can be related either to naive pregerminal center B cells or, more commonly, to germinal center/postgerminal center-memory B cells.4-9
A plasma cell reactive monoclonal antibody (MoAb) B-B4, which specifically recognizes plasma cells among hematopoietic cells, has recently been developed and characterized.10 Among hematopoietic neoplasms, the B-B4 MoAb specifically identifies multiple myeloma cells.10 Thus, B-B4 can be considered a bona fide specific marker for terminally differentiated B cells, although its expression has been detected, at a low cellular density, in some human leukemic cell lines of pre-B phenotype.10 The cDNA of the B-B4 antigen encodes syndecan-1,10 a member of a family of transmembrane heparan sulfate proteoglycans thought to be involved in binding cells of the B lineage to the interstitial matrix.11 Notably, among the various stages of B-cell differentiation, high levels of syndecan-1 mRNA have been detected only at pre-B cell and plasma cell stages,12 13 fully reflecting the reactivity patterns of the B-B4 MoAb.10
This study was aimed at characterizing the expression pattern of the B-B4 antigen on the B-cell–related nodular LP subtype of HD, classical HD, and HD-derived cell lines, and at assessing its potential use to further define the differentiation stage of the putative tumor cells of HD. B-B4 reactivity was also evaluated on a series of primary non-Hodgkin's lymphomas (NHL) of B-cell phenotypes at different stages of differentiation. Furthermore, the B-B4 reactivity was correlated with the expression of syndecan-1 in HD-derived cell lines, as detected by both reverse transcriptase polymerase chain reaction (RT-PCR) studies and Western blotting. Our results showed the presence of the B-B4 antigen on RS cells of all classical HD cases tested, albeit at a varying degree of cellular expression. In contrast, the putative tumor cells of nodular LP subtype of HD never reacted with the B-B4 MoAb. Finally, both B-B4 reactivity and syndecan-1 expression were detected on HD-derived human cell lines.
MATERIALS AND METHODS
Samples.The study included tissue samples of 56 cases of HD (nodular LP, eight cases; NS, 32 cases; MC, 16 cases) with a non-T–cell phenotype (Table 1). Tissues were fixed in Bouin solution or neutral buffered formalin. In all cases a portion of unfixed tissue was snap frozen in liquid nitrogen and stored at −80°C. As a control, cryopreserved cell samples from 81 B-cell lymphomas/leukemias and 25 multiple myelomas were studied by flow cytometry. Furthermore, tissue samples of 38 B-cell lymphomas/leukemias, including 15 cases previously tested by flow cytometry, and three extramedullary plasmacytomas were analyzed by immunohistochemistry (Table 2).
B-B4 Antigen Expression in RS Cells
| Histologic Type . | Phenotype* . | Percentage of B-B4 Positive RS Cells . | |||||
|---|---|---|---|---|---|---|---|
| (no. of cases) . | (no. of cases) . | 0 . | <10 . | 10-25 . | 25-50 . | 50-75 . | >75 . |
| NLP HD (8) | B (8) | 8 | |||||
| NS HD (32) | B (5) | 2† | 1† | 2‡ | |||
| U (27) | 2† | 9ρ | 7ρ | 8‡ | 1‡ | ||
| MC HD (16) | B (2) | 1† | 1ρ | ||||
| U (14) | 5† | 4† | 5ρ | ||||
| Histologic Type . | Phenotype* . | Percentage of B-B4 Positive RS Cells . | |||||
|---|---|---|---|---|---|---|---|
| (no. of cases) . | (no. of cases) . | 0 . | <10 . | 10-25 . | 25-50 . | 50-75 . | >75 . |
| NLP HD (8) | B (8) | 8 | |||||
| NS HD (32) | B (5) | 2† | 1† | 2‡ | |||
| U (27) | 2† | 9ρ | 7ρ | 8‡ | 1‡ | ||
| MC HD (16) | B (2) | 1† | 1ρ | ||||
| U (14) | 5† | 4† | 5ρ | ||||
Abbreviations: HD, Hodgkin's disease; NLP, nodular lymphocyte predominance; NS, nodular sclerosis; MC, mixed cellularity.
U, undetermined.
Weak expression.
Strong expression in most RS cells.
ρ Weak to moderate expression.
B-B4 Expression in Human B-Cell Non-Hodgkin's Lymphomas/Leukemias and Myeloma/Plasmacytomas as Assessed by FC and Frozen Section IHC
| Diagnosis* . | No. of Positive/ . | |
|---|---|---|
| . | Tested Cases . | |
| . | FC† . | IHC† . |
| B-cell chronic lymphocytic leukemia/prolymphocytic leukemia/small lymphocytic lymphoma | 0/48 | 0/5 |
| Lymphoplasmacytoid lymphoma | 2/2 | — |
| Mantle cell lymphoma | 0/4 | 0/5 |
| Follicular center cell lymphoma | 0/7 | 0/7 |
| Hairy cell leukemia | 0/9 | — |
| Extranodal marginal zone B-cell lymphoma (low-grade B-cell lymphoma of MALT type) | — | 0/2 |
| Plasmacytoma/myeloma | 25/25 | 3/3 |
| Diffuse large B-cell lymphoma | ‡2/11 | 0/14 |
| Burkitt's lymphoma | — | 0/4 |
| High-grade B-cell lymphoma, Burkitt-like | — | 0/1 |
| Diagnosis* . | No. of Positive/ . | |
|---|---|---|
| . | Tested Cases . | |
| . | FC† . | IHC† . |
| B-cell chronic lymphocytic leukemia/prolymphocytic leukemia/small lymphocytic lymphoma | 0/48 | 0/5 |
| Lymphoplasmacytoid lymphoma | 2/2 | — |
| Mantle cell lymphoma | 0/4 | 0/5 |
| Follicular center cell lymphoma | 0/7 | 0/7 |
| Hairy cell leukemia | 0/9 | — |
| Extranodal marginal zone B-cell lymphoma (low-grade B-cell lymphoma of MALT type) | — | 0/2 |
| Plasmacytoma/myeloma | 25/25 | 3/3 |
| Diffuse large B-cell lymphoma | ‡2/11 | 0/14 |
| Burkitt's lymphoma | — | 0/4 |
| High-grade B-cell lymphoma, Burkitt-like | — | 0/1 |
Abbreviations: FC, flow cytometry; IHC, immunohistochemistry.
According to the revised European-American lymphoma classification.2
Cells from 15 B-cell lymphoma/leukemia cases encompassing the different histotypes (3 B-cell chronic lymphocytic leukemia; 2 mantle cell lymphoma; 3 follicular center cell lymphoma; 7 diffuse large B-cell lymphoma) were analyzed by both FC and IHC.
Immunoblastic plasmacytoid.
The nodular LP subtype of HD was diagnosed according to morphologic and immunophenotypic criteria, as previously reported.1,2 The CD30+, CD45−, CD15+, EMA− diagnostic profile was required for the diagnosis of classical HD.2 The Rye modification of the Lukes and Butler classification was used to classify classical HD.14
The diagnosis of B-cell NHL/leukemia was based on correlative analysis of the morphologic, immunophenotypic and, when necessary, genotypic characteristics. The cases were categorized according to the Revised European-American Lymphoma Classification.2
Frozen and/or paraffin-embedded tissues from 11 clinical samples with non-neoplastic lymphoid proliferations (seven reactive lymph nodes without obvious cause, two lymph nodes with dermatopathic lymphadenopathy, and two reactive lymph nodes in infectious mononucleosis) were also included in the study.
Immunohistochemistry.Anti-B–B4 MoAb (Immuno Quality Products, Lagitre, Milan, Italy) was applied to frozen tissues from all 56 HD cases included in the study. The MoAb was also applied to paraffin-embedded tissues from a representative subset of HD cases (nodular LP, four cases; NS, 15 cases; MC, six cases) for morphologic control purposes. Air dried frozen sections were kept under vacuum for 3 hours and then fixed in a 1:1 solution of acetone and chloroform for 10 minutes; sections were hydrated with phosphate buffered saline (PBS) and then incubated with normal rabbit serum (1:50 for 20 minutes at room temperature) and then with anti-B–B4 MoAb (5 μg/mL) for 1 hour at room temperature. Paraffin-embedded tissue sections were pretreated in a microwave oven (Jet 900 W; Philips, Eindhoven, The Netherlands) twice for 5 minutes at 650 W in citrate buffer (pH 6); immunostaining was performed by incubating anti-B–B4 MoAb, with the addition of 3% normal human serum, for 1 hour at room temperature.
Deparaffinized and cryostat sections were used for further immunophenotyping and lineage assignment of HD cases. Source and specificities of antibodies used in this study have been reported in detail previously.15 16
Immunohistochemistry was performed with the alkaline phosphatase antialkaline phosphatase (APAAP) method as described.17 Naphthol AS-MX phosphate along with Fast Red TR salt (with the addition of 4% polyvinyl alcohol to the solution) was used for the development of alkaline phosphatase. The endogenous alkaline phosphatase was blocked by adding Levamisole to the substrate solution at the final concentration of 1 mmol/L. Negative controls, which were invariably negative, consisted of omission of the primary antibody, substitution with PBS, and staining with an irrelevant isotype-matched control MoAb.
Cytospin smears of HD-derived cell lines were fixed in acetone-chloroform at room temperature for 10 minutes and immunostained with anti-B–B4 and anti-CD30 MoAbs by the APAAP method.17
Assessment of B-B4 staining in HD samples.At least 100 neoplastic cells/per section, as defined by histologic and immunohistologic criteria (CD30 positivity), were independently counted by two of us (A.C. and A.G.). The percentage of B-B4+ neoplastic cells was assigned into one of the following categories: 0, less than 10%, 10% to 25%, 25% to 50%, 50% to 75%, and greater than 75%. Only definite and unambiguous staining on unequivocal malignant cells was accepted as positive. The intensity of staining (weak, moderate, or strong) was also recorded.
Cell lines.The characteristics of the human HD cell lines L428, KM-H2, and HDLM-2 were described in detail previously.18 HD-derived cell lines, KG-1A (acute myeloid leukemia), U266 and LP-1 (multiple myeloma) cell lines were obtained through the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).
Flow cytometry.Indirect immunofluorescence of cell lines and primary NHL/leukemia cell samples was performed as previously described.19 As a second step reagent, fluorescein (FITC)-conjugated F(ab′ )2 fragments of goat antimouse Ig (H+L) (Jackson Immunoresarch Laboratories, West Grove, PA) were employed. Nonspecific binding of MoAbs was assessed by labeling cells with isotype-matched control mouse Igs (Becton-Dickinson, Mountain View, CA). Viable, antibody-labeled cells were identified according to their forward and right angle scattering, electronically gated, and analyzed for surface fluorescence on a FACScan flow cytometer (Becton-Dickinson). Analyzed cells were considered as positively stained by the B-B4 MoAb (Immuno Quality Products) if exhibiting a fluorescence intensity greater than 95% of cells stained with negative control antibodies.20
RNA isolation and RT-PCR.Total RNA (1 μg), extracted by the guanidium thiocyanate method,21 was reverse-transcribed by avian myeloblastosis virus (AMV) reverse transcriptase (Promega Co, Madison, WI) in a 20- μL reaction mix containing hexadeoxyribonucleotides random primers (0.5 μg) for 1.0 hours at 42°C. Two microliters of the same cDNA preparations were amplified in a 50-μL volume of final reaction mix in a Perkin Elmer 9600 thermal cycler (Perkin Elmer Cetus, Emeryville, CA), with 25 pmol/L of primers specific for syndecan-1 (sense, 5′-GAT GGC TCT GGG GAT GAC TC-3′, region 308-327; antisense, 5′-TGT TTG GTG GGC TTC TGG TAG-3′, region 1099-1119),22 and β-actin (Clontech Laboratories Inc, Palo Alto, CA; sense, region 578-609; antisense, region 1415-1384). Conditions of hot start PCR23 for syndecan-1 were 4 minutes at 94°C followed by 35 cycles of 45 seconds at 94°C and 2.0 minutes at 70°C, and a final elongation of 5.0 minutes at 72°C. In the case of β-actin, amplification was performed for 30 cycles according to the manufacturer's guidelines. A total of 10 μL of amplified cDNAs were run in 1.5% agarose gels, blotted onto nylon membranes (Boehringer Mannheim, Mannheim, Germany) and hybridized with 2 × 106 cpm/mL of 5′ end-labelled oligoprobes, specifically designed to recognize PCR products. The syndecan-1 probe spanned nucleotide positions 338-360.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.Cells were washed twice in ice-cold PBS, 80 mmol/L Na2HPO4 , 20 mmol/L NaH2PO4 , 100 mmol/L NaCL, pH 7.4), and lysed on ice for 30 minutes in a buffer containing 10 mmol/L Tris-HCL pH 8.0, 1% Nonidet P-40, 150 mmol/L NaCl, 1.0 mmol/L EDTA, 1.0 mmol/L phenylmethylsulphonyl fluoride and 10% glycerol. Following centrifugation for 5 minutes at 14,000 rpm (4°C), the supernatant was collected and samples were separated on a 5.0% SDS-PAGE after boiling in unreduced Laemmli sample buffer. Proteins were blotted onto polyvinylidene difluoride (PVDF ) membrane (Bio Rad Laboratories, Hercules,CA) and blocked with PBS containing 5% nonfat milk and 0.02% sodium azide (PBS-NM–NaN3 ) for 12 hours at 4°C under shaking. The membrane was then incubated for 4 hours at room temperature with PBS-NM–NaN3 containing 2.0 μg/mL of MoAb B-B4 (Serotec, Oxford, UK) or an irrelevant mouse IgG1 MoAb, washed three times with PBS-NM–NaN3 and incubated with peroxidase-conjugated goat antimouse Ig (Amersham Life Science, Amersham, UK). Blots were shown by enhanced chemiluminescence (ECL) system (Amersham), following the manufacturer's instructions.
RESULTS
Expression of B-B4 in non-neoplastic lymph nodes.In tissue sections of reactive lymph nodes, the anti B-B4 MoAb showed a strong cytoplasmic and membrane staining of plasma cells, being overall unreactive with other cell populations (not shown). Blood vessels were usually negative.
Expression of the B-B4 antigen in HD.Table 1 summarizes data on the expression of the B-B4 antigen by RS cells of 56 cases of HD representative of the different histologic subtypes. A positive B-B4 staining was observed in RS cells of all classical (NS + MC) HD cases examined (Fig 1A through E), whereas B-B4 reactivity was consistently negative in nodular LP HD. Among classical HD cases, there was no apparent correlation between B-B4 expression and the antigenic phenotypes of RS cells (B, undetermined) (Table 1). In positive cases, cell membrane positivity was associated with a diffuse cytoplasmic staining of variable intensity (Table 1). There was no evidence of nuclear staining and there was no difference between the results obtained in frozen and paraffin sections stained with the B-B4 MoAb (Fig 1A through E).
(A), (B), and (D) Hodgkin's disease, nodular sclerosis subtype. Variants of the RS cell (the lacunar cells) show cytoplasmic and membrane staining of variable intensity for B-B4 antibody (arrows) both in frozen (A ) and in Bouin-fixed paraffin-embedded tissue sections (B) and (D). (C) Hodgkin's disease, mixed cellularity subtype. RS cells of classical type show cytoplasmic and membrane staining for B-B4 antibody (arrows) in frozen (C, [C inset]) sections. (E) A mononuclear variant of the RS cell displays homogeneous cytoplasmic staining in a Bouin-fixed paraffin-embedded tissue section. Note positive staining of bystander plasma cells (asterisks) (A), (B), and (C). (F ) Hodgkin's disease, nodular sclerosis subtype. Frozen section. Sclerosis-trapped spindle cells show intense staining for B-B4 antibody (top left). APAAP immunostaining; hematoxylin counterstain. Original magnification × 250 (A), (C); × 400 (B), (C inset), (D), (E), and (F ).
(A), (B), and (D) Hodgkin's disease, nodular sclerosis subtype. Variants of the RS cell (the lacunar cells) show cytoplasmic and membrane staining of variable intensity for B-B4 antibody (arrows) both in frozen (A ) and in Bouin-fixed paraffin-embedded tissue sections (B) and (D). (C) Hodgkin's disease, mixed cellularity subtype. RS cells of classical type show cytoplasmic and membrane staining for B-B4 antibody (arrows) in frozen (C, [C inset]) sections. (E) A mononuclear variant of the RS cell displays homogeneous cytoplasmic staining in a Bouin-fixed paraffin-embedded tissue section. Note positive staining of bystander plasma cells (asterisks) (A), (B), and (C). (F ) Hodgkin's disease, nodular sclerosis subtype. Frozen section. Sclerosis-trapped spindle cells show intense staining for B-B4 antibody (top left). APAAP immunostaining; hematoxylin counterstain. Original magnification × 250 (A), (C); × 400 (B), (C inset), (D), (E), and (F ).
Among NS HD, the majority of RS cells (range, 50% to 100%) showed an intense staining in approximately 30% (11 of 32) of the cases. RS cells (range, 10% to 50%) from 19 of 32 NS HD and 10 of 16 MC HD cases showed weak to moderate or weak B-B4 positivity. In the remaining 8 classical HD cases (2 NS and 6 MC), the percentage of B-B4+ RS cells was very low (about 10%) and the intensity of staining was weak. The staining pattern of RS cells was comparable to that of reactive plasma cells in the background.
Taken together, these data demonstrate that, throughout the histologic spectrum of HD, a high reactivity and staining intensity for the B-B4 MoAb was restricted to tumor cells from NS HD.
In cases in which residual normal lymphoid tissue was present, only plasma cells were stained with the anti-B–B4 MoAb. In cases of NS HD, B-B4 positivity was also found in sclerosis-trapped spindle cells (fibrocytes/fibroblasts) (Fig 1F ).
Expression of B-B4 in B-cell NHLs/leukemias and multiple myelomas/plasmacytomas.NHL/leukemia cases were classified as shown in Table 2. The expression of B-cell–associated markers was found in all cases. Flow cytometry immunoreactivity and immunohistochemical staining of anti-B–B4 MoAb in the total series is listed in Table 2.
Anti-B–B4 MoAb reacted with the cases of lymphoplasmacytoid lymphoma (2 of 2), the cases of diffuse large B-cell lymphoma of the immunoblastic plasmacytoid type (2 of 2), and the cases of multiple myeloma/plasmacytoma (28 of 28). The B-B4 antigen was not detected in all other B-cell malignancies, including different subsets of NHL, chronic lymphocytic leukemia, and hairy cell leukemia.
The use of tissue immunohistochemistry, by the APAAP technique, confirmed flow cytometry results by showing that tumor cells in the great majority of NHLs do not express B-B4 both at the cell surface and in the cytoplasm.
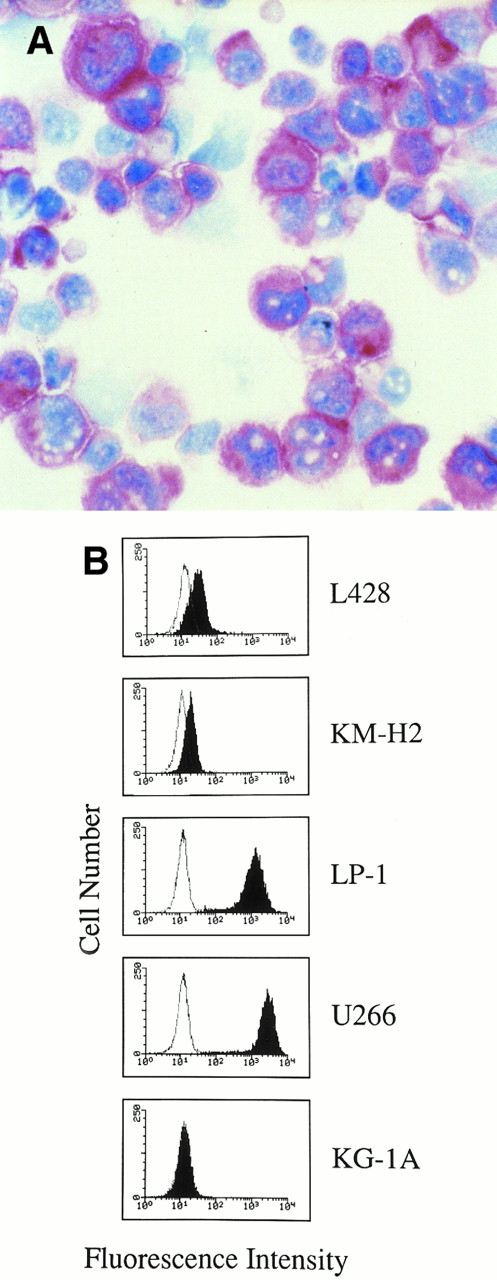
Expression of B-B4 and syndecan-1 in human HD-derived and myeloma/leukemia cell lines.On cytospin preparations, HD cell lines were consistently stained by anti-B–B4 MoAb irrespective of their antigenic profiles, which encompassed B (KM-H2) and undetermined (non-T, non-B) (L428) phenotypes. Cultured RS cells were characterized by a strong granular or diffuse cytoplasmic staining with anti-B–B4 MoAb (Fig 2A); on a number of cells, this staining was associated with labelling on the cell membranes, leading to an overall picture closely resembling that observed by us on RS cells in HD tissues.
(A) Hodgkin's disease-derived cell line L428. Cultured RS cells show staining for B-B4 antibody. Cytospin preparation; APAAP immunostaining; hematoxylin counterstain. (Original magnification × 400.) (B) Flow cytometry profiles showing B-B4 expression in human HD-derived cell lines (L428, KM-H2), myeloma cell lines (LP-1, U266), and myeloid leukemia cells KG-1A. Cells were incubated with the MoAb B-B4 (closed histograms) and an irrelevant isotype-matched mouse antibody (open histograms), followed by FITC-labeled goat antimouse Ig. The X and Y axes indicate the logarithm of the relative intensity of green fluorescence and relative cell numbers, respectively.
(A) Hodgkin's disease-derived cell line L428. Cultured RS cells show staining for B-B4 antibody. Cytospin preparation; APAAP immunostaining; hematoxylin counterstain. (Original magnification × 400.) (B) Flow cytometry profiles showing B-B4 expression in human HD-derived cell lines (L428, KM-H2), myeloma cell lines (LP-1, U266), and myeloid leukemia cells KG-1A. Cells were incubated with the MoAb B-B4 (closed histograms) and an irrelevant isotype-matched mouse antibody (open histograms), followed by FITC-labeled goat antimouse Ig. The X and Y axes indicate the logarithm of the relative intensity of green fluorescence and relative cell numbers, respectively.
To determine whether the MoAb B-B4 was able to identify syndecan-1 localized at the surface of cultured RS cells, we stained HD cell lines and compared the results with other human tumor cell lines. Analysis of flow cytometry profiles (Fig 2B) indicated that L428 and KM-H2 cells were stained by the MoAb B-B4 at a high and intermediate fluorescence intensity, respectively. Myeloma cell lines U266 and LP-1 were both stained at a very high fluorescence intensity, while KG-1A leukemic cells did not react with the B-B4 MoAb (Fig 2B).
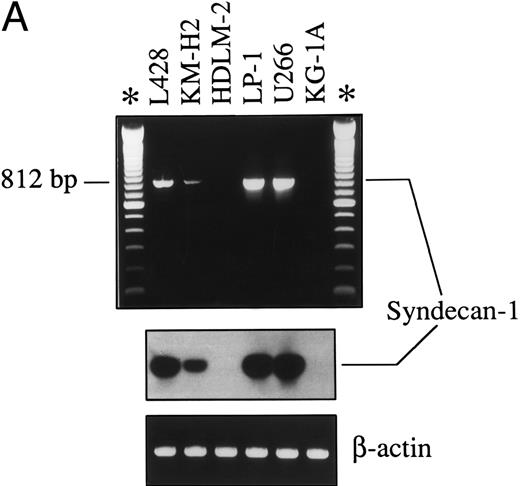
Expression of syndecan-1 mRNA was also studied by RT-PCR. As shown in Fig 3A, an 812-bp amplified cDNA product specific for syndecan-1 was detectable in both HD-derived cell lines (L428, KM-H2) and in myeloma cell lines (U266, LP-1) expressing surface B-B4, but not in myeloid leukemic cells (KG-1A), which were unreactive with the B-B4 MoAb and in a HD-derived cell line of T-cell phenotype (HDLM-2). The specificity of the PCR amplified products was also assessed by Southern blot hybridization with a syndecan-1 oligoprobe (Fig 3A).
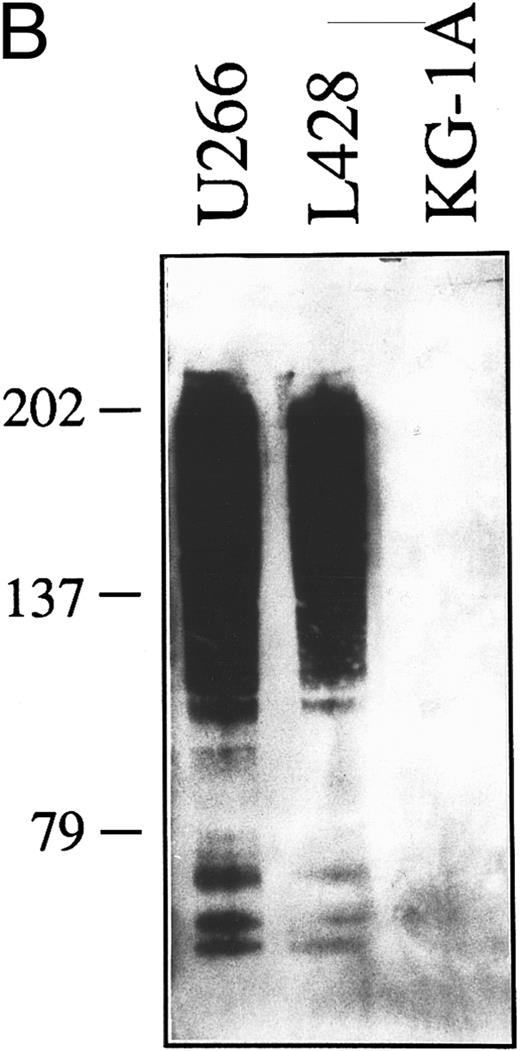
(A) Expression of syndecan-1 mRNA in human HD-derived cell lines (L428, KM-H2, HDLM-2), myeloma cell lines (LP-1, U266), and myeloid leukemia cells KG-1A, as detected by RT-PCR. cDNA bulks were prepared and amplified with specific primers for human syndecan-1 (upper panel) or β-actin (lower panel). A total of 10 μL of amplified cDNAs was also run on 1.5% agarose gels, blotted onto nylon membranes, and hybridized with a syndecan-1–specific cDNA probe (center panel). (B) Western blot of cell lysates from the human myeloma cell line U266 (1.0 × 106 cells), the HD-derived cell line L428 (6.0 × 106 cells), and the myeloid leukemia cell line KG-1A (6.0 × 106 cells). The blot was incubated with 2.0 μg/mL of the MoAb B-B4 and shown by chemiluminescence. The position of the molecular weight markers is indicated.
(A) Expression of syndecan-1 mRNA in human HD-derived cell lines (L428, KM-H2, HDLM-2), myeloma cell lines (LP-1, U266), and myeloid leukemia cells KG-1A, as detected by RT-PCR. cDNA bulks were prepared and amplified with specific primers for human syndecan-1 (upper panel) or β-actin (lower panel). A total of 10 μL of amplified cDNAs was also run on 1.5% agarose gels, blotted onto nylon membranes, and hybridized with a syndecan-1–specific cDNA probe (center panel). (B) Western blot of cell lysates from the human myeloma cell line U266 (1.0 × 106 cells), the HD-derived cell line L428 (6.0 × 106 cells), and the myeloid leukemia cell line KG-1A (6.0 × 106 cells). The blot was incubated with 2.0 μg/mL of the MoAb B-B4 and shown by chemiluminescence. The position of the molecular weight markers is indicated.
To further characterize the molecular structures recognized by the B-B4 MoAb on cultured RS cells, we have analyzed cell lysates subjected to SDS-PAGE and Western blotting analysis. On cell lysates from human myeloma cells U266 and the HD cell line L428 (Fig 3B), the MoAb B-B4 showed a pattern consisting of a predominant broad smear starting at about 200 kD plus other less represented components of about 77 to 75 kD. This pattern is consistent with the detection of syndecan-1 molecules predominantly bearing different amounts of glycosaminoglycanes, with the lower molecular weight components being related to the core protein.10,11 24 A similar profile was observed in KM-H2 cells (not shown). In contrast, no syndecan-1–related molecular components were immunodected by MoAb B-B4 on cell lysates from negative control cells KG-1A (Fig 3B) or when the blot was incubated with an irrelevant isotype-matched control mouse MoAb (not shown). Taken together, these data demonstrate that cultured RS cells of B and undetermined phenotype express syndecan-1–specific mRNA and produce a form of syndecan-1 recognized by the B-B4 MoAb that is predominantly associated with glycosaminoglycans and is present at the cell surface.
DISCUSSION
Although the cellular origin of RS cells of classical HD has not been totally clarified, recent immunophenotypic and molecular studies4-9 have suggested that RS cells of a subset of classical HD cases might represent a B-cell–derived monoclonal population. Presumably, the precise stage of B-cell differentiation of RS cells varies among different cases, even within the same histologic subtype of classical HD.
To further define the differentiation stage of RS cells and their variants, we have investigated the expression of B-B4, a specific marker for terminally differentiated B cells,10 in a large series of HD samples representative of different histologic subtypes and immunophenotypes. For comparison, B-B4 reactivity was also assessed in a panel of B-cell malignancies at different stages of differentiation and in non-neoplastic lymph nodes. We have shown that RS cells and their morphologic variants of classical HD expressed B-B4 in 48 of 48 cases, irrespective of the HD histologic subtype (NS and MC) and of their antigenic phenotype (B and undetermined). Conversely, RS cells of nodular LP HD were consistently unreactive with the B-B4 MoAb. Among classical HD cases we have observed, however, a certain heterogeneity in the number of reactive tumor cells and the intensity of staining, with the percentage of RS cells and variants expressing B-B4 ranging from 10% to more than 75%, among different cases. The staining of RS cells with the anti-B–B4 MoAb was of variable intensity on the cytoplasms being always associated with cell membranes reactivity. Despite this heterogeneity, RS cells from NS HD appeared to display a higher reactivity and a stronger staining intensity than tumor cells of the MC subtype.
Among B-cell NHL/leukemia and multiple myeloma/plasmaytoma samples, the reactivity of B-B4 MoAb was restricted to cases of lymphoplasmacytoid lymphoma, immunoblastic plasmacytoid lymphoma, and multiple myeloma/plasmaytoma, confirming the specificity of B-B4 for the terminally differentiated B cell.10 In keeping with these findings, B-B4 MoAb was highly reactive with normal plasma cells of all non-neoplastic lymph node samples and with plasma cells in the context of HD biopsies. B-B4 MoAb stained HD-derived cell lines L428 and KM-H2 and the myeloma cell lines U266 and LP-1, as assessed by immunocytochemistry and flow cytometry. Furthermore, mRNA for syndecan-1 was detected in HD cell lines of B and undetermined phenotype (KM-H2, L428), and Western blotting with the MoAb B-B4 visualized in the same cell lines a pattern consistent with the presence of syndecan-1 molecules bearing glycosaminoglycans.10,13 Taken together, our data demonstrate that primary RS from classical HD of NS and MC subtypes, but not tumor cells of nodular LP HD, and HD-derived human cell lines produce a form of syndecan-1, as detected by the B-B4 MoAb, that is predominantly associated with glycosaminoglycans and expressed at the cell surface.
The frequent and selective occurrence of B-B4 expression in classical HD provides further evidence favoring that the disease may frequently represent a neoplasm of postgerminal center B-cell origin, and thus supporting previous molecular findings.6-9 In addition, we provide the first phenotypic evidence that expression of the plasma cell-specific antigen B-B4 is a feature of RS cells and their morphologic variants of classical HD, but not of tumor cells of nodular LP HD that consistently express B-cell antigens and are thought to derive from germinal center cells.1 With respect to classical HD, it has been proposed that a small fraction of cases display a genetic asset compatible with naive pre-germinal center B cells.7,9 Notably, such putative derivation is consistent with the expression of the B-B4 antigen (syndecan-1), that is transiently expressed on pre-B cells, lost on further B-cell maturation and reexpressed on terminally differentiated plasma cells.10,13 24
Our findings also raise some points as to the biologic significance of B-B4 expression on tumor cells of HD. During development syndecan-1 is usually expressed at sites where cells associate with each other and with the extracellular matrix,11,12 being usually lost or downregulated on normal and tumor cell types before they can be released from tissues, invade the matrix and migrate to peripheral compartments.11,13,24-27 Accordingly, binding of cellular syndecan-1 to interstitial and stromal matrix components has been specifically implicated in the process of anchoring and sequestring of pre-B cells and plasma cells within the bone marrow and lymphoid organs.13,24 Interestingly, we have detected the highest cellular density of syndecan-1 on RS from NS cases and in a cell line (L428) derived from a patient with NS HD. Because an increased deposition of collagen types I and III and fibronectin, all known ligands for cellular syndecan-1, have been evidenced in tissues of NS HD,28,29 our present data suggest that RS cells could use syndecan-1 to remain anchored within the cellular and stromal microenvironment typical of NS HD.30-33
The extensive fibrosis typical of NS HD has been mainly related to the production of transforming growth factor-β1 and tumor necrosis factor.34-36 Our detection of a high density of the B-B4 antigen (syndecan-1) on reactive fibroblasts and fibrocytes within and around tumor nodules, may suggest an additional mechanism for sclerosing changes in NS HD, given the ability of syndecan-1 to act as a coreceptor for the basic fibroblast growth factor.11,37,38 This view is further supported by previous studies that upregulation of syndecan-1 on fibroblastic cells, during the wound repair processes, is associated with active fibroblast proliferation and the occurence of fibrosis.37-40
Based on the above considerations, the presence of surface syndecan-1 on RS cells in HD tissues may be consistent with the tendency of malignant cells to remain confined within their typical microenvironment and the highly compartimentalized and nondestructive pattern of growth of this lymphoma.30,31,33 41
In conclusion, our detection of the plasma cell-specific antigen B-B4 (syndecan-1) on tumor cells of classical HD further supports that RS cell progenitors may be related to germinal/postgerminal center mature B cells,6-9 and suggests that expression of syndecan-1 may contribute to some of the typical biologic and histopathologic features of HD, with a special regard to the NS subtype.
Supported in part by the Ministero della Sanità, Ricerca Finalizzata I.R.C.C.S., Rome, Italy; the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy; and the Consiglio Nazionale delle Ricerche, PF-ACRO, Italy.
Address reprint requests to Antonino Carbone, MD, Division of Pathology, Centro di Riferimento Oncologico, IRCCS, via Pedemontana Occidentale, Aviano I-33081, Italy.

![Fig. 1. (A), (B), and (D) Hodgkin's disease, nodular sclerosis subtype. Variants of the RS cell (the lacunar cells) show cytoplasmic and membrane staining of variable intensity for B-B4 antibody (arrows) both in frozen (A ) and in Bouin-fixed paraffin-embedded tissue sections (B) and (D). (C) Hodgkin's disease, mixed cellularity subtype. RS cells of classical type show cytoplasmic and membrane staining for B-B4 antibody (arrows) in frozen (C, [C inset]) sections. (E) A mononuclear variant of the RS cell displays homogeneous cytoplasmic staining in a Bouin-fixed paraffin-embedded tissue section. Note positive staining of bystander plasma cells (asterisks) (A), (B), and (C). (F ) Hodgkin's disease, nodular sclerosis subtype. Frozen section. Sclerosis-trapped spindle cells show intense staining for B-B4 antibody (top left). APAAP immunostaining; hematoxylin counterstain. Original magnification × 250 (A), (C); × 400 (B), (C inset), (D), (E), and (F ).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3787/3/m_bl_0043f1.jpeg?Expires=1767831919&Signature=3IAMld6WxI9p5auVqD3~vzbQYOJJRJIXN6Jjy02kfqI9~nsOKLkWwe49N-SM2xSFoMn94CSLDGCIODzWVbH5GPRV5675fBTnj71yi7GTN7ifkM21cBjC1FQCs1KhgMW3DDSPZvpLOo7U-2Qq9jvwD4Rcm-Q6vNf~hC23acr1gawEgNYT~kXt9Wd7tCXuOJZ5deGFrWmCP3UJKe2i1yoBrS93ab6d4m3xSeTigybhpuo8~F2WERE1-t65d027tOCZ7yZYxNzUQAcEjfZyvKUH479y15AWfQ52PFisZXJnl2KnuRlSpn3EJQGl7ssLMtsulizLxOPUwnNkcJKQwcJb-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal