Abstract
We used a stroma-supported culture method to study the prevalence and growth characteristics of malignant stem cells in acute lymphoblastic leukemia (ALL). In 51 of 108 B-lineage ALL samples, bone marrow-derived stroma not only inhibited apoptosis of ALL cells but also supported their proliferation in serum-free medium. When single leukemic cells were placed in the stroma-coated wells of microtiter plates, the percentage of wells with leukemic cell growth after 2 to 5 months of culture ranged from 6% to 20% (median, 15%; 5 experiments). The immunophenotypes and genetic features of cells recovered from these cultures were identical to those noted before culture. All cells maintained their stroma dependency and self-renewal capacity. Leukemic clones derived from single cells contained approximately 103 to 106 cells after 1 month of culture; other clones became detectable only after prolonged culture. Cell growth in stroma-coated wells correlated with the number of initially seeded cells (1 or 10; r = .87). However, the observed percentages of positive wells seeded with 10 cells always exceeded values predicted from results with single-cell–initiated cultures (P < .003 by paired t-test), suggesting stimulation of leukemic cell growth by paracrine factors. In conclusion, the proportion of ALL cells with clonogenic potential may be considerably higher than previously thought.
CENTRAL TO UNDERSTANDING neoplastic growth is the ability to study malignant cells with self-renewal capacity, the so-called cancer stem cells. This approach to cancer investigation has been impeded by the lack of suitable methods to support the long-term in vitro growth of neoplastic cells from patients. Hence, the frequency, growth requirements, and proliferative status of cancer stem cells remain largely unknown. In acute lymphoblastic leukemia (ALL), the most common cancer of children, these issues have been addressed almost entirely with short-term colony assays in semisolid medium.1-15 The results suggest that leukemic lymphoblasts with self-renewal capacity constitute only a minute fraction of ALL cells (ie, one or two per thousand) and that the remainder of leukemic blasts are incapable of generating self-sustaining clones.16 17
We would argue that this method of assessment vastly underestimates the growth potential of leukemic cell populations for two reasons. First, the majority of such cells rapidly die in vitro by apoptosis, which is not prevented by the animal sera or exogenous cytokines commonly used in colony assays.18 Thus, the pool of both resting and proliferating leukemic cells may be drastically reduced within hours of culture. Second, it is conceivable that a proportion of leukemic stem cells are quiescent, as suggested by clinical observations of leukemic relapse years after cessation of therapy.19 20 It seems unlikely that these cells could be driven into vigorous proliferative activity by several days of culture in a suboptimal in vitro microenvironment.
We have observed that bone marrow-derived stromal layers suppress apoptosis of leukemic lymphoblasts from cases of childhood B-lineage ALL.18,21 22 In the study reported here, we tested whether these culture conditions could be adapted to study the self-renewal potential of individual leukemic cells. Thus, we evaluated 108 samples of B-lineage ALL and found that, in approximately half of the cases, stromal cell layers supported the growth and expansion of leukemic cells without addition of exogenous cytokines or animal sera. We then applied flow cytometric cell sorting to seed defined numbers of cells on stroma and determine the frequency and growth patterns of leukemic stem cells in long-term culture. Our findings indicate that ALL blast cells with self-renewing potential are much more abundant than previously thought.
MATERIALS AND METHODS
Cells.Bone marrow samples were collected at diagnosis from 108 children with B-lineage ALL. Immunophenotyping established the diagnosis in each case (>80% of the blast cells were CD19+, HLA-DR+ and lacked surface Ig). Five samples, four collected at diagnosis and one at the time of relapse, were used for further clonogenic studies. These cases were selected from cases with proliferative activity in short-term cultures (see Results) because of the availability of abundant cryopreserved material. Their immunophenotypic and karyotypic features are summarized in Table 1. Mononuclear cells were obtained after centrifugation on a density gradient (Lymphoprep; Nycomed, Oslo, Norway) and washed three times in phosphate-buffered saline (PBS). Samples were cryopreserved and used immediately after thawing. Continuously growing ALL cell lines (Nalm6, REH, 380, RS4; 11, and CEM-C7) were obtained from the institution's cell bank and maintained in RPMI-1640 (BioWhittaker, Walkersville, MD) with 10% fetal calf serum (FCS; BioWhittaker), L-glutamine, and antibiotics. In all experiments, the cells' viability before culture exceeded 95% by trypan-blue dye exclusion.
Immunophenotypic and Karyotypic Features of the Samples Used for Single-Cell Sorting Experiments
| Case No. . | Immunophenotype* . | Karyotype . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CD19 . | CD10 . | CD34 . | HLA-Dr . | TdT . | cμ . | CD7 . | CD3 . | CD13 . | CD33 . | . |
| 1 | 98 | 4 | 39 | 98 | 96 | <1 | <1 | 2 | 5 | 5 | 46,XX,t(4; 11)(q21; q23) |
| 2 | 97 | 20 | 10 | 96 | 1 | 67 | <1 | 1 | <1 | 31 | 46,XY,t(11; 19)(q23; p13) |
| 3 | 96 | 95 | <1 | 95 | 90 | 99 | 1 | 3 | <1 | 1 | 46,XY,t(1; 19)(q23; p13) |
| 4 | 95 | 96 | 95 | 96 | 96 | <1 | 3 | 2 | <1 | 46 | 46,XY,t(9; 22)(q34; q11) |
| 5† | 96 | 91 | 95 | 98 | 98 | <1 | 4 | 4 | 89 | 68 | 46,XY,t(9; 22)(q34; q11), multiple random structural |
| rearrangements | |||||||||||
| Case No. . | Immunophenotype* . | Karyotype . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CD19 . | CD10 . | CD34 . | HLA-Dr . | TdT . | cμ . | CD7 . | CD3 . | CD13 . | CD33 . | . |
| 1 | 98 | 4 | 39 | 98 | 96 | <1 | <1 | 2 | 5 | 5 | 46,XX,t(4; 11)(q21; q23) |
| 2 | 97 | 20 | 10 | 96 | 1 | 67 | <1 | 1 | <1 | 31 | 46,XY,t(11; 19)(q23; p13) |
| 3 | 96 | 95 | <1 | 95 | 90 | 99 | 1 | 3 | <1 | 1 | 46,XY,t(1; 19)(q23; p13) |
| 4 | 95 | 96 | 95 | 96 | 96 | <1 | 3 | 2 | <1 | 46 | 46,XY,t(9; 22)(q34; q11) |
| 5† | 96 | 91 | 95 | 98 | 98 | <1 | 4 | 4 | 89 | 68 | 46,XY,t(9; 22)(q34; q11), multiple random structural |
| rearrangements | |||||||||||
Values are the percentages of cells recognized by antibodies to the listed antigens.
Same case as case no. 4, except that cells were collected at relapse.
To prepare bone marrow-derived stromal layers, we obtained mononucleated cells from bone marrow transplantation donors.18,21-24 Preliminary studies indicated that such cells had similar capacities to maintain ALL cell viability regardless of the donor from whom they were collected.22,23 Mononuclear cells were separated as described above and washed three times in RPMI-1640. They were resuspended at 5 × 106/mL in RPMI-1640, 10% FCS, and 10−6 mol/ L hydrocortisone (Sigma, St Louis, MO) as previously described.18 21-24 Twenty-milliliter aliquots of the suspension were distributed in 75-cm2 flasks (Nunc, Roskilde, Denmark) and placed in an incubator set at 37°C and 5% CO2 with 90% humidity. The cells were fed every 7 days by replacing 90% of the supernatant with fresh medium with additives. When a confluent layer of stromal cells had formed, the cells were detached by treatment with trypsin (BioWhittaker), washed once in RPMI-1640 with 10% FCS, resuspended in fresh medium containing additives, and placed in an identical flask. This procedure was repeated at least three times to remove hematopoietic cells. Finally, after T-cell depletion with anti-CD3 complement-fixing monoclonal antibody and rabbit complement, the cells were distributed into 96-well microtiter plates (Costar, Cambridge, MA) precoated with fibronectin (3.3 μg/cm2; Boehringer Mannheim, Indianapolis, IN).
Cell culture experiments and cell sorting.Before the culture experiments were begun, media were removed from the bone marrow stromal cultures and the feeder layers were washed seven times with AIM-V media (GIBCO, Grand Island, NY), a chemically defined, serum-free medium that contains human insulin and transferrin. Leukemic lymphoblasts, depleted of T cells with CD4-and CD8-conjugated immunomagnetic beads (Dynal, Oslo, Norway), were resuspended at a final concentration of 1.5 × 106/mL in AIM-V. Two-hundred microliters of the suspension was then seeded onto marrow stromal cells that had been extensively washed to remove FCS and hydrocortisone or were placed in identical plates without stroma. Neutralizing antibodies to interleukin-7 (IL-7) and stem-cell factor (SCF ), purchased from R & D (Minneapolis, MN), were used at a final concentration of 10 μg/mL. After 7 days of culture in an incubator set at 37°C, 5% CO2 , and 90% humidity, the cells were harvested, incubated with fluorescein isothiocyanate (FITC) conjugated CD19 and phycoerythrin (PE) conjugated CD3 monoclonal antibodies (both from Becton Dickinson, San Josè, CA), and analyzed with a FACScan flow cytometer equipped with Lysis II software (Becton Dickinson) as previously described.18 21-24 The percentage of cell recovery was calculated as the number of CD19+ lymphoblasts after 7 days of culture × 100/number of CD19+ lymphoblasts after 1 hour of culture. Estimates of cell recovery are reported as the mean of at least duplicate experiments.
Cell sorting was performed with a FACSstarPlus cell sorter, equipped with an Argon ion laser tuned at 488 nm, an automatic cell deposition unit, and Lysis II software (Becton Dickinson). B-lineage ALL cells were resuspended in AIM-V medium and data of 2,000 events were recorded. In the light scattering dot plot, we defined a gate that encompassed the majority of lymphoblasts. To eliminate apoptotic cells and debris we excluded cells with small forward light scatter; doublets and cell aggregates were eliminated by excluding events with large forward and orthogonal light scatter. Predetermined numbers of cells within this gate were deposited in the wells of 96-well U-bottomed microtiter plates coated with bone marrow stroma containing approximately 100 μL of AIM-V tissue culture medium. Fifty microliters of medium per well was added after completion of the sorting procedure. All cell cultures were performed in an incubator set at 37°C, 5% CO2 , and 90% humidity. Fifty microliters of medium was replaced every 1 to 2 weeks.
Immunophenotyping and flow cytometric analysis of apoptosis.Cells were immunophenotyped at the beginning and end of the cultures according to standard procedures. Briefly, they were washed in PBS containing 0.2% bovine serum albumin and 0.2% sodium azide (PBSA) before incubation with monoclonal antibodies conjugated to FITC or PE (all purchased from Becton Dickinson or Dako, Carpinteria, CA). Goat antihuman IgM and Ig light-chain antisera conjugated to FITC (Southern Biotechnology Associates, Birmingham, AL), as well as monoclonal antibodies to human terminal deoxynucleotidyl transferase (TdT) conjugated to FITC (Supertech Inc, Bethesda, MD), were also used in some experiments. Isotype-matched fluorochrome-conjugated nonreactive antibodies served as controls. Cells were analyzed with a FACScan flow cytometer (Becton Dickinson). To detect intracellular antigens, we treated cells with Ortho Permeafix (Ortho, Raritan, NJ), after which they were analyzed by flow cytometry.25 Cells were also cytocentrifuged, fixed in acetone:methanol (1:1) for 15 minutes at 4°C, stained, and viewed with a Zeiss Axioscope fluorescence microscope (Carl Zeiss, Oberkochen, Germany). To detect occurrence of apoptosis, we examined changes in the light-scattering properties of the cells. In addition, we labeled cells with Annexin-V conjugated to FITC (Trevigen, Gaithersburg, MD) to assess phosphatidylserine exposure on the surface of leukemic lymphoblasts (a marker of apoptosis).26 To detect cell membrane permeabilization, cells were also labeled with propidium iodide using standard procedures.
Southern blot analysis.Genomic DNA was extracted from cultured ALL cells according to standard procedures. Control DNA was extracted from the human epidermoid carcinoma cell line KB (obtained from Dr G. Kitchingman, St Jude Children's Research Hospital, Memphis, TN). DNA (8 μg) digested with the restriction enzymes EcoRI, HindIII, Bgl II, or BamHI was electrophoresed in 0.8% agarose gel and transferred to Hybond-N filter membranes (Amersham, Arlington Heights, IL). After hybridization to 32P-labeled DNA probes and high stringency washing, the filters were autoradiographed with Kodak XAR-5 film (Kodak, New Haven, CT). Two DNA probes were used: JH , a 5.6-kb genomic fragment containing the joining region of the human Ig heavy-chain gene (Oncor, Gaithersburg, MD), and MLL, a 0.8-kb cDNA containing exons V to XI of the human MLL gene (gift of Dr J. Downing, St Jude Children's Research Hospital). These probes were labeled with [α-32P] dCTP by the multiprime DNA labeling system (Amersham) to a specific activity of 1 × 109 cpm per microgram of DNA.
Determination of Epstein-Barr virus (EBV) infection.The expression of EBV-associated molecules was investigated with immunologic and molecular techniques. Cytocentrifuged preparations of cultured cells were fixed in acetone:methanol (1:1) and stained with a specific monoclonal antibody to Epstein-Barr virus nuclear antigen-2 (EBNA-2; a gift of Dr M. Rowe, Birmingham, UK),27 followed by a goat antimouse Ig antibody conjugated to FITC; the Raji cell line was used as a positive control. EBNA expression in cultured cells was also assessed by the polymerase chain reaction (PCR).28 The 100-μL reaction mixture contained 1 μg of genomic DNA; 10 pmol each of 5′ and 3′ primer; 200 μmol/L each of dATP, dCTP, dGTP, and dTTP; 10 mmol/L Tris-HCl (pH 8.3); 50 mmol/L KCl; 1.5 mmol/L MgCl2 ; 0.001% (wt/vol) gelatine; and 2.5 U of Taq DNA polymerase (Perkin-Elmer, Branchburg, NJ). The sequences of the oligonucleotide primers for EBNA 2a (5′-AACTTCAACCCACACCATCA-3′, 5′-TTCTGGACTATCTGGATCAT-3′) and EBNA 2b (5′-TACTCTTCCTCAACCCAGAA-3′, 5′-GGTGGTAGACTTAGTTGATG-3′) amplification were synthesized with an automatic DNA synthesizer (Applied Biosystems, Foster City, CA). We performed 35 cycles of denaturing (94°C for 1 minute), annealing (56°C for 1 minute), and extension (72°C for 2 minutes) with a Thermo-Cycler 480 (Perkin-Elmer). Ten microliters of the PCR product was subjected to 2.5% agarose gel electrophoresis and visualized by ethidium bromide staining (0.5 μg/mL). This protocol allows the detection of as few as two copies of EBV genome per cell.28
RESULTS
Stromal cells support leukemic cell proliferation.In experiments with 108 B-lineage ALL samples, the percentages of cells recovered after 7 days of culture on bone marrow-derived stroma, but without animal sera and exogenous cytokines, ranged from less than 1% to 292% (median, 95.5%) of the cells originally seeded. By contrast, in parallel cultures without stroma performed in 107 cases, the median cell recovery was only 1% owing to apoptosis. In 51 of the 108 cases (47.2%), the number of leukemic cells recovered after 7 days of culture on stroma exceeded that originally seeded, indicating that the stromal cells not only suppressed apoptosis, but also supported cell proliferation.
Previous studies had indicated that two stroma-derived cytokines, IL-7 and SCF, stimulate the proliferation of leukemic immature B cells.29-32 To determine whether leukemic cell growth on stromal layers depends on the secretion of either or both cytokines, we cultured three ALL samples in the presence or absence of neutralizing antibodies to IL-7 and SCF. Cell recoveries after 7 days of culture in the absence of the neutralizing antibodies, using an isotype-matched nonreactive antibody as a control, were 185%, 118%, and 127%, respectively. These values did not decrease in parallel stroma-supported cultures containing anti–IL-7, anti-SCF, or both antibodies at 10 μg/mL, a concentration known to completely neutralize cytokine activity. Thus, the growth of ALL cells on stroma does not appear to depend on stimulation by IL-7 or SCF.
Proportion of clonogenic cells in ALL.To determine the number of leukemic lymphoblasts with self-renewal capacity, we sorted 1, 10, and 100 cells by flow cytometry and deposited them into the stroma-coated wells of microtiter plates.33 We first studied four bone marrow samples collected at diagnosis and another collected at the time of relapse. In these five samples the cell recovery after 7 days of culture on stroma (median, 178%; maximum, 244%) overlapped that of the 51 cases that proliferated in vitro (median, 141%; maximum, 292%). After cell sorting, the plates were not further manipulated until the end of the cultures except for weekly changes of 50 μL of tissue culture medium and monthly inspection with an inverted microscope. In preliminary experiments we placed leukemic cells in log increments into microtiter plates that were identical to those used in the assays. Leukemic cells were unequivocally detected by two observers when they reached a density at least 1,000 cells in an individual stroma-coated U-bottomed well. Thus, for the clonogenic assay, any concentration of 1,000 or more cells was then taken to indicate leukemic growth.
The results of plating 1, 10, and 100 cells into single-culture wells coated with stroma are presented in Table 2. Leukemic cell growth was readily detected after 1 month of culture in all wells seeded with 100 cells, indicating that at least 1% of the lymphoblasts in each sample had self-renewal capacity. This estimate was confirmed by results obtained when wells were seeded with 10 cells. After 1 month of culture, leukemic cell growth was detectable in 20% to 95% of wells (median, 90%) in all five cases. After 2 months of culture, the proportion of productive cultures remained stable in two cases and increased further in the remaining three.
Leukemic Cell Growth in Stroma-Coated Wells Seeded With Defined Numbers of B-Lineage ALL Cells
| Case . | No. of . | No. of . | Wells Showing Leukemic Cell Growth* . | ||||
|---|---|---|---|---|---|---|---|
| No. . | Cells Plated . | Wells . | After 1 to 5 mo of Culture (%) . | ||||
| . | per Well . | Plated . | 1 . | 2 . | 3 . | 4 . | 5 . |
| 1 | 100 | 10 | 100 | 100 | NA | NA | NA |
| 10 | 20 | 90 | 90 | NA | NA | NA | |
| 1 | 110 | 8 | 12 | 14 | NA | NA | |
| 2 | 100 | 20 | 100 | 100 | NA | NA | NA |
| 10 | 20 | 40 | 50 | NA | NA | NA | |
| 1 | 80 | 2 | 6 | NA | NA | NA | |
| 3 | 100 | 10 | 100 | 100 | 100 | 100 | 100 |
| 10 | 20 | 20 | 45 | 45 | 60 | 55 | |
| 1 | 90 | 2 | 4 | 6 | 11 | 16 | |
| 4 | 100 | 10 | 100 | 100 | 100 | NA | NA |
| 10 | 30 | 93 | 93 | 93 | NA | NA | |
| 1 | 60 | 10 | 15 | 15 | NA | NA | |
| 5 | 100 | 10 | 100 | 100 | NA | NA | NA |
| 10 | 20 | 95 | 100 | NA | NA | NA | |
| 1 | 90 | 17 | 20 | NA | NA | NA | |
| Case . | No. of . | No. of . | Wells Showing Leukemic Cell Growth* . | ||||
|---|---|---|---|---|---|---|---|
| No. . | Cells Plated . | Wells . | After 1 to 5 mo of Culture (%) . | ||||
| . | per Well . | Plated . | 1 . | 2 . | 3 . | 4 . | 5 . |
| 1 | 100 | 10 | 100 | 100 | NA | NA | NA |
| 10 | 20 | 90 | 90 | NA | NA | NA | |
| 1 | 110 | 8 | 12 | 14 | NA | NA | |
| 2 | 100 | 20 | 100 | 100 | NA | NA | NA |
| 10 | 20 | 40 | 50 | NA | NA | NA | |
| 1 | 80 | 2 | 6 | NA | NA | NA | |
| 3 | 100 | 10 | 100 | 100 | 100 | 100 | 100 |
| 10 | 20 | 20 | 45 | 45 | 60 | 55 | |
| 1 | 90 | 2 | 4 | 6 | 11 | 16 | |
| 4 | 100 | 10 | 100 | 100 | 100 | NA | NA |
| 10 | 30 | 93 | 93 | 93 | NA | NA | |
| 1 | 60 | 10 | 15 | 15 | NA | NA | |
| 5 | 100 | 10 | 100 | 100 | NA | NA | NA |
| 10 | 20 | 95 | 100 | NA | NA | NA | |
| 1 | 90 | 17 | 20 | NA | NA | NA | |
Abbreviation: NA, not assessed.
Defined as 103 cells or more.
Thus, after 2 months of culture, 45% to 100% of the wells seeded with 10 cells were positive. Finally, in attempts to initiate cell growth with single lymphoblasts, the percentages of wells with 1,000 or more cells after 1 month of culture ranged from 2% to 17% (median, 8%; Table 2). Notably, in all cases, the number of positive wells continued to increase as cultures were extended. New clones were visible even after 5 months of culture. When cultures were terminated after 2 to 5 months, the percentage of positive wells in the five experiments ranged from 6% to 20% (median, 15%). These percentages strongly correlated with values obtained in parallel wells seeded with 10 cells (r = .87; P = .001). The vast majority of cells in the productive cultures were viable and lacked morphologic signs of apoptosis (Fig 1). They also appeared to adhere tightly to stroma and could not be dislodged by shaking the plates. Randomly picked clones continued to grow in direct contact with stroma for more than 1 year.
Bone marrow-derived stromal layers support the in vitro growth of leukemic B lymphoblasts. Inverted microscopy photographs ([A] 4× objective; [B] 20× objective) show leukemic lymphoblasts derived from one single progenitor cell after 2 months of culture on stroma (case no. 4). Leukemic cells are viable and grow in close contact with stromal elements.
Bone marrow-derived stromal layers support the in vitro growth of leukemic B lymphoblasts. Inverted microscopy photographs ([A] 4× objective; [B] 20× objective) show leukemic lymphoblasts derived from one single progenitor cell after 2 months of culture on stroma (case no. 4). Leukemic cells are viable and grow in close contact with stromal elements.
We used established ALL cell lines to validate the accuracy of the cell sorting procedures described above. In experiments with Nalm6, REH, 380, and RS4; 11 cells, growth was detectable after 1 month of culture in 70% to 100% (median, 80%) of the wells that had been seeded with one cell. We also sorted single cells from a mixture of REH (CD19+, CD7−) and CEM-C7 (CD19−, CD7+) cells and immunophenotyped the growing clones. Of the 38 wells tested after 1 month of culture, 25 contained only CD19+ cells and 13 contained only CD7+ cells; no mixed cell populations were detected. Thus, our methodology appears to have deposited leukemic cells in the vast majority of wells and to have ensured that no doublets or triplets were present in wells ostensibly seeded with single cells.
Immunophenotypic and genotypic features of blast cell progeny.We wished to confirm that the growing cells on stroma represented the progeny of the leukemic lymphoblasts originally seeded and not the outgrowth of normal cells that may have been present at low frequency in the stromal layers or among the initially seeded leukemic cells. There was no detectable growth in parallel cultures of stromal cells maintained in serum-free medium without seeding of ALL blasts. In wells that contained the progeny of leukemic lymphoid cells, phenotypic profiles were consistent with those of the originally seeded blasts (Fig 2). EBNA-2 expression was absent as determined by staining with a specific antibody (cases no. 1 and 4) using the Raji cell line as a positive control. EBNA-2a and EBNA-2b were undetectable by PCR in all three cases studied (cases nos. 1, 3, and 4), whereas EBV-transformed lymphoblastoid cells (positive controls) had either the 116-bp or the 119-bp amplification product of EBNA-2a or EBNA-2b, respectively (Fig 3). These results exclude the possibility that cell proliferation in culture wells was driven by EBV infection.
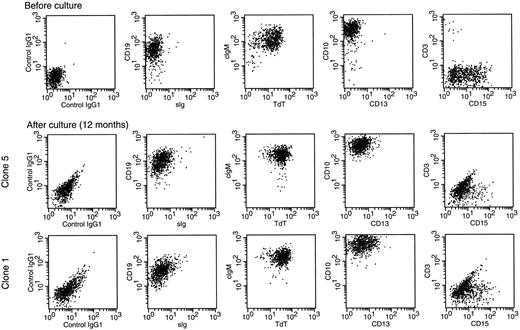
Leukemic cells retain their immunophenotypic features after long-term culture on stroma. Double-color flow cytometric dot-plots illustrate the cell marker profile of cells from case no. 3 before culture (top panels) and after 12 months of culture (middle and bottom panels). Cells after culture were obtained from clones initiated by single leukemic cells. Antibodies were conjugated to FITC (X axes) or PE (Y axes). Most leukemic cells expressed CD19, cytoplasmic μ (cIgM), terminal deoxynucleotidyl transferase (TdT), and CD10; a proportion also expressed CD15. Expression of TdT and cytoplasmic μ was confirmed by fluorescence microscopy (not shown). Cells did not express sIg, CD13, and CD3. The immunophenotype of the other three clones was identical to that of the clones shown.
Leukemic cells retain their immunophenotypic features after long-term culture on stroma. Double-color flow cytometric dot-plots illustrate the cell marker profile of cells from case no. 3 before culture (top panels) and after 12 months of culture (middle and bottom panels). Cells after culture were obtained from clones initiated by single leukemic cells. Antibodies were conjugated to FITC (X axes) or PE (Y axes). Most leukemic cells expressed CD19, cytoplasmic μ (cIgM), terminal deoxynucleotidyl transferase (TdT), and CD10; a proportion also expressed CD15. Expression of TdT and cytoplasmic μ was confirmed by fluorescence microscopy (not shown). Cells did not express sIg, CD13, and CD3. The immunophenotype of the other three clones was identical to that of the clones shown.
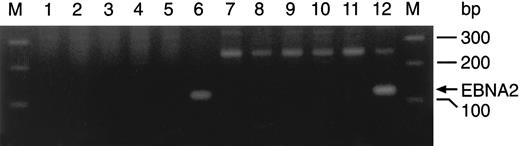
Lack of EBNA-2 expression in cultured leukemic lymphoblasts. PCR amplification of EBNA-2a (lanes 1 through 6) and EBNA-2b (lanes 7 through 12) in 3 clones initiated from single leukemic cells (cases no. 1, 3, and 4) after 5 months of culture (lanes 2 through 4 and 8 through 10), in the EBNA-2a– (AD; lanes 6 and 11) and EBNA-2b– (C2BL16; lanes 5 and 12) positive cell lines, and in placenta DNA (negative control; lanes 1 and 7). The 116-bp (EBNA-2a) and 119-bp (EBNA-2b) PCR products could only be detected in the EBNA-2–positive lines. External lanes contain a molecular weight marker.
Lack of EBNA-2 expression in cultured leukemic lymphoblasts. PCR amplification of EBNA-2a (lanes 1 through 6) and EBNA-2b (lanes 7 through 12) in 3 clones initiated from single leukemic cells (cases no. 1, 3, and 4) after 5 months of culture (lanes 2 through 4 and 8 through 10), in the EBNA-2a– (AD; lanes 6 and 11) and EBNA-2b– (C2BL16; lanes 5 and 12) positive cell lines, and in placenta DNA (negative control; lanes 1 and 7). The 116-bp (EBNA-2a) and 119-bp (EBNA-2b) PCR products could only be detected in the EBNA-2–positive lines. External lanes contain a molecular weight marker.
In three experiments, cells randomly picked from wells seeded with single lymphoblasts were expanded on stroma until approximately 3 to 5 × 106 cells were available for DNA extraction and Southern blot analysis. DNA blot hybridization with a JH probe showed biallelic IgH rearrangement in cells from case no. 3 (Fig 4) and monoallelic IgH rearrangement with loss of the other allele in cells from case no. 4 (Fig 5). After 7 and 2 months of culture, respectively, the blast cell progeny carried identically rearranged IgH genes (Figs 4 and 5). Moreover, the gene rearrangements in case no. 4 were also seen in a sample obtained from the same patient at the time of relapse and in a stroma-independent cell line (OP-1) developed from the diagnostic sample (Fig 5). In case no. 3, the IgH gene rearrangements detected at diagnosis were also identified in cells grown from single cells deposited in four separate stroma-coated wells (Fig 4). Finally, the MLL gene alterations that defined case no. 1 were reiterated in blasts cells examined before and after culture (Fig 6). Collectively, these findings indicate that cell growth in our stroma-based system represents the progeny of originally seeded leukemic cells.
Leukemic lymphoblasts after culture retain Ig gene rearrangement in the same configuration determined before culture. Shown is the Southern blot analysis of Ig gene rearrangements in leukemic cells from case no. 3 obtained before culture and after 7 months of culture on stroma. Four clones derived in vitro from single leukemic cells were studied. DNA was digested with the restriction enzymes indicated and probed with a JH probe. Arrows point to the rearranged bands; G corresponds to the germline band. The KB epidermoid carcinoma cell line, which has Ig genes in germline configuration, was used as a control. The faint germline bands seen in some of the clones after culture are probably derived from residual stromal cells.
Leukemic lymphoblasts after culture retain Ig gene rearrangement in the same configuration determined before culture. Shown is the Southern blot analysis of Ig gene rearrangements in leukemic cells from case no. 3 obtained before culture and after 7 months of culture on stroma. Four clones derived in vitro from single leukemic cells were studied. DNA was digested with the restriction enzymes indicated and probed with a JH probe. Arrows point to the rearranged bands; G corresponds to the germline band. The KB epidermoid carcinoma cell line, which has Ig genes in germline configuration, was used as a control. The faint germline bands seen in some of the clones after culture are probably derived from residual stromal cells.
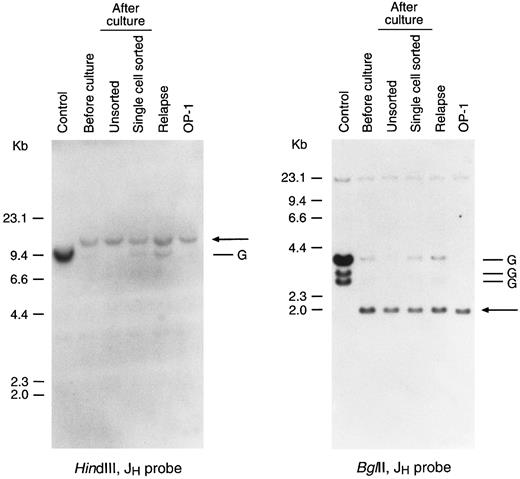
Leukemic lymphoblasts after culture retain Ig gene rearrangement in the same configuration determined before culture. Shown is the Southern blot analysis of Ig gene rearrangements in leukemic cells from case no. 4 obtained before culture and after 2 months of culture on stroma. The latter cells were derived from a bulk culture and from a culture initiated by a single leukemic cell. DNA obtained from the relapse sample and from a stroma-independent cell line (OP-1) originated from the diagnostic sample were also analyzed. DNA was digested with the restriction enzymes indicated and probed with a JH probe. Arrows point to the rearranged bands; G corresponds to the germline bands. KB epidermoid carcinoma line was used as a control.
Leukemic lymphoblasts after culture retain Ig gene rearrangement in the same configuration determined before culture. Shown is the Southern blot analysis of Ig gene rearrangements in leukemic cells from case no. 4 obtained before culture and after 2 months of culture on stroma. The latter cells were derived from a bulk culture and from a culture initiated by a single leukemic cell. DNA obtained from the relapse sample and from a stroma-independent cell line (OP-1) originated from the diagnostic sample were also analyzed. DNA was digested with the restriction enzymes indicated and probed with a JH probe. Arrows point to the rearranged bands; G corresponds to the germline bands. KB epidermoid carcinoma line was used as a control.
MLL gene rearrangements are retained in cultured leukemic lymphoblasts. DNA was extracted from cells of case no. 1 before culture and from a clone initiated by a single leukemic cell after 3 months of culture. DNA was digested with the restriction enzymes indicated and probed with an MLL cDNA probe. Arrows indicate the rearranged bands; G corresponds to the germline bands. The KB epidermoid carcinoma line, which has normal MLL alleles, was used as a control.
MLL gene rearrangements are retained in cultured leukemic lymphoblasts. DNA was extracted from cells of case no. 1 before culture and from a clone initiated by a single leukemic cell after 3 months of culture. DNA was digested with the restriction enzymes indicated and probed with an MLL cDNA probe. Arrows indicate the rearranged bands; G corresponds to the germline bands. The KB epidermoid carcinoma line, which has normal MLL alleles, was used as a control.
Stroma-dependency and self-renewal of ALL blasts growing in vitro.To determine the stroma dependency of leukemic cells derived from single progenitors after prolonged in vitro culture (ie, 3 to 5 months), we replated such progeny (representing cases no. 1, 3, and 4) in the absence of stroma. Most cells from all three cases died within 7 days showing apoptotic changes, such as typical morphologic and light scattering deterioration, as well as binding to Annexin-V and staining with propidium iodide (Fig 7).
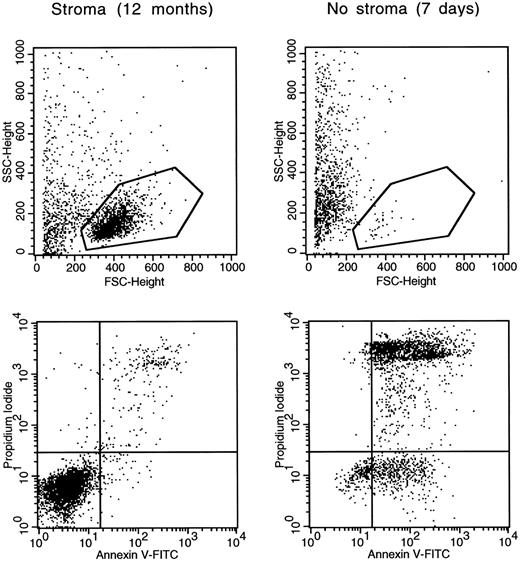
Leukemic lymphoblasts generated by a single leukemic cell in vitro undergo apoptosis when separated from stroma. Cells from case no. 3 cultured for 12 months on stroma were studied before and after detachment from stroma for 7 days. Light scattering dot plots (top) illustrate measurements of forward light scattering (FSC; X axis), which indicates cell size, and side light scattering (SSC; Y axis), which indicates cell granularity. The gates shown encompass morphologically undamaged lymphoblasts (72% of all cells in the stroma-supported culture; 5% after detachment from stroma). Double color dot plots (bottom) illustrate binding of Annexin V (X axis), a marker of apoptosis, and staining with propidium iodide (Y axis), an indicator of cell membrane permeability, of cells within the gate. Viable cells are confined to the bottom left quadrants.
Leukemic lymphoblasts generated by a single leukemic cell in vitro undergo apoptosis when separated from stroma. Cells from case no. 3 cultured for 12 months on stroma were studied before and after detachment from stroma for 7 days. Light scattering dot plots (top) illustrate measurements of forward light scattering (FSC; X axis), which indicates cell size, and side light scattering (SSC; Y axis), which indicates cell granularity. The gates shown encompass morphologically undamaged lymphoblasts (72% of all cells in the stroma-supported culture; 5% after detachment from stroma). Double color dot plots (bottom) illustrate binding of Annexin V (X axis), a marker of apoptosis, and staining with propidium iodide (Y axis), an indicator of cell membrane permeability, of cells within the gate. Viable cells are confined to the bottom left quadrants.
A second issue was whether the progeny of singly seeded leukemic cells retained self-renewal capacity. Thus, we sorted single cells retrieved from single clones in cases no. 1 and 4 after 3 months of culture and replated them on stromal layers. After 1 month of culture, 5% and 3% of the wells, respectively, showed leukemic cell growth. When cultures were extended to 3 months, the percentage of positive wells representing case no. 4 increased approximately threefold (to 10%). Thus, even in long-term cultures, a substantial proportion of blast cell progeny have self-renewal potential.
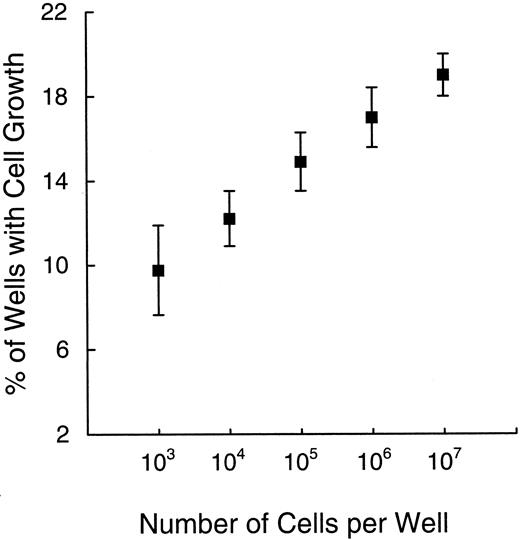
Kinetics of leukemic cell growth and influence of paracrine stimuli.To define the kinetics of leukemic cell growth in vitro, we estimated the number of cells in cultures that had been seeded with single or multiple lymphoblasts. Among clones growing from a single implant, the cell numbers ranged from 103 to 106 after 1 month of culture (data not shown), as estimated by visually comparing the cultures with leukemic cells placed in log increments in identical microtiter plates. Using these values, we calculated the minimum doubling times for five clinical samples: 1.5 days, cases no. 1 and 5; 2.3 days, case no. 4; and 3 days, cases no. 2 and 3. In most instances, the number of cells per clone increased progressively as culture times were extended. Notably, the propensity to grow in vitro was reflected not only by the number of positive wells, but also by the cell numbers in each well. These two parameters were strikingly correlated (Fig 8), ie, the higher the percentage of positive wells, the higher the number of cells in each well. The observation that after the same time in culture different clones were composed by variable numbers of cells and the emergence of detectable leukemic clones at different times during culture suggest that some leukemic stem cells persist in a quiescent state before entering a proliferative phase and/or traverse through the active phases of the cell cycle at a slower rate.
The percentage of wells with leukemic cell growth (Y axis) correlates with the number of cells per well (X axis). Values obtained after 2 month of culture in five experiments are illustrated. Symbols and bars indicate means ± standard deviation.
The percentage of wells with leukemic cell growth (Y axis) correlates with the number of cells per well (X axis). Values obtained after 2 month of culture in five experiments are illustrated. Symbols and bars indicate means ± standard deviation.
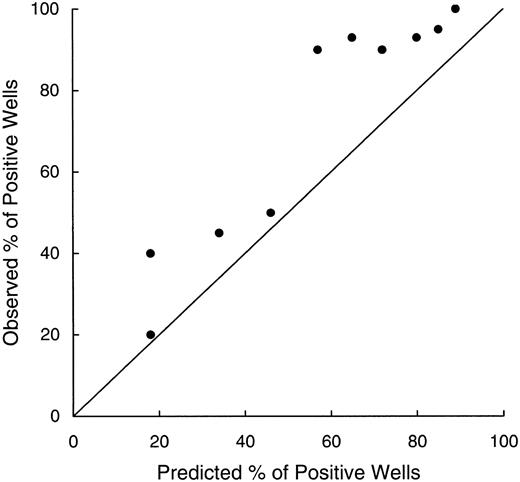
It is often observed that cell density influences the growth of hematopoietic cells in culture in that high densities tend to be associated with more vigorous growth. This relationship might be attributed to the production of paracrine factors that stimulate contiguous cells.15 34 We, therefore, tested whether the growth of leukemic lymphoblasts in our assay might be enhanced by such factors. Using cumulative binomial probabilities, based on the percentage of positive cultures when wells were seeded with one lymphoblast, we predicted the frequencies of leukemic cell growth for a hypothetical seeding of 10 cells per well. The results were then compared with the observed experimental values (Fig 9). Although showing a good correlation with the predicted values (r = .91), the observed frequencies were consistently higher (P < .003 by paired t-test), suggesting a growth advantage in wells with greater cell densities. Hence, ALL cell growth may be stimulated by factors produced by the leukemic cells themselves.
Relationship between observed percentages of positive wells seeded with 10 cells after 1 and 2 months of culture (Y axis) and percentages predicted from results of culture initiated by individual cells (X axis). Values were correlated (r = .91), but the percentages observed always exceed those predicted, suggesting growth stimulation from factors secreted by the leukemic cells themselves. The line of identity is indicated.
Relationship between observed percentages of positive wells seeded with 10 cells after 1 and 2 months of culture (Y axis) and percentages predicted from results of culture initiated by individual cells (X axis). Values were correlated (r = .91), but the percentages observed always exceed those predicted, suggesting growth stimulation from factors secreted by the leukemic cells themselves. The line of identity is indicated.
DISCUSSION
Attempts to study the clonogenicity of leukemic cells have been severely hampered by the inability to prevent apoptotic death. We have observed that apoptosis can be suppressed in a substantial proportion of B-lineage ALL cases by placing leukemic lymphoblasts on bone marrow-derived stromal layers.18,21,22 We show here that bone marrow-derived stromal cells not only provide indispensable survival signals to leukemic lymphoblasts, but they also support their proliferation. Although it is known that direct contact between lymphoblasts and stroma layers is essential,21 the stromal cell factors responsible for the survival and proliferation of immature B cells are still unknown. Stroma-derived cytokines such as IL-7 and SCF do provide growth stimulatory effects on normal B lymphoblasts and a subset of ALL samples,29-32 but, in our experiments, neutralizing antibodies to IL-7 and SCF affected neither the survival nor the proliferation of leukemic cells when added to stroma-supported cultures. Thus, ALL cell growth did not depend on these factors.
Most previous attempts to evaluate the clonogenicity of ALL cells have compared the number of colonies formed after 1 week of culture in semisolid medium with the number of leukemic cells plated. In two thirds of 300 ALL samples examined by this approach, the estimated percentages of clonogenic leukemic cells ranged from 0.001% to 2.76% of the number of cells plated; in the remaining third, no colonies were generated.1-15 The overall median value for those 15 studies was 0.28%, with all medians not exceeding 0.65%. From these results, one might conclude that virtually all leukemic blasts have lost their self-renewal capacity.16 17 A corollary of this interpretation is that disease eradication depends on the elimination of exceptionally rare clonogenic cells, which may have biological features distinct from the bulk of leukemic cells.
Our results indicate a relatively large proportion of leukemic cells with self-renewal capacity in cases of B-lineage ALL. Percentages of leukemic stem cells ranged from 6% to 20%, with a median of 15%, approximately 50-fold greater than the corresponding value for short-term cultures. The conservative criterion for leukemic cell growth in this study, 1,000-cell colonies as opposed to a 20-cell minimum in most other colony assays, makes this difference even more striking. Because the majority of samples analyzed for specific growth features were from high-risk cases (eg, unfavorable chromosomal abnormalities, high white blood cell counts, or both),35 with poor response to chemotherapy (3 of the 4 cases studied at diagnosis subsequently relapsed), one could argue that our findings apply only to ALL with unfavorable prognosis. Clearly this issue requires further investigation. In the meantime, we would stress that previous reports of plating efficiency also included high-risk cases.
The marked discrepancy between our results and previous findings can be explained in at least two ways. First, and perhaps most important, the marrow-derived stromal cells used to coat the surfaces of culture wells likely provided essential survival factors not available in typical colony assays. Second, we delayed assessment of cultures for as long as 5 months, in contrast to periods of 1 to 2 weeks in conventional tests, which may have allowed quiescent stem cells to enter the cell cycle. This latter consideration is important because of ALL recurrences that do not become clinically apparent until long after cessation of treatment.19,20 Such relapses could arise from leukemic stem cells in which proliferative activity is forestalled by extended periods of quiescence. Indeed, despite their derivation from comparable numbers of stem cells, many of the leukemic clones showed different doubling times, supporting the concept of delayed stem cell activity. Although this heterogeneity could reflect differences in stroma among individual culture wells, we consider this unlikely, because all cultures in each experiment were prepared at the same time and appeared to contain morphologically identical cells. In previous experiments, different stroma preparations, even from different donors, have shown a remarkably similar ability to support ALL cells.22 23 Thus, the cells used to seed culture wells appear to have been in different phases of the cell cycle, with some remaining dormant for several days before entering a proliferative state.
In the cases described here, 100 leukemic cells invariably produced colonies of 103 cells or more, suggesting that equivalent numbers of residual blasts could cause leukemic relapse in patients. This finding has clear implications for the development of new systemic therapies, as well as the extent to which autologous bone marrow must be purged before it is returned to the patient.
ACKNOWLEDGMENT
We thank Dr Motoyasu Hirao for technical advice on EBV detection and John Gilbert for editorial assistance.
Supported by Grants No. RO1-CA58297, UO1-CA60419, P30-CA21765 (CORE), and CA20180 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Dario Campana, MD, PhD, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105.

![Fig. 1. Bone marrow-derived stromal layers support the in vitro growth of leukemic B lymphoblasts. Inverted microscopy photographs ([A] 4× objective; [B] 20× objective) show leukemic lymphoblasts derived from one single progenitor cell after 2 months of culture on stroma (case no. 4). Leukemic cells are viable and grow in close contact with stromal elements.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3735/3/m_bl_0031f1.jpeg?Expires=1765935144&Signature=IxstTIGyli4BXiqCLw4vHgpG3hHi3hTxytowcfkdJpinDTV7gQG7EZtODn3KOA~2P21oD2WLXYKAPIWm1zZHC6f0jCzyuXDQd7CiPdKpDdYQpLZHzjI66KesiAhOYXz-e9IAkwyeFHdo6x-1BFAZxYKbSmeQqGvlRAI6CXxfmm9WRGwvBgabWQz7FBaUeC4jm4pU8DeSFDBwxzMRMdlEDmeo5uG24FvKhzMTYDPQhwD3kazTvqjvLpt2WLCH6cEB8wnJWSVxUYPaX6hrZpODli0HWzb--Dw~rcMwZ9j9lRtZqiQP8lUoo1fa0Yq~Po0R6k1yzz8iZmCO6~IF7TUTtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal