Abstract
Rapid recovery of CD4+ T cells after intensive chemotherapy is limited by an age-dependent decline in thymopoiesis. Here we sought to determine whether similar limitations exist for CD8+ T-cell regeneration. After intensive chemotherapy, CD8+ T cells had a faster effective doubling time than CD4+ T cells (median, 12.6 v 28.2 days, P < .05). Accordingly, at 3 months posttherapy, mean CD8+ T-cell number had returned to baseline, whereas mean CD4+ T-cell number was only 35% of pretherapy values (P < .05). These differences were primarily due to very rapid expansion of CD8+CD57+ and CD8+CD28− subsets. At 3 months posttherapy, there was no relationship between age and CD8+ T-cell number (R = −.02), whereas CD4+ T-cell number was inversely related to age (R = −.66) and there were no discernible differences in CD8+ recovery among patients with or without thymic enlargement, whereas CD4+ recovery was enhanced in patients with thymic enlargement after chemotherapy (P < .01). Therefore thymic-independent pathways of T-cell regeneration appear to rapidly regenerate substantial numbers of CD8+, but not CD4+ T cells, resulting in prolonged T-cell subset imbalance after T-cell depletion. These inherent distinctions between CD4+v CD8+ T-cell regeneration may have significant implications for immunotherapeutic strategies undertaken to eradicate minimal residual neoplastic disease after cytoreductive chemotherapy.
IMPAIRED T-CELL regeneration is a central obstacle to clinical progress for human immunodeficiency virus (HIV)-1 infection, post-bone marrow transplantation (BMT), and for developing immune-based therapies for residual neoplastic disease after intensive chemotherapy. Prevailing concepts regarding T-cell regeneration have emphasized the importance of the recapitulation of primary developmental pathways.1 The primary developmental pathway for αβ+ T cells begins with thymic homing of primitive bone marrow-derived progenitors; triple negative thymocytes then undergo T-cell receptor (TCR) gene rearrangement followed by expression of both CD4 and CD8 coreceptors.2 A minority of CD4+CD8+ thymocytes are positively selected resulting in high levels of CD3 expression and CD4 or CD8 lineage commitment via downregulation of the CD8 or CD4 coreceptor, respectively. Thus, CD4 and CD8+ T cells share a common primary developmental pathway until relatively late in thymopoiesis.
Using animal models, thymic-independent pathways of T-cell regeneration have also been described. Peripheral expansion of mature T-cell populations plays a central role in CD4+ and CD8+ T-cell regeneration in athymic hosts.3,4 In addition, extrathymic lymphopoiesis appears important for the generation of CD4−CD8−TCR+ cells and CD8+TCR+ cells, but similar pathways have not been identified for CD4+TCR+ cells. In peripheral lymphoid organs, CD8+TCR+ cells are found in aged nude mice5 and athymic radiation chimeras3, and evidence for the extrathymic generation of CD4−CD8−αβTCR+ cells from small numbers of hematopoietic pluripotential progenitor cells has recently been generated.6 Further, in situ generation of TCR+ cells in the gut,7 liver,8 and bone marrow9 has been reported, although such cells are not generally considered to recirculate. Therefore, potential thymic-independent pathways for regeneration of CD8+ T-cell populations include both peripheral expansion of mature CD8+ T cells and extrathymic lymphopoiesis from hematopoietic precursors.
In humans, there is evidence for an age-dependent decline in the capacity of the adult immune system to regenerate CD4+ T cells. After intensive chemotherapy and post-BMT, the rate of CD4+ T-cell regeneration is relatively rapid in children who regenerate CD4+ T cells bearing high molecular weight CD45 isoforms and who show thymic enlargement on completion of chemotherapy.10-12 In contrast, adults show prolonged CD4+ depletion after T-cell depleting chemotherapy,12 post-BMT,10,13 and after monoclonal antibody-mediated CD4+ cell depletion in patients with rheumatoid arthritis.14 Such results suggest that thymic-dependent pathways are required for rapid CD4+ regeneration in humans, but that such pathways are diminished with advancing age.
With regard to CD8+ T-cell regeneration in humans, little is known about the relative contribution of thymic-dependent versus thymic-independent pathways of regeneration. Several studies have reported differences in the rate of CD4+ versus CD8+ cells post-BMT.13,15,16 While such differences could be due to inherent biologic differences in CD4+ versus CD8+ regenerative pathways, unique clinical circumstances and methodologic limitations in the analyses also provide plausible explanations. For example, because CD8+ T-cell numbers are highest in patients with graft-versus-host disease (GVHD)17,18 and/or cytomegalovirus infection,19 the relatively rapid CD8+ regeneration observed may be due to high level antigenic stimulation and unique to the post-BMT setting. In addition, a lack of quantification of the inocula of mature T cells administered within the bone marrow graft have made interpretation of these data difficult. Further, the CD8+ subset is heterogeneous and comprised of both CD3+ and CD3− subpopulations. Many reports have shown recovery of only total CD4+ and CD8+ numbers post-BMT leaving open the possibility that the observed differences are due to rapid recovery of CD3−CD8+ subsets, which are part of the natural killer (NK) cell lineage and which follow different developmental pathways than CD3+CD8+ T cells.20 21 Hence, the degree to which inherent biologic differences in the CD4+ versus CD8+ T-cell regenerative pathways contribute to these observations remain unclear.
To study biological distinctions between CD4+ and CD8+ T-cell regenerative pathways, we have compared CD4+ and CD8+ T-cell regeneration after intensive chemotherapy. Using mathematical modeling, we provide estimates of CD4+ and CD8+ T-cell doubling times during a period of T-cell regeneration and show that there are significant quantitative differences between CD4+ and CD8+ T-cell regeneration. We also report that unlike CD4+ T-cell regeneration, CD8+ T-cell regeneration is not impaired by age related declines in thymopoiesis suggesting that thymic-independent pathways contribute substantially to CD8+ T-cell regeneration in humans. Such biologic distinctions between CD4+ and CD8+ T-cell regeneration result in a prolonged imbalance of the immune system after T-cell depletion, characterized by a relative excess of distinct CD8+ subsets and a prolonged deficiency of CD4+ cells.
MATERIALS AND METHODS
Patients and protocols.Sixteen patients with histologic evidence of malignancy were enrolled on one of the following protocols: NCI PB 90-C-211 for brain tumors, NCI PB 86-C-169 or 93-C-125 for sarcomas, NCI PB 89-C-41 or 93-C-207 for non-Hodgkin's lymphomas. In each protocol, the dosage of cyclophosphamide was substantial and ranged from 1.2 g/m2/cycle to 4.5 g/m2/cycle. A detailed description of the chemotherapy administered in NCI PB 90-C-211, 86-C-169, and 89-C-41 has been published previously.22 Patients treated on NCI PB 93-C-125 received 12 weekly doses of vincristine 2 mg/m2, and five sequential cycles of doxorubicin 90 mg/m2, and cyclophosphamide 2.4 g/m2. Patients treated on NCI PB 93-C-207 received three sequential cycles of cyclophosphamide 1.2 g/m2 and methotrexate 3.36 g/m2. In all protocols except 86-C-169, successive cycles ensued as soon as possible after hematologic recovery. For protocol 86-C-169, successive cycles began 21 days after the previous cycle if hematologic recovery had occurred. Three patients received radiation therapy as part of their treatment: patient no. 7, 6 Gy to the right arm; patient no. 8, 6.6 Gy to the pelvis; and patient no. 13, 3 Gy to the cranium and spine. No patient had detectable bone marrow involvement with tumor. All patients were rendered free of detectable neoplastic disease on completion of chemotherapy. Two patients (nos. 1 and 15, both 8 months after completion of therapy) developed recurrent disease during the first year after completion of therapy, and lymphocyte analysis was discontinued at the time of recurrence. The remainder of the patients remained free of disease for at least 1 year after completion of therapy. Patient no. 1 also had vertically acquired HIV infection. Before the development of lymphoma, his HIV disease manifestations were limited to eczema and thrombocytopenia. All protocols were approved by the Institutional Review Board of the National Cancer Institute and informed consent was obtained from all patients or their parents before protocol enrollment. CD4+ T-cell number at 6 months posttherapy from 15 of the patients reported here was published previously as part of a separate report.12
Flow cytometry.Peripheral blood specimens were obtained during routine clinic visits, which generally occurred every 2 to 3 months for the first year after completion of chemotherapy. Specimens were handled according to established clinical guidelines. Cells were stained for flow cytometry using the whole blood lysis technique and analyzed on a FACScan using Lysis II software (Becton Dickinson, San Jose, CA) as previously described.22 To calculate absolute numbers of each lymphocyte subset, the percentage of cells staining positive was multiplied by the absolute peripheral blood lymphocyte count. This was determined by a Coulter counter (Hialeah, FL) and leukocyte differential on a blood sample obtained simultaneously. A control sample obtained from normal volunteers was analyzed concurrently with each experimental sample.
The following monoclonal antibodies were used: anti-CD3 (Leu 4) -CD4 (Leu 3), -CD8(Leu 2), -CD28(Leu 28), -CD57 (Leu 7), -CD16(Leu 11), -CD56(Leu 19), -CD19(Leu 12), and -CD20(Leu 19) were obtained from Becton Dickinson (San Jose, CA). Anti-CD45RA(Alb11) was obtained from Gentrak (Plymouth Meeting, PA). Irrelevant murine monoclonal antibodies directly fluorochrome conjugated of the IgG1, IgG2a, and IgG2b subclass obtained from Becton Dickinson were used to define background staining.
Lymphocytes were identified by forward and side-scatter analyses and the lymphocyte gate was checked using the Leucogate (CD45/CD14) reagent from Becton Dickinson. List mode parameters were collected for 10,000 cells within the lymphocyte gate and positive staining was calculated based on the subclass control specimens. CD4+ T cells were defined as CD4+CD3+ cells, B cells as CD19+ cells, and NK cells as CD3− cells, which were CD16+ and/or CD56+. To minimize the possibility that CD8+ enumeration in these studies would include non-T cells, CD8+ expression was considered positive only when high levels of CD8 expression was present compared with background staining. Cells with low level CD8 expression were not considered CD8+ cells in these studies. In this report “CD8+ T cells” are defined as CD8+CD3+, while “CD8+ cells” expressed high levels of CD8 with or without concomitant CD3 expression.
Radiographic imaging.All patients, except patient no. 13, underwent radiographic imaging, which included the thymus as part of routine follow-up for their disease. Patients no. 4, 5, 7, 8, 10, 11, 12, and 16 underwent computed tomography (CT) scanning of the chest and patients no. 1, 2, 3, 5, 6, 9, 11, 14, 15, and 16 underwent gallium-67 scanning. Thymic imaging was analyzed serially from the time of initial presentation until 1 year after completion of therapy, except for patients no. 1 and 15 in whom analysis of thymic imaging was discontinued after tumor recurrence.
Analyses for radiographic evidence of thymic rebound were made by radiologists who were blinded with respect to the degree of T-lymphocyte recovery for individual patients at the time of analyses. Thymic volumes via CT were calculated as previously described.23 Thymic rebound was defined as a twofold or greater increase in thymic volume compared with thymic volume at presentation. For patients who underwent imaging with gallium-67, thymic rebound was defined as uptake of gallium-67 in the anterior mediastinum showing the characteristic size, shape, and location of thymic activity, which was not seen on previous studies.24 For those patients who underwent imaging using both modalities, analyses were made from each modality and the results were concordant in all cases. Because radiographic distinction of thymic rebound from recurrent tumor is difficult,25 radiographic studies were analyzed serially to ascertain resolution of the increased thymic size by CT scanning or increased gallium uptake by radionuclide scanning. Spontaneous resolution of thymic enlargement over time was regarded as sufficient evidence that the increased thymic size was benign in nature.
Mathematical analysis.Mathematical analyses used to calculate rate constants and effective cell doubling times were generated using the Scientist software program (Micromath, Salt Lake City, UT). Exponential patterns of cell number changes were fitted to the solution of the differential equation: dT/dt = gT(1-iT)(the logistic model), where T is the number of T cells, t = time, g = growth rate constant and i = inhibitory (regulatory) constant. The growth rate constant is related to the effective cell doubling time (td), td = ln2/g = 0.69/g. Although the true cell doubling time cannot be calculated without an accurate measurement of cell death rate, the effective cell doubling time as described here describes the net rate of cell number increase over time. The regulatory constant i is inversely proportional to the maximal limiting number of cells (Tm) allowed to grow under the homeostatic regulator, Tm = 1/i. The linear pattern of cell number change was fitted to the differential equation: dT/dt = s, where s is the source of cells. Upon integration, it yields a linear relationship between the number of cells and time: T = st + a, where a is the number of cells at time 0. The linear relationship can be obtained as limiting case of the exponential when gt < 1. In this case, the number of cells T = aexp(g*t) is approximately equal to ag*t + a, where g* can be considered as an effective growth constant for the linear type of cell number change: g* = s/a.
For statistical analyses, Spearman correlation coefficients, Wilcoxon Signed Rank test for paired comparisons, and Mann-Whitney U test for unpaired comparisons were calculated where described. All P values are two-sided.
RESULTS
Sixteen patients aged 1 to 24 years were treated with intensive chemotherapy for cancer. We calculated the percent recovery of individual lymphocyte subsets at multiple timepoints postchemotherapy. All lymphocyte subsets were depleted by chemotherapy, but there was rapid recovery of total CD8+, B-cell, and NK cell numbers. In contrast, CD4+ recovery remained incomplete up to 12 months posttherapy (Table 1). The relatively rapid recovery of CD8+ versus CD4+ cells was not due to significant numbers of CD3−CD8+ cells, as the mean percent recovery for CD3+CD8+ T cells was also significantly higher than for CD4+ T cells at 3, 6, and 9 months and approximated the rate of recovery for the total CD8+ population (Table 1).
Percent Recovery of Peripheral Blood Lymphocyte Subsets During and After Intensive Chemotherapy
| Timepoint . | CD4+ . | CD8+ . | CD8+CD3+ . | B Cells . | NK Cells . |
|---|---|---|---|---|---|
| Pretherapy* | 100 | 100 | 100 | 100 | 100 |
| Nadir† | 16 ± 3 | 27 ± 5‡ | 26 ± 6 | 3 ± 2‡ | 27 ± 25 |
| 3 mos | 35 ± 7 | 98 ± 24‡ | 113 ± 34‡ | 164 ± 43‡ | 86 ± 18‡ |
| 6 mos | 49 ± 9 | 89 ± 11‡ | 94 ± 15‡ | 197 ± 28‡ | 94 ± 28 |
| 9 mos | 48 ± 12 | 105 ± 23‡ | 109 ± 29‡ | 191 ± 41‡ | 148 ± 71 |
| 12 mos | 78 ± 19 | 85 ± 16 | 84 ± 16 | 124 ± 30 | 69 ± 15 |
| Timepoint . | CD4+ . | CD8+ . | CD8+CD3+ . | B Cells . | NK Cells . |
|---|---|---|---|---|---|
| Pretherapy* | 100 | 100 | 100 | 100 | 100 |
| Nadir† | 16 ± 3 | 27 ± 5‡ | 26 ± 6 | 3 ± 2‡ | 27 ± 25 |
| 3 mos | 35 ± 7 | 98 ± 24‡ | 113 ± 34‡ | 164 ± 43‡ | 86 ± 18‡ |
| 6 mos | 49 ± 9 | 89 ± 11‡ | 94 ± 15‡ | 197 ± 28‡ | 94 ± 28 |
| 9 mos | 48 ± 12 | 105 ± 23‡ | 109 ± 29‡ | 191 ± 41‡ | 148 ± 71 |
| 12 mos | 78 ± 19 | 85 ± 16 | 84 ± 16 | 124 ± 30 | 69 ± 15 |
Mean pretherapy values (CD4+: 807 ± 152, CD8+: 641 ± 139, CD8+CD3+: 520 ± 123, B: 215 ± 50, NK: 262 ± 56 cells/μL) are arbitrarily designated as 100%. Percent change was calculated for individual patients by dividing the values obtained at each timepoint specified by the pretherapy value × 100. Mean percent change and SEM for all patients studied is shown.
Nadir is defined as the timepoint when the lowest counts for each individual subset was recorded. This generally occurred at or near the completion of chemotherapy.
P < .05 when compared with percent change for CD4+ cells at the same timepoint using Wilcoxon Signed Rank Test.
The mean CD4/CD8 ratio decreased from 1.82 ± 0.23 pretherapy to 1.08 ± 0.20 immediately posttherapy. The higher baseline value for CD4+ T cells and the greater degree of chemotherapy-induced depletion for CD4+ versus CD8+ T cells could result in an earlier return to baseline for CD8+ T cells, even if the actual rate of regeneration for the two subsets were similar. To study whether the differences shown in Table 1 are related to differences in the rate of cellular regeneration, we used mathematical analyses of lymphocyte kinetics to generate quantitative measurements of the rate of CD4+ versus CD8+ T-cell regeneration. Because the most dramatic differences between CD4+ and CD8+ cells were seen in the initial 3 months posttherapy, after which time CD8+ cell number stabilized (Table 1), we focused on this time period.
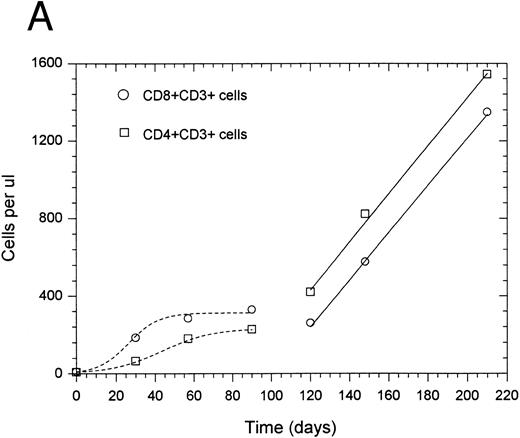
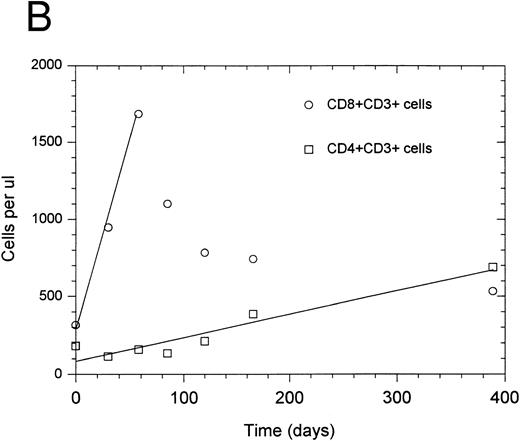
In general, the change in CD4+ and CD8+ T-cell number was best described by one of two types of equations: exponential with a regulator of growth (10 of 14 patients) or linear (four of 14 patients). For two patients, accurate rate constants for the initial 3 months posttherapy could not be determined due to insufficient data points. Data from a representative patient, shown in Fig 1A, illustrate the more rapid rate of CD8+ versus CD4+ T-cell recovery during the initial exponential phase of lymphocyte regeneration. Several patients who had an initial exponential pattern subsequently showed a linear pattern of regeneration during the period between 3 months and 1 year after completion of therapy as shown in Fig 1A. For those patients who showed an initial linear phase of T-cell recovery, there was a similarly increased rate of recovery for CD8+ versus CD4+ T cells during the initial phase of T-cell regeneration as shown from a representative patient in Fig 1B. The mean growth rate constant (Table 2) for CD8+ T cells (0.085 ± 0.036 day−1) was higher than that for CD4+ T cells (0.027 ± 0.007 day−1) (P = .007). This corresponds to a median effective doubling time of 12.6 and 28.2 days for CD8+ and CD4+ T cells, respectively (P = .016 using an arbitrary effective doubling time of 500 days for patient no. 14 whose measured effective doubling time was infinite). Thus, CD8+ T cells regenerate more rapidly than CD4+ T cells after chemotherapy-induced depletion.
Recovery kinetics of CD4+ and CD8+ T cells from individual patients after intensive chemotherapy. Cell numbers were measured in the peripheral blood at serial timepoints after completion of chemotherapy and fitted to either an exponential or linear growth equation. The lines represent predicted values calculated by either the regulated exponential growth model - - - - - or the linear growth model ——. (A) Patient no. 1; (B) patient no. 5.
Recovery kinetics of CD4+ and CD8+ T cells from individual patients after intensive chemotherapy. Cell numbers were measured in the peripheral blood at serial timepoints after completion of chemotherapy and fitted to either an exponential or linear growth equation. The lines represent predicted values calculated by either the regulated exponential growth model - - - - - or the linear growth model ——. (A) Patient no. 1; (B) patient no. 5.
Regeneration Rate Constants and Effective Doubling Times for CD4+ and CD8+ T Cells After Intensive Chemotherapy
| Patient No. . | Age . | CD4 g . | CD8 g . | CD4 Td† . | CD8 Td† . |
|---|---|---|---|---|---|
| . | (yrs) . | (day−1)* . | (day−1)* . | (days) . | (days) . |
| 1 | 1 | 0.077 | 0.130 | 9 | 5 |
| 2 | 3 | 0.035 | 0.038 | 20 | 18 |
| 3 | 7 | 0.055 | 0.072 | 12 | 10 |
| 4 | 8 | NA | NA | NA | NA |
| 5 | 11 | 0.008‡ | 0.075‡ | 83 | 9 |
| 6 | 13 | 0.084 | 0.070 | 8 | 10 |
| 7 | 13 | NA | NA | NA | NA |
| 8 | 14 | 0.026 | 0.050 | 28 | 14 |
| 9 | 18 | 0.021‡ | 0.091‡ | 33 | 8 |
| 10 | 19 | 0.035 | 0.037 | 20 | 19 |
| 11 | 19 | 0.003‡ | 0.003‡ | 230 | 230 |
| 12 | 21 | 0.010 | 0.010 | 69 | 69 |
| 13 | 23 | 0.030 | 0.060 | 23 | 11 |
| 14 | 24 | 0 | 0.010 | ∞ | 69 |
| 15 | 24 | 0.024‡ | 0.530‡ | 29 | 1 |
| 16 | 24 | 0.003 | 0.011 | 230 | 63 |
| Patient No. . | Age . | CD4 g . | CD8 g . | CD4 Td† . | CD8 Td† . |
|---|---|---|---|---|---|
| . | (yrs) . | (day−1)* . | (day−1)* . | (days) . | (days) . |
| 1 | 1 | 0.077 | 0.130 | 9 | 5 |
| 2 | 3 | 0.035 | 0.038 | 20 | 18 |
| 3 | 7 | 0.055 | 0.072 | 12 | 10 |
| 4 | 8 | NA | NA | NA | NA |
| 5 | 11 | 0.008‡ | 0.075‡ | 83 | 9 |
| 6 | 13 | 0.084 | 0.070 | 8 | 10 |
| 7 | 13 | NA | NA | NA | NA |
| 8 | 14 | 0.026 | 0.050 | 28 | 14 |
| 9 | 18 | 0.021‡ | 0.091‡ | 33 | 8 |
| 10 | 19 | 0.035 | 0.037 | 20 | 19 |
| 11 | 19 | 0.003‡ | 0.003‡ | 230 | 230 |
| 12 | 21 | 0.010 | 0.010 | 69 | 69 |
| 13 | 23 | 0.030 | 0.060 | 23 | 11 |
| 14 | 24 | 0 | 0.010 | ∞ | 69 |
| 15 | 24 | 0.024‡ | 0.530‡ | 29 | 1 |
| 16 | 24 | 0.003 | 0.011 | 230 | 63 |
The growth rate constant (g) was calculated from the equation dT/dt = gT(1-iT) where T is the number of T cells, t = time, g = growth rate constant and i = inhibitory (regulatory) constant.
Effective cell doubling time was calculated by 0.69/g or 0.69/g* as described in Materials and Methods.
The linear growth rate constant (g*) was calculated by dividing the slopes of the linear time dependences by the initial number of cells (g* = s/a) as described in Materials and Methods.
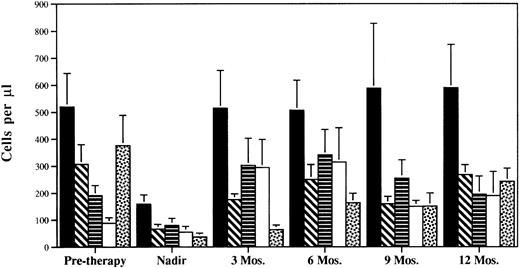
Analysis of individual CD8+ subsets showed significant heterogeneity in the pattern of recovery after chemotherapy. CD8+CD57+ and CD8+CD28− cells increased rapidly to levels at 3 months posttherapy, which were higher than pretherapy levels (Fig 2). In contrast, CD8+CD28+ cells showed a moderate rate of recovery attaining near pretherapy levels approximately 6 months posttherapy. The CD8+CD45RA+ subset showed the slowest pattern with 17%, 43%, and 64% recovery at 3, 6, and 12 months after completion of chemotherapy, respectively. Indeed, the growth rate constants for CD28+CD8+ (0.056 ± 0.02) or CD45RA+CD8+ T cells (0.034 ± 0.01) were not significantly higher than the growth rate constants for CD4+ T cells (P > .01). Therefore, the more rapid recovery of CD8+ versus CD4+ T cells immediately following chemotherapy is primarily due to the very rapid recovery of the CD8+CD28− and CD8+CD57+ subsets.
CD8+ subset composition during and after intensive chemotherapy. CD8+ subsets were measured in the peripheral blood at serial timepoints using two and three color flow cytometry. Mean values ± standard error of mean (SEM) for all patients analyzed at each timepoint are shown. (▪) CD8+CD3+; (▧ CD8+CD28+; (▤) CD8+CD28−; (□) CD8+CD57+; (▧) CD8+CD45RA+.
CD8+ subset composition during and after intensive chemotherapy. CD8+ subsets were measured in the peripheral blood at serial timepoints using two and three color flow cytometry. Mean values ± standard error of mean (SEM) for all patients analyzed at each timepoint are shown. (▪) CD8+CD3+; (▧ CD8+CD28+; (▤) CD8+CD28−; (□) CD8+CD57+; (▧) CD8+CD45RA+.
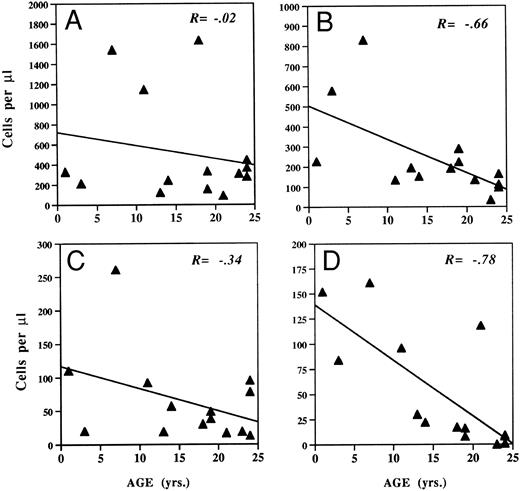
To study whether thymic-dependent pathways play a central role in CD8+ T-cell regeneration, the recovery of total CD8+ cells and CD8+ subsets was compared with age and the presence or absence of thymic rebound. There was no significant relationship between age and CD8+ T-cell number 3 months after completion of chemotherapy (R = −0.02, P = .93), whereas CD4+ T-cell number was inversely correlated with age at that time (R = −0.66, P = .017) (Fig 3). Similarly, there was no significant relationship between age and the CD8+ growth rate constants shown in Table 2 (R = −.37, P = .18), while there was an inverse correlation between age and CD4 growth rate constants (R = −.62, P = .02). Further, at 3 months posttherapy, the absolute number of CD8+CD45RA+ T cells did not correlate with age (R = −0.34, P = .21), while there was an inverse correlation between total CD4+CD45RA+ T-cell number and age (R = −0.78, P = .005). We also analyzed the relationship between age and the other CD8+ subsets quantified in this study (CD8+CD28+,CD8+CD28− and CD8+CD57+), and no significant correlations were found. Therefore, age-related changes result in deficient CD4+ T-cell regeneration after intensive chemotherapy, but deficiencies in CD8+ T-cell regeneration are not seen with advancing age.
Relationships between age and T-cell subsets after intensive chemotherapy. The absolute number of each lymphocyte subset was measured in the peripheral blood of patients approximately 3 months after completion of chemotherapy. Correlation coefficient was calculated by the Spearman rank correlation method. (A) CD8+CD3+ cells. (B) CD4+CD3+ T cells. (C) CD8+CD45RA+ cells. (D) CD4+CD45RA+ cells.
Relationships between age and T-cell subsets after intensive chemotherapy. The absolute number of each lymphocyte subset was measured in the peripheral blood of patients approximately 3 months after completion of chemotherapy. Correlation coefficient was calculated by the Spearman rank correlation method. (A) CD8+CD3+ cells. (B) CD4+CD3+ T cells. (C) CD8+CD45RA+ cells. (D) CD4+CD45RA+ cells.
Similarly, radiographic evidence of thymic rebound did not result in significant differences in the absolute number of CD8+ T cells or CD45RA+CD8+ T cells 3 months or 6 months after completion of chemotherapy (Table 3). In contrast, patients with radiographic evidence of thymic rebound had significantly higher numbers of CD45RA+CD4+ T cells 3 months and 6 months and higher total CD4+ T cells 6 months after completion of chemotherapy. Thus, while there is a substantial age-related decline in the rate of CD4+ T-cell regeneration that correlates with the lack of thymic enlargement after intensive chemotherapy, advancing age and absent thymic enlargement after intensive chemotherapy do not result in impaired regeneration of CD8+ T-cell number.
Lymphocyte Subsets After Completion of Chemotherapy in Patients With and Without Thymic Rebound
| Subset . | Time . | Thymic Rebound3-150 . | ||
|---|---|---|---|---|
| . | Posttherapy . | + . | − . | P Value . |
| CD8+CD3+ | 3 mos | 594 ± 244 | 476 ± 197 | .78 |
| CD8+CD3+ | 6 mos | 695 ± 197 | 331 ± 184 | .20 |
| CD8+CD45RA+ | 3 mos | 93 ± 37 | 46 ± 12 | .32 |
| CD8+CD45RA+ | 6 mos | 217 ± 52 | 116 ± 43 | .25 |
| CD4+CD3+ | 3 mos | 343 ± 119 | 182 ± 25 | .47 |
| CD4+CD3+ | 6 mos | 507 ± 77 | 203 ± 393-151 | .006 |
| CD4+CD45RA+ | 3 mos | 106 ± 21 | 13 ± 43-151 | .004 |
| CD4+CD45RA+ | 6 mos | 320 ± 123 | 23 ± 93-151 | .001 |
| Subset . | Time . | Thymic Rebound3-150 . | ||
|---|---|---|---|---|
| . | Posttherapy . | + . | − . | P Value . |
| CD8+CD3+ | 3 mos | 594 ± 244 | 476 ± 197 | .78 |
| CD8+CD3+ | 6 mos | 695 ± 197 | 331 ± 184 | .20 |
| CD8+CD45RA+ | 3 mos | 93 ± 37 | 46 ± 12 | .32 |
| CD8+CD45RA+ | 6 mos | 217 ± 52 | 116 ± 43 | .25 |
| CD4+CD3+ | 3 mos | 343 ± 119 | 182 ± 25 | .47 |
| CD4+CD3+ | 6 mos | 507 ± 77 | 203 ± 393-151 | .006 |
| CD4+CD45RA+ | 3 mos | 106 ± 21 | 13 ± 43-151 | .004 |
| CD4+CD45RA+ | 6 mos | 320 ± 123 | 23 ± 93-151 | .001 |
Thymic rebound was determined based on either a twofold increase in thymic volume during the first year after chemotherapy compared with thymic volume pretherapy as measured by CT scanning or thymic uptake by gallium67 scanning as delineated in Materials and Methods.
P value using Mann-Whitney U test.
DISCUSSION
The data presented here show that among lymphocytes, CD4+ T cells are unique in their propensity for prolonged deficiencies after depletion in vivo. Intensive chemotherapy significantly depleted all lymphocyte subsets, but only CD4+ T cells remained depleted 6 months after completion of therapy. Because there are major differences in the developmental pathways for B cells, NK cells, and T cells, differential rates of recovery are not unexpected. However, most peripheral CD4+ and CD8+ T cells are thought to share a common pathway until relatively late in development raising the question of why there are significant differences in the rate of recovery of CD4+ versus CD8+ T-cell subsets.
One possibility is that CD4+ and CD8+ T-cell regeneration both occur essentially exclusively via thymic-dependent pathways, but in situations of suboptimal thymic regenerative capacity, the ability to generate CD8+ T cells is maintained, while CD4+ T-cell regeneration is impaired. This could explain the absence of significant relationships between age, postchemotherapy thymic enlargement, and CD8+ T-cell regeneration, while such relationships are obvious for CD4+ regeneration. However, none of the hypotheses, which have been generated to explain the process of lineage commitment within the thymus, predict this to occur. In the instructional model, TCR specificity ultimately determines commitment to the CD4 or CD8 lineage26; as TCR rearrangement occurs randomly, TCR specificities with affinity for major histocompatability complex (MHC) class I versus MHC class II should be equally represented in states of suboptimal thymic regenerative capacity. In the stochastic model, CD4 versus CD8 commitment occurs stochastically,27 and in the asymmetric model, CD4 commitment is the default pathway and CD8 commitment requires specific TCR/MHC/Class I interactions28; because CD8 commitment is a more complex process, it would be predicted to be more susceptible to impairment than CD4 lineage commitment. Further, in mice undergoing BMT, CD4+ and CD8+ T-cell regeneration is equally reduced in aged compared with young hosts.29 Therefore, existing evidence suggests that suboptimal thymic regenerative capacity would not preferentially effect CD4+ populations.
Several lines of evidence suggest that thymic-independent pathways of CD8+ T-cell regeneration are responsible for the differences observed. This could potentially involve extrathymic generation of CD8+ cells from hematopoietic precursors and/or peripheral expansion of mature CD8+ T cells, which remain in the secondary lymphoid tissues after completion of chemotherapy. In this report, CD8+ T-cell populations were predominantly comprised of CD8+CD28− cells for up to 9 months after completion of chemotherapy. Similar expansions of CD8+CD28− cells have been observed in several clinical settings associated with impaired thymic function: HIV infection,30 post-BMT,31,32 GVHD,33 and aged hosts.34 In mice, extrathymically-derived intraepithelial CD8+ T cells lack CD28 expression,35 and CD8+CD28− T cells present in the peripheral blood of normal humans express high levels of CD8α and relatively low levels of CD8β reminiscent of the phenotype of extrathymically derived CD8αα cells.36 Hence, the predominance of CD8+CD28− cells suggests that extrathymic lymphopoiesis may be involved. In addition, while CD28 expression is not lost as a result of cellular proliferation,36 previous reports have shown that CD8+CD28− cells have shorter telomeres than CD8+CD28+ cells,37,38 suggesting that the peripheral expansion of the CD8+CD28− subset may be substantial. Similarly, restricted T-cell repertoire diversity has been described for CD8+CD28− and CD8+CD57+ subsets suggesting that such populations could be derived from expansions of a limited number of mature cells.39 40 Taken together, the data suggest that the differences observed between CD4+ and CD8+ T-cell regenerative rates are due to the existence of thymic-independent pathways that efficiently regenerate distinct CD8+ subsets, but which are not available for CD4+ regeneration. The relative contribution of extrathymic lymphopoiesis, peripheral expansion of mature CD8+CD28−, and CD8+CD57+ cells, or a combination of these pathways to this process is not known.
The data, as presented here, do not rule out that thymic-dependent pathways are important for regeneration of certain CD8+ subsets. We have previously reported that recovery of CD8+CD28+ T cells paralleled increases in CD4+ T-cell populations in rhesus monkeys after autologous BMT.32 Similarly, a progressive decline in “naive” CD8+ cells parallels the loss of CD4+ populations in HIV infection.41,42 The relatively slow recovery of the CD8+CD28+ and CD8+CD45RA+ subsets in this report (which tended to parallel the recovery of CD4+ T cells) suggest that regeneration of at least some CD8 subsets may require thymic-dependent pathways. It remains a possibility that very careful delineation of “naive” CD8+ T-cell populations using flow cytometric analysis of multiple cell surface markers concurrently (eg, L-selectin, CD44, CD45RA, CD11a) might uncover similar relationships between age, thymic enlargement, and recovery of particular CD8+ T-cell subsets as has been reported previously for “naive” CD4+ T cells.10-12
In this report, we have generated a quantitative estimate of the rate of CD4+ and CD8+ cell regeneration after intensive chemotherapy. Such data may prove useful for comparison with T-cell growth rates in other clinical settings. For example, these data can now be compared with published cell growth rates for CD4+ regeneration in patients with HIV-1 infection. In the adults studied here (all of whom were HIV negative), the mean CD4+ growth rate constant was 0.016 ± 0.007, which correlates with a mean effective cell doubling time of 43 days (range, 8.0 to ∞). Using similar methods, Ho et al43 calculated a mean CD4 growth rate constant of 0.047 ± 0.006 in a series of adults after antiretroviral therapy, which correlated with a reported mean CD4+ cell doubling time of 14.7 days (range, 1 to 173). Taken at face value, these data suggest that adults with HIV-1 infection have a greater CD4+ regenerative capacity than cancer patients after intensive chemotherapy and implicate high level CD4+ cell destruction rather than limitations on CD4+ regeneration as the primary factor involved in HIV-1–related CD4+ depletion. However, because the effective cell doubling time is a measure of the net result of cell death and cell regeneration, it is difficult to understand why patients with high levels of HIV-1–induced CD4+ destruction would actually show a more rapid CD4+ regenerative rate than cancer patients. Further, such differences in regenerative capacity would be surprising in light of the apparent importance of the thymus for CD4+ regeneration and the adverse effects of HIV-1 on thymic tissue.44
The differences could also be explained if the increases in CD4+ T-cell number reported in HIV-1 are confounded by transient changes in lymphocyte trafficking with or without true increases in total body CD4+ T-cell number as suggested by others.45-47 While changes in lymphocyte trafficking could also effect our observations, the changes described here were not transient, but occurred in a consistent pattern for each lymphocyte subset over a prolonged time period. Further, in some individuals, chemotherapy-induced lymphocyte depletion was associated with clinical evidence of immunodeficiency,3 12 which uniformly resolved posttherapy as increases in peripheral blood lymphocytes occurred. The significant interpatient variation in both reports, however, suggest that important variables, which substantially effect changes in peripheral blood CD4+ number exist, but are presently unaccounted for. Further work to generate quantitative estimates of the CD4+ and CD8+ regenerative rates in other clinical settings would be very helpful in determining “normal” T-cell regenerative rates.
The alterations in T-cell subsets described here are likely to have important functional consequences. CD8+CD28− and CD8+CD57+ subsets have impaired proliferation after CD3 crosslinking36 and appear to function primarily as negative regulatory populations.31,48,49 In addition, the restricted TCR repertoire diversity,39 40 which has been described for the CD8+CD28− and CD8+CD57+ subsets likely limits the effectiveness of these populations as effectors in vivo. The combination of altered CD8+ subset composition and prolonged depletion of CD4+ populations would be predicted to result in a prolonged impairment of immune function after intensive chemotherapy limiting the ability of the immune system to eradicate minimal residual neoplastic disease. In addition, the T-cell subset abnormalities described here will no doubt impede the efficacy of immune-based antineoplastic therapies (such as tumor vaccines) designed to eradicate minimal residual disease after intensive chemotherapy.
In summary, we have shown that biologic distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. CD8+ T cells show rapid regeneration without relationships to age or thymic enlargement, while rapid CD4+ T-cell regeneration is only observed in younger patients with thymic enlargement after completion of chemotherapy. These observations are best explained by the rapid recovery of CD8+CD28− and CD8+CD57+ subsets via thymic-independent pathways, which are not available for regeneration of CD4+ T cells.
ACKNOWLEDGMENT
The authors would like to thank Dr Jenni Punt for her careful review of the manuscript. The authors would also like to thank the nurses of the Star Team, 13 West and 13th floor Outpatient Clinic, Pediatric Branch, National Cancer Institute for their diligence in obtaining specimens for analysis.
Address reprint requests to Crystal L. Mackall, MD, Bldg 10, Rm 13N240, 10 Center Dr, MSC 1928, Bethesda MD 20892-1928.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal