Abstract
Acetylcholinesterase (AChE) is expressed in murine megakaryocytes (MK), where its antisense inhibition suppresses differentiation, yet was never detected in human MK. Here, we report that AChE is produced in normal human bone marrow MK and in cell lines derived thereof. Reverse transcriptase-polymerase chain reaction (RT-PCR) amplification showed two ACHEmRNA forms in human megakaryoblastic DAMI cells. In situ hybridization demonstrated ACHEmRNA surrounding the nucleus of small DAMI cells and the nuclear lobes of large, polyploid cells. Differentiation induction with phorbol ester and exposure to recombinant human thrombopoietin suppressed both ACHEmRNA and AChE activity. The residual AChE in mature differentiated cells acquired higher stability and detergent-sensitivity as compared with AChE in small proliferating cells. AChE activity was primarily associated with nuclei of both DAMI cells and small (10 μm) primary proliferating human bone marrow MK identified with GPIIb/IIIa antibodies. This activity was significantly reduced in medium size MK (10 to 25 μm) and was almost undetectable in large MK (<25 μm), yet was twofold more abundant in some large MK from idiopathic thrombocytopenia purpura (ITP) patients with accelerated MK maturation. The loss of AChE activity at the transition from proliferating to differentiating MK highlights species-specific differences in its expression, suggesting a distinct role for AChE in human MK development.
THE ACETYLCHOLINE hydrolyzing enzyme, acetylcholinesterase (AChE, Acetylcholine acetylhydrolase, EC 3.1.1.7), is notably expressed in the nervous system where it is involved in terminating the neurotransmission signal.1,2 AChE is also expressed in tissues with no cholinergic innervation, such as nonneuronal tumors and hematopoietic cells.1,3 AChE was shown to affect morphogenic changes in chick embryo neurons4,5 and to induce differentiation when transfected into glioma cells6 and in transgenic Xenopus embryos.7 The ubiquitous AChE expression in hematopoietic systems from all known vertebrates,8 therefore, indicated that AChE may effect hematopoiesis, as well.9 In humans, prominent AChE activities are associated with the erythrocyte membranes,10 while mature megakaryocytes (MK) are not known to possess this enzyme. In contrast, rodents display sparse erythrocyte AChE activity accompanied by intense MK and platelet activity. An extreme example for this cell type discordance was displayed in cats, which have high levels of platelet AChE and no detectable erythrocyte AChE activity.11 Therefore, Zajicek12 postulated that in mammals this enzyme is distributed in an inverse order between erythrocytes and platelets. In view of recent reports,13 this suggests that changes in AChE expression levels occur during the commitment step leading from the common bipotential progenitor, which responds to both erythroid and megakaryocytic growth factors erythropoietin (EPO) and thrombopoietin (TPO) towards the MK and erythrocyte lineages. Early expression of AChE during megakaryocytopoiesis was further supported by findings of others. Jackson14 reported that the proportion of AChE-positive marrow cells increased following induction of severe thrombocytopenia in rats. This suggested that AChE may serve as a possible marker for early murine MK precurssors, a possibility executed by Paulus et al.15 Burstein and Harker16 subsequently demonstrated enhanced platelet production in mice injected in vivo with the AChE inhibitor physostigmine. In situ hybridization studies performed in our laboratory demonstrated an increase in ACHEmRNA during mouse MK maturation.17 In contrast to this pronounced expression of AChE in murine MK, its absence in human MK remained an enigma.
While reexamining the notion that human MKs are AChE-deficient, we considered several possibilities. First, we assumed that the level of AChE in human MK could have been too low to be detected by standard techniques or in fixed cells. Therefore, detection methods had to be modified and improved. Second, we wished to search for this enzyme in subcellular compartments where it had not been seen before. For this purpose we needed subcellular fractionation approaches. Our third and most important hypothesis was that AChE production is controlled differently in human and murine MK. For example, AChE would not be detected in normal bone marrow (BM) smears, which contain few immature MK, if its levels go down with human MK maturation. This last difficulty could be solved by the use of transformed cell lines of human MK origin, such as the DAMI megakaryoblastic cell line,18 or the established MK cell lines CHRF-288-1119 and MEGO1.20 To determine if any of these assumptions were correct, we developed several highly sensitive cellular, molecular, and biochemical techniques to search for human AChE expression and activity. We have found ACHE mRNA surrounding the nuclear lobes in DAMI megakaryoblastic cells and AChE activity in DAMI and normal BM MK, which was diminished in more differentiated MK. This dramatic decrease in AChE activity that occurs during MK maturation was attenuated in BM of idiopathic thrombocytopenic purpura (ITP), which shows enhanced megakaryocytopoiesis, suggesting that AChE may be involved in human MK maturation.
MATERIALS AND METHODS
Cell cultures.DAMI, CHRF-288-11, and MEGO1 cells were kindly supplied by Dr S. Greenberg via the ATCC (Rockville, MD), Dr S. Lieberman (Chicago, IL), and Dr K. Preissner (Bad Neuheim, Germany), respectively. Cells were grown in Iscove's Minimal Dulbecco's Medium (IMDM), supplemented with 15% heat-inactivated horse serum in a fully humidified atmosphere at 37°C in 5% CO2 . Phorbol ester (PMA; Sigma Chemical Corp, St Louis, MO) was used at a concentration of 10 ng/mL. Recombinant human TPO21 (Zymogenetics, Inc, Seattle, WA) obtained from Dr K. Kaushansky (University of Washington, Seattle, WA) was used at a concentration of 930 U/mL.
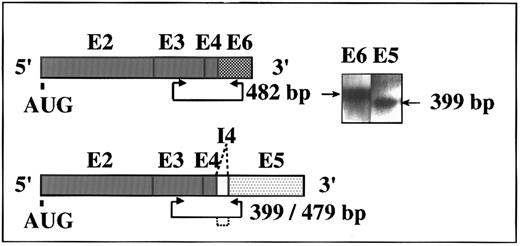
Two alternative ACHEmRNA forms in human DAMI cells. Putative transcripts and PCR primer positions are schematically shown for the human ACHE gene. Exons (E) are represented by boxes. I, intron. AUG, site of initiator methionine. Inset: RT-PCR amplification of DAMI cells RNA followed by DNA blot hybridization using exons E2-E6 DNA as a probe. Two of three possible alternative forms of ACHEmRNA were detected. Length of expected PCR fragments is marked by arrowed line and deleted intron by dotted lines. Note that the E5-specific primers yielded a 399-bp, but not a 479-bp fragment, indicating complete exclusion of the I4 intron from DAMI ACHEmRNA.
Two alternative ACHEmRNA forms in human DAMI cells. Putative transcripts and PCR primer positions are schematically shown for the human ACHE gene. Exons (E) are represented by boxes. I, intron. AUG, site of initiator methionine. Inset: RT-PCR amplification of DAMI cells RNA followed by DNA blot hybridization using exons E2-E6 DNA as a probe. Two of three possible alternative forms of ACHEmRNA were detected. Length of expected PCR fragments is marked by arrowed line and deleted intron by dotted lines. Note that the E5-specific primers yielded a 399-bp, but not a 479-bp fragment, indicating complete exclusion of the I4 intron from DAMI ACHEmRNA.
Bone marrow samples.Fresh BM smears and fresh BM mononuclear cells were obtained from normal allogenic transplantation donors after informed consent. Mononuclear cells were separated on Ficoll Hypaque (Pharmacia, Uppsala, Sweden; specific gravity, 1.077) and grown in suspension cultures at a concentration of 1 to 2 × 106 cells/mL in IMDM containing 10% normal serum of the AB blood group, 5% horse serum, 930 U/mL TPO as previously described.21 ITP BM smears were obtained from patients diagnosed at the Hematology Institute of the Tel-Aviv Medical Center.
Flow cytometry.Cells in suspension were centrifuged (1,000 rpm, 5 minutes) and pellets were resuspended in 100% ethanol for 1 hour of fixation at 4°C, spun, and again resuspended in 450 μL phosphate-buffered saline (PBS) containing RNase A (0.5 mg/mL; Sigma) for 30 minutes at 37°C. Propidium iodide was added at a final concentration of 0.05 ng/mL. Samples were analyzed by flow cytometry in a FACScan (Becton Dickinson, Oxnard, CA). The percentages of cells in the different ploidy classes were calculated for 5,000 to 10,000 cells per sample by setting markers at the nadirs between peaks. The ploidy distribution of the cells was compared with that of freshly prepared lymphocytes as a 2N standard. Cell cycle analysis was managed using the SFIT program (Becton Dickinson).
Reverse transcriptase-polymerase chain reaction (RT-PCR).RNA was extracted with RNAzol B (Cinna/Biotecx Lab, Inc, Houston, TX) from 7 × 106 DAMI cells with or without PMA, 12 and 24 hours posttreatment. RT-PCR analysis was performed as described elsewhere17 using selective primers for ACHEmRNA.3 To detect the “synaptic” ACHEmRNA form including exons E2-E3-E4-E6,7 we employed the 1522(+)/2003(−) pair (expected fragment size 482 bp). For the ACHEmRNA encoding the glycophospholipid-anchored AChE including E2-E3-E4-E5-E6, typically found in red blood cells,3 we used the 1522(+)/1917(−) pair. This reaction is also expected to detect the “readthrough” form including the I4 pseudointron, E2-E3-E4-I4-E5-E6 (with fragment sizes 399 and 478 bp, respectively). To detect the splicing factor SF2/ASF mRNA, we used the same RT-PCR procedure except that the annealing temperature in the PCR cycles was 55°C. The primer pair for the SF2/ASF gene was (+): TCGAGTCCGCGCTTTTCG and (−): GCTTCGAGGAAACTCCAC.22
AChE cytochemical staining.Cytochemical staining for AChE activity was performed on pooled adherent and nonadherent DAMI cells following trypsinization (Biological Industries, Beit Haemek, Israel) and air-drying on slides, as well as on fresh human bone marrow smears. Thiocholine complexes with copper and ferrous ions were created in situ essentially according to Karnovsky and Roots23 as modified recently7 except that we increased the sensitivity by omitting the fixation step and extending the staining time to 48 hours in a humid box. Quantitative image analysis of the brownish signal resulting from AChE staining was performed as previously described17 using the software package INSITARE (Applied Imaging, Dukesway, UK). Statistical evaluation using the Mann-Whitney U test considered the results to be significant where P < .002.
Homogenate preparations and catalytic activity measurements.Hydrolysis rates of acetylthiocholine iodide (ATCh) were determined in a multiwell assay as detailed elsewhere.24 Adherent and nonadherent cells were pooled and cell extracts prepared essentially as described25 in extraction buffer (50 mmol/L NaCl, 50 mmol/L Tris HCl, pH 7.4, 250 mmol/L sucrose, 5 mmol/L MgCl2 , 5 μg/mL leupeptin and 0.05 to 0.1 trypsin inhibitor units [TIU]/mL of aprotinin [Sigma]). Nuclei were separated by a 10-minute centrifugation of homogenized cells at 2,000g, 4°C, pelleted, and separated from the supernatant fraction by washing in extraction buffer and repeated centrifugation under the same conditions. 4′,6′-Diamidine-2-phenylindole-dihydrochloride (DAPI; Boehringer, Mannheim, Germany) staining was used to count the intact separated nuclei. To assay for AChE activities, the nuclear and supernatant fractions were further homogenized with extraction buffer including 0.1% Triton X-100. Total cell homogenates were extracted in high salt buffer including 1 mol/L NaCl, 1% Triton X-100, 10 mmol/L Tris HCl, pH 7.5, and 1 mmol/L EDTA. Homogenates were spun for 10 minutes at 100,000 rpm in a TL-100 rotor (Beckman, Palo Alto, CA) at 4°C. Catalytic activity was measured as described above.
In situ hybridization in suspended DAMI cells.DAMI cells in suspension were concentrated by repeated centrifugation at 4°C for 5 minutes at 1,000 rpm and resuspension cycles. Cells were fixed for 20 minutes in 4% paraformaldehyde in PBS, washed twice with PBT (PBS with 0.1% Tween-20), treated with 0.6% hydrogen peroxide in PBS for 2 minutes and washed in PBT. Prehybridization was performed for 1 hour at 65°C with hybridization buffer (50% formamide, 750 mmol/L sodium chloride, 75 mmol/L sodium citrate at pH 4.5, 50 μg/mL heparin [Sigma], and 50 μg/mL tRNA [Boehringer]). Hybridization was performed overnight in Eppendorf microtubes at 65°C with 1 μg/mL digoxigenin (DIG; Boehringer)-labeled in vitro transcribed human ACHEcRNA spanning exons E2-E3-E4-E6, trimmed by carbonate buffer to a final length of 500 bp. Cells were then resuspended in fresh hybridization buffer for 30 minutes at 65°C.
To minimize the loss of cells during posthybridization processing and to maximize the signal to noise ratios, the cells were injected into dialysis cassettes (Slide-A-Lyzer; Pierce, Rockford, IL) for the washing procedure. Loaded cassettes were submerged twice in solution 1 (50% formamide, 750 mmol/L sodium chloride, 75 mmol/L sodium citrate, pH 4.5) for 1 hour at 65°C; and once in solution 2 (10 mmol/L Tris-HCl, pH 7.5, 0.5 mol/L NaCl) for 1 hour at room temp. RNase A (1 mg/mL) was then injected directly into the dialysis cassettes for 1 hour of incubation at 37°C. The cassettes were further submerged three times in solution 2 for 1 hour and then twice in TBST (25 mmol/L Tris-HCl, pH 7.5, 136 mmol/L NaCl, 2.7 mmol/L KCl, 0.1% Tween-20 with 2 mmol/L Levamisole) for 1 hour, both at room temperature. To avoid nonspecific absorption, lamb serum (GIBCO BRL, Life Technologies, Paisley, UK) was injected to the dialysis cassettes at a final concentration of 1% in TBST for 3 hours at room temperature. Alkaline phosphatase (AP)-conjugated anti-DIG antibody (1 U; Boehringer) was then injected and left overnight in TBST. Cells were then transferred back to Eppendorf microtubes and washed by centrifugation-resuspension cycles in TBST (5 times for 10 minutes at room temperature). AP detection was performed using the fluorogenic ELF 6605 substrate (Molecular Probes, Eugene, OR) diluted 1:20 with 1:1,000 dilutants of each of the substrate additives I and II. The fluorogenic reaction was developed for 10 minutes and products observed at ×1,000 magnification under Zeiss Axioplan microscope covered with Vectra Shield (H-1000) antifade medium (Vector, Burlingame, CA) using ultraviolet (UV) excitation and emission at 480 nm.
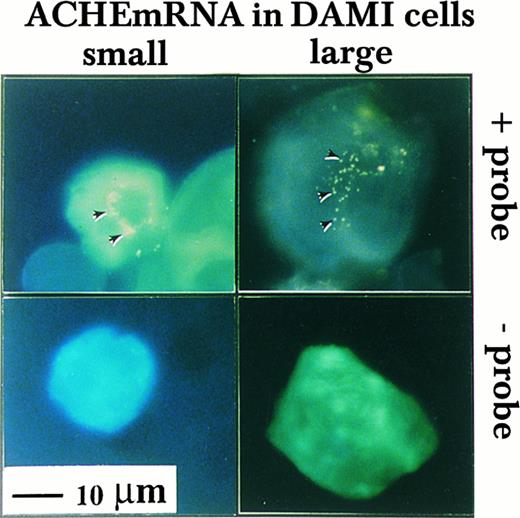
Nuclear association of ACHEmRNA in DAMI cells. High resolution in situ hybridization was performed on cells in suspension using a DIG-labeled cRNA probe. Note that the yellow fluorescent signal (marked by arrows in the top panel) appeared in the presence of probe around multiple lobes in both small (left-hand side) and large MK (right-hand side) and was less pronounced in the cytoplasm, particularly in large cells. The absence of signal in cells treated similarly, but with no probe in the hybridization solution (bottom panel), demonstrated that these fluorogenic signals reflect cellular mRNA.
Nuclear association of ACHEmRNA in DAMI cells. High resolution in situ hybridization was performed on cells in suspension using a DIG-labeled cRNA probe. Note that the yellow fluorescent signal (marked by arrows in the top panel) appeared in the presence of probe around multiple lobes in both small (left-hand side) and large MK (right-hand side) and was less pronounced in the cytoplasm, particularly in large cells. The absence of signal in cells treated similarly, but with no probe in the hybridization solution (bottom panel), demonstrated that these fluorogenic signals reflect cellular mRNA.
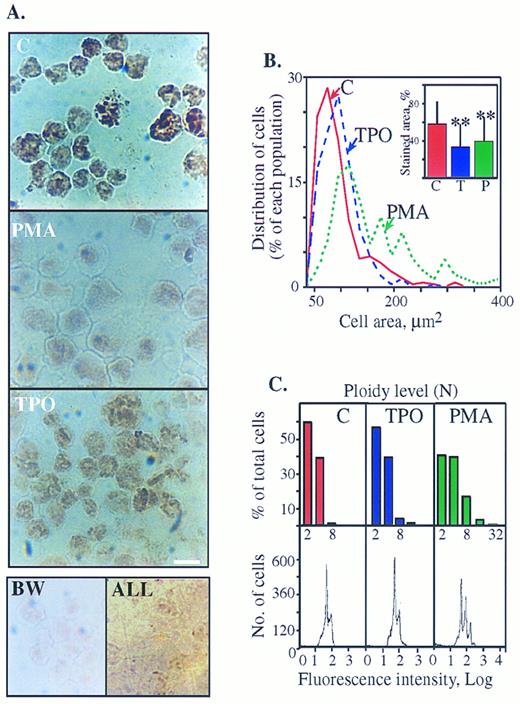
Cytochemical staining of AChE activity in human megakaryoblasts is suppressed with maturation. (A) AChE cytochemical staining is reduced in PMA- more than in TPO-treated cells. Top: DAMI cells treated for 3 days with or without PMA or TPO were stained for AChE activity. A nuclear-association pattern of the cytochemical staining in the presence of 1 × 10−4 mol/L of the BuChE inhibitor (IO) was observed in all cells with or without PMA and TPO. Note the prominent decrease in color intensity within PMA-treated cells as compared with control and the moderate decrease in TPO cultures. Bottom (left panel): Untreated DAMI cells were subjected to AChE staining in the presence of 1 × 10−5 mol/L of the AChE inhibitor BW284C57 (BW); (right panel): BM cells derived from ALL patients were subjected to AChE staining without specific inhibitors. Cells in both bottom panels are unstained, reflecting the specificity of both the staining procedure on DAMI megakaryoblasts and the lineage-specific expression of AChE in megakaryoblastic, but not lymphoid cells. Size bar = 10 μm. Shown are representative pictures of one experiment of five in each panel. (B) PMA treatment increases cell areas (an analysis of the cells shown in Fig 3A, top panels). Percent of each cell population out of total cells analyzed as classified by their area is presented. Cell areas were grouped at 20 μm2 intervals and each point represents the largest area measured within that interval. Average cell areas expressed as mean μm2 ± standard deviation (SD) were: control cells (n = 360) 79.7 ± 45.4; PMA-treated cells (n = 189) 153.56 ± 87.6 (significantly larger than control cells, P < .0005), and TPO-treated cells (n = 281) 86.9 ± 54.5 (P < .1). Inset: Decreased AChE stained areas in PMA- and TPO-treated cells. Intracellular areas stained above threshold level were assessed as fractions of total cell areas using the INSITARE software (Applied Imaging, Dukesway, UK). The relative stained area for each DAMI cells preparation is presented in percentages as mean ± SD for each of the noted cell populations. ** P < .0005 as compared with control cells. (C) Flow cytometry analysis of DAMI cells treated for 3 days with PMA or TPO or without treatment (C) was performed by propidium iodide staining. Signals reflecting nuclei in each subclass of cells are presented as numbers (bottom) or in percentages of total cells (top). The first major peak represents the fraction of cells with a normal 2N complement of DNA. Subsequent peaks represent the proportion of cells with ploidies of 4N, 8N, 16N, and greater levels of DNA as shown on a log scale of the DNA contents and detailed in the above columns. Note that the peak distribution corresponds to that of the measured cell areas in Fig 3B. The results represent one experiment of more than 10 performed.
Cytochemical staining of AChE activity in human megakaryoblasts is suppressed with maturation. (A) AChE cytochemical staining is reduced in PMA- more than in TPO-treated cells. Top: DAMI cells treated for 3 days with or without PMA or TPO were stained for AChE activity. A nuclear-association pattern of the cytochemical staining in the presence of 1 × 10−4 mol/L of the BuChE inhibitor (IO) was observed in all cells with or without PMA and TPO. Note the prominent decrease in color intensity within PMA-treated cells as compared with control and the moderate decrease in TPO cultures. Bottom (left panel): Untreated DAMI cells were subjected to AChE staining in the presence of 1 × 10−5 mol/L of the AChE inhibitor BW284C57 (BW); (right panel): BM cells derived from ALL patients were subjected to AChE staining without specific inhibitors. Cells in both bottom panels are unstained, reflecting the specificity of both the staining procedure on DAMI megakaryoblasts and the lineage-specific expression of AChE in megakaryoblastic, but not lymphoid cells. Size bar = 10 μm. Shown are representative pictures of one experiment of five in each panel. (B) PMA treatment increases cell areas (an analysis of the cells shown in Fig 3A, top panels). Percent of each cell population out of total cells analyzed as classified by their area is presented. Cell areas were grouped at 20 μm2 intervals and each point represents the largest area measured within that interval. Average cell areas expressed as mean μm2 ± standard deviation (SD) were: control cells (n = 360) 79.7 ± 45.4; PMA-treated cells (n = 189) 153.56 ± 87.6 (significantly larger than control cells, P < .0005), and TPO-treated cells (n = 281) 86.9 ± 54.5 (P < .1). Inset: Decreased AChE stained areas in PMA- and TPO-treated cells. Intracellular areas stained above threshold level were assessed as fractions of total cell areas using the INSITARE software (Applied Imaging, Dukesway, UK). The relative stained area for each DAMI cells preparation is presented in percentages as mean ± SD for each of the noted cell populations. ** P < .0005 as compared with control cells. (C) Flow cytometry analysis of DAMI cells treated for 3 days with PMA or TPO or without treatment (C) was performed by propidium iodide staining. Signals reflecting nuclei in each subclass of cells are presented as numbers (bottom) or in percentages of total cells (top). The first major peak represents the fraction of cells with a normal 2N complement of DNA. Subsequent peaks represent the proportion of cells with ploidies of 4N, 8N, 16N, and greater levels of DNA as shown on a log scale of the DNA contents and detailed in the above columns. Note that the peak distribution corresponds to that of the measured cell areas in Fig 3B. The results represent one experiment of more than 10 performed.
Coupled MK immunofluorescent — cytochemical staining.Staining was performed on freshly prepared bone marrow aspirates or following MK expansion ex vivo. To this end, mononuclear cells from normal human BM depleted of adherent cells were cultured ex vivo in IMDM containing 10% horse serum and 5% human AB serum, as well as TPO (930 U/mL) for 3 days. Cytochemical staining of active AChE was followed by 1 hour of immunoreactivity staining under parafilm, with the fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody CD41 (Pharmingen, San Diego, CA; 2 μg/mL in PBS), which specifically labels the GPIIb/IIIa complex. Fluorescent emission was observed under Zeiss Axioplan microscope at 525 nm.
RESULTS
DAMI cells express two alternative ACHEmRNAs.To determine whether the human ACHE gene is expressed in immature human MK, we applied a highly sensitive RT-PCR method using selective primers for each of the alternatively spliced forms of human ACHEmRNA.3 Two of the three potential fragments, 482 and 399 bp long, were detected with these two primer pairs in RNA preparations from the megakaryoblastic DAMI cells. These reflected production of the synaptic ACHEmRNA form terminated with exon E67 and the alternative ACHEmRNA species terminated with exon E5. This latter mRNA is potentially translated into the glycophospholipid-anchored form of the enzyme dominantly associated with the erythrocyte membrane (Fig 1). To further explore for the third readthrough form including pseudointron I4,6 we employed DNA blot hybridization with a plasmid DNA probe containing the E2-E6 exons and the pseudointron I4 region. This analysis confirmed that in DAMI cells, the ACHE gene is expressed and spliced into two 3′ alternative mRNA forms, which include either the E6 or the E5 exons, both excluding the I4 intron. The major detectable transcript was the ACHE E6 form, similar to the situation in tumor cells of hematopoietic origin.3 Semiquantitative follow-up of the kinetics of this RT-PCR amplification showed approximately 1 × 106 ACHEmRNA molecules per 200 ng total DAMI cells RNA (see calibration curve3 ). A yield of 10 to 15 μg total RNA was obtained from 1 × 106 cells, which implies 50 to 75 ACHEmRNA molecules for each DAMI cell.
Nuclear association of ACHEmRNA in DAMI cells.To investigate the subcellular distribution of DAMI cells ACHEmRNA transcripts, we developed a high resolution fluorescent in situ hybridization protocol (see Materials and Methods). This analysis showed approximately 50 to 100 fluorescent yellow dots per cell reflecting ACHEmRNA molecules. Hybridization signals depended on the presence of probe and were all concentrated in a small region, surrounding what appeared to be the nuclear lobes in both small and large cells (Fig 2). The nuclear-associated distribution of ACHEmRNA transcripts demonstrated similar depositions around different nuclear lobes, suggesting equal transcriptional activity for the ACHE gene and/or uniform mRNA stability around various nuclear lobes in both small and large MK. Similar patterns of nuclear-associated ACHEmRNA labeling were previously observed in mouse MK in vivo, albeit with a lower resolution level due to the radioactive probe employed in that study.17 In contrast, in spinal cord sections, ACHEmRNA chains are dispersed throughout the cell body of neurons,26 suggesting that the nuclear association pattern of these transcripts may be characteristic of MKs.
Catalytically active AChE is associated with MK nuclei.To test if AChE expression is common to human megakaryoblasts, we also employed the CHRF-288-1119 and MEGO120 cell lines. ATCh hydrolysis rates in total cell homogenates showed cholinesterase activity with prominent sensitivity to the selective AChE inhibitor 1,5-bis (4-allyldimethyl–ammonium phenyl) pentane-3–1 dibromide (BW284C51) at a concentration of 1 × 10−5 mol/L in all of these cell lines, as well as lower hydrolytic activity attributed to butyrylcholinesterase (BuChE), which was sensitive to tetraisopropyl-pyrophosphoramide (iso-OMPA) at a concentration of 1 × 10−4 mol/L, in all of these lines (Table 1). The subcellular distribution of catalytically active AChE was further examined in the DAMI cell line, which displayed the highest hydrolytic activity. Intact DAMI cell nuclei possessed 70% ± 5% of total cell AChE activity in three separate experiments (not shown). Subtle permeabilization of the nuclear membrane with 0.1% Triton X-100, did not release enzyme activity from these isolated nuclei. Assuming a turnover number of 1 × 104 molecules ATCh/second/AChE subunit,27 the measured specific activity of 7.8 × 10−7 nmol ATCh hydrolyzed/min/DAMI nucleus, implied approximately 130 AChE molecules associated with each nucleus.
AChE Activity in Human Megakaryoblastic Cell Lines
| Cell Line . | mOD/min . | mOD/min + BW . | mOD/min + IO . | nmol/min/cell . | nmol/min/cell . | Calc. No. of . |
|---|---|---|---|---|---|---|
| . | None . | . | . | BChE . | AChE . | Molecules/Cell . |
| DAMI | 3.630 | 0.607 | 3.589 | 0.67 × 10−7 | 5.05 × 10−7 | 84 |
| MEG01 | 1.529 | 0.300 | 0.925 | 0.22 × 10−7 | 1.14 × 10−7 | 19 |
| CHRF | 0.932 | 0.320 | 0.804 | 0.26 × 10−7 | 0.96 × 10−7 | 16 |
| Cell Line . | mOD/min . | mOD/min + BW . | mOD/min + IO . | nmol/min/cell . | nmol/min/cell . | Calc. No. of . |
|---|---|---|---|---|---|---|
| . | None . | . | . | BChE . | AChE . | Molecules/Cell . |
| DAMI | 3.630 | 0.607 | 3.589 | 0.67 × 10−7 | 5.05 × 10−7 | 84 |
| MEG01 | 1.529 | 0.300 | 0.925 | 0.22 × 10−7 | 1.14 × 10−7 | 19 |
| CHRF | 0.932 | 0.320 | 0.804 | 0.26 × 10−7 | 0.96 × 10−7 | 16 |
Cell extracts were prepared in high salt detergent buffer and AChE activities in total cell homogenates were determined as detailed in Materials and Methods. Raw data are presented as mOD/min of 1 mmol/L acetylthiocholine hydrolyzed24 in the presence of the AChE inhibitor BW284C51 (BW) at 1 × 10−5 mol/L, the BuChE inhibitor iso-OMPA (IO) at 1 × 10−4 mol/L, or without inhibitors (none). Linearity of the reaction24 was stable throughout detection (correlation coefficient > 0.99). Because of the low-leveled activity values, spontaneous substrate hydrolysis in extraction buffer (0.151 mOD/min) was subtracted and net activity divided by cell numbers.
AChE activity is nuclear associated.To further explore the association between ACHE gene expression in human megakaryoblasts and production of the catalytically active AChE protein, we subjected air-dried, nonfixed DAMI cells to cytochemical staining of AChE activity. To this end, we developed a modified method for AChE staining that included a prolonged incubation for 48 hours in a humid chamber. Under these conditions, clear enzyme activity was evident as a brown precipitate in DAMI cells. Most of the reaction product was microscopically detected in association with the cell nuclei, particularly in small cells (Fig 3A, upper panel). AChE activity was barely detectable in the cytoplasm, unlike the striking cytoplasmic stain found in mouse MK.28,29 Confirmation that this staining product indeed reflected AChE activity involved the use of the selective AChE inhibitor BW284C51 (BW), which completely abolished staining at a concentration of 1 × 10−5 mol/L (Fig 3A, lower panel). In contrast, approximately 90% of this staining remained unchanged in the presence of the selective inhibitor suppressing BuChE activity, iso-OMPA (IO) at a concentration of 1 × 10−4 mol/L (Fig 3A, upper panel). To find out if AChE expression is specific to the megakaryoblastic lineage, we employed the cytochemical staining method on lymphoid cells derived from acute lymphatic leukemia (ALL) BM. Our results show that lymphoid cells are AChE-deficient (Fig 3A, lower panel) and confirm previous observations demonstrating a lymphoid cell line that does not possess ACHEmRNA.3
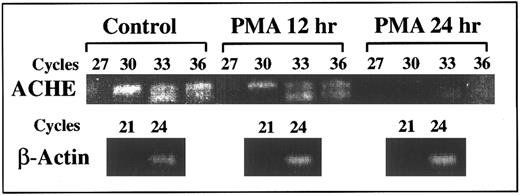
Suppression of E6-ACHEmRNA levels under PMA. Presented is a semiquantitative kinetic follow-up of RT-PCR amplification of cell cultures RNA. Later appearance of ACHEmRNA-derived products reflects lower levels (by approximately eightfold for a three cycle difference3 ). Continuous decrease in the level of E6 ACHEmRNA caused progressively later appearance of the corresponding PCR product of mRNA from cell cultures treated for 12 and 24 hours with PMA as compared with control cells. The appearance of actin mRNA products remained unchanged, excluding the possibility of total mRNA destruction. PCR cycle numbers at which samples were withdrawn are noted above.
Suppression of E6-ACHEmRNA levels under PMA. Presented is a semiquantitative kinetic follow-up of RT-PCR amplification of cell cultures RNA. Later appearance of ACHEmRNA-derived products reflects lower levels (by approximately eightfold for a three cycle difference3 ). Continuous decrease in the level of E6 ACHEmRNA caused progressively later appearance of the corresponding PCR product of mRNA from cell cultures treated for 12 and 24 hours with PMA as compared with control cells. The appearance of actin mRNA products remained unchanged, excluding the possibility of total mRNA destruction. PCR cycle numbers at which samples were withdrawn are noted above.
Megakaryoblastic AChE activity is suppressed with differentiation.In cultures that underwent differentiation induction by PMA,18 AChE staining efficiency around the nuclear domain was reduced (Fig 3A). Average stained area divided by total cell area as measured by computer-assisted image analysis was smaller than that of untreated cells (P < .0005, Fig 3B [inset]). Image analysis also demonstrated that the total cell areas increased under PMA treatment from 80 μm2 to 154 μm2 (P < .0005, Fig 3B). The measured ploidy levels of these cells as obtained by fluorescence-activated cell scanner (FACS) analysis showed discrete peaks that corresponded well to cell populations with the different size subclasses discerned by image analysis (compare Fig 3B with 3C). While the discrete peak of 2N-4N was the major one in control and TPO-treated cells, the proportion of cells with this ploidy was lowered in PMA-treated cells, which displayed 8N and 16N peaks (Fig 3C, bottom panels). The FACS analysis is schematically presented in the upper bar graph (Fig 3C, upper panels) for simplicity. PMA-treated cells underwent complete proliferation arrest, so that the total number of cells remained unchanged and the number of 2N nuclei was reduced from 60% in control to 40% under PMA due to the proportional increase in higher ploidy cells (Fig 3C). In contrast, TPO induced only limited suppression of AChE activity as observed by the intensity of the brownish color (Fig 3A). The relative proportion of 2N nuclei remained the same as in control cultures and the total number of cells increased (Fig 3C). Thus in these cultures, like in control cultures, most of the cells were small proliferating 2N-4N MK, the measured average size remained statistically unchanged, and only 5% of the population displayed incremented ploidy levels (>8N).
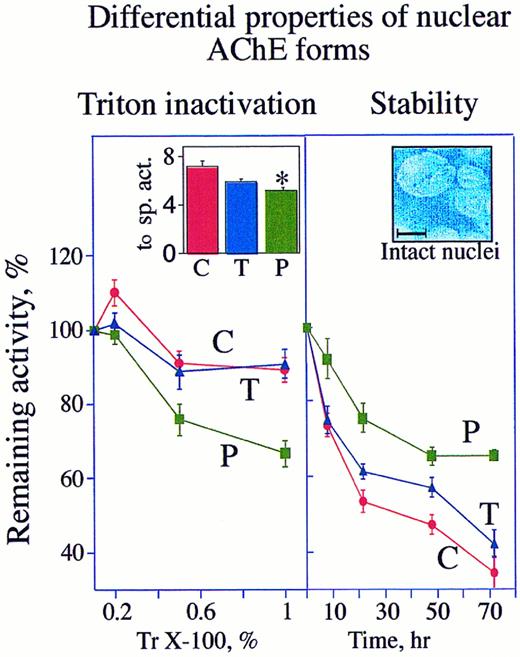
DAMI cells possess two nuclear-associated AChE forms that vary in their detergent-vulnerability and stability. Left: AChE activity in intact nuclei of PMA-treated cells is detergent sensitive. Cells were grown for 3 days in culture with or without (C) 10 ng/mL PMA (P) or 930 U/mL TPO (T). Intact nuclei separated from supernatant as detailed in Materials and Methods were homogenized with 0.2%, 0.5%, or 1% Triton (Tr) X-100. AChE activities in these homogenates are presented as average ± standard error in percentages of initial activity measured at 0 time in the presence of 0.1% Triton X-100 (left inset). The data represent the average of three experiments each performed in triplicate assays. Note that following PMA induction, AChE becomes more vulnerable to Triton X-100. Inset: The specific activities (sp. act.) calculated at 0 time in the presence of 0.1% Triton for control, PMA, and TPO cultures are presented as average ± SD hydrolyzed ATCh (10−7 nmol per minute per nucleus). Note that the PMA-treated cells lost a significant fraction (* P < .005) of their enzyme activity. Right: Nuclear-associated AChE in proliferating MK is unstable. Nuclear-associated AChE activities were measured in enzyme preparations from intact nuclei of cells treated 3 days in culture with or without PMA or TPO. The percentage of remaining AChE activities following the noted periods of incubation at 37°C in the presence of 0.1% Triton were calculated as compared with the initial activity at 0 time. The data represent the average of three experiments each performed in triplicate assays. Inset: High magnification of separated intact nuclei stained with DAPI. Size bar = 10 μm.
DAMI cells possess two nuclear-associated AChE forms that vary in their detergent-vulnerability and stability. Left: AChE activity in intact nuclei of PMA-treated cells is detergent sensitive. Cells were grown for 3 days in culture with or without (C) 10 ng/mL PMA (P) or 930 U/mL TPO (T). Intact nuclei separated from supernatant as detailed in Materials and Methods were homogenized with 0.2%, 0.5%, or 1% Triton (Tr) X-100. AChE activities in these homogenates are presented as average ± standard error in percentages of initial activity measured at 0 time in the presence of 0.1% Triton X-100 (left inset). The data represent the average of three experiments each performed in triplicate assays. Note that following PMA induction, AChE becomes more vulnerable to Triton X-100. Inset: The specific activities (sp. act.) calculated at 0 time in the presence of 0.1% Triton for control, PMA, and TPO cultures are presented as average ± SD hydrolyzed ATCh (10−7 nmol per minute per nucleus). Note that the PMA-treated cells lost a significant fraction (* P < .005) of their enzyme activity. Right: Nuclear-associated AChE in proliferating MK is unstable. Nuclear-associated AChE activities were measured in enzyme preparations from intact nuclei of cells treated 3 days in culture with or without PMA or TPO. The percentage of remaining AChE activities following the noted periods of incubation at 37°C in the presence of 0.1% Triton were calculated as compared with the initial activity at 0 time. The data represent the average of three experiments each performed in triplicate assays. Inset: High magnification of separated intact nuclei stained with DAPI. Size bar = 10 μm.
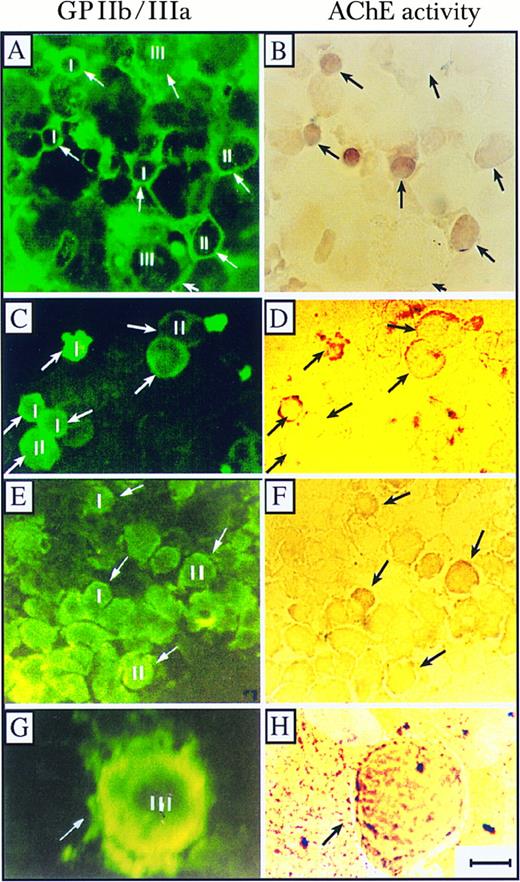
AChE activity in normal and abnormal BM MK. Presented is cytochemical staining of AChE activity (right) and immunochemical staining with GPIIb/IIIa antibodies (left) of normal, normal TPO-expanded, and ITP BM cells. (A and B) Normal BM grown ex vivo in the presence of TPO; (C and D) normal BM cells; and (E and H) ITP BM cells. Small (I, diameter < 10 μm), intermediate (II, diameter 10 to 25 μm), and large (III, diameter < 25 μm) MK are marked. Note the pronounced staining of AChE activity in small megakaryoblasts and the gradual decrease in AChE activities from cells at stage I to cells at stages II and III towards nonproliferating MK in the normal BM MK and the visible activity staining in a representative mature stage III MK in BM from an ITP patient (G and H). Pictures are representative of one of three tested normal, one of three tested ITP preparations, and one of five normal TPO-expanded BM cultures. Size bar = 10 μm.
AChE activity in normal and abnormal BM MK. Presented is cytochemical staining of AChE activity (right) and immunochemical staining with GPIIb/IIIa antibodies (left) of normal, normal TPO-expanded, and ITP BM cells. (A and B) Normal BM grown ex vivo in the presence of TPO; (C and D) normal BM cells; and (E and H) ITP BM cells. Small (I, diameter < 10 μm), intermediate (II, diameter 10 to 25 μm), and large (III, diameter < 25 μm) MK are marked. Note the pronounced staining of AChE activity in small megakaryoblasts and the gradual decrease in AChE activities from cells at stage I to cells at stages II and III towards nonproliferating MK in the normal BM MK and the visible activity staining in a representative mature stage III MK in BM from an ITP patient (G and H). Pictures are representative of one of three tested normal, one of three tested ITP preparations, and one of five normal TPO-expanded BM cultures. Size bar = 10 μm.
DAMI cells ACHEmRNA is suppressed under PMA-induced differentiation.Kinetic follow-up of RT-PCR amplification was further used to evaluate ACHEmRNA levels in RNA samples from cell cultures treated with PMA. A 10-fold to 100-fold decrease in the level of PCR-derived ACHE E6 cDNA was observed within 24 hours after PMA addition. The level of β-actin mRNA-derived DNA fragment remained unchanged under PMA treatment, verifying the integrity of the RNA preparations and the selectivity of the observed changes (Fig 4). Thus the RT-PCR analysis confirmed transcriptionally or posttranscriptionally controlled differentiation-associated suppression of DAMI cells ACHEmRNA, which takes place within hours after PMA addition. To elucidate the mechanisms regulating the differential decrease occurring in spliced ACHEmRNA levels but not in β-actin mRNA, we examined in these cells the involvement of the SF2/ASF splicing factor, which promotes inclusion of tissue-specific exons.22 Selective primers for SF2/ASF mRNA showed a 100-fold decrease at 24 hours post-PMA induction as compared with control cells (data not shown), suggesting that ACHEmRNA suppression under PMA may be due to diminished support of E6 splicing activity.
Nuclear-associated AChE eliminated under PMA treatment is relatively unstable.PMA and TPO caused decreases of 30% (P < .005) and 17% (P < .09), respectively, in the nuclear-associated AChE activities (Fig 5 [left inset]). That both cytochemical staining and nuclear-associated AChE activity decreased under PMA more than under TPO treatment indicates that the reduction in AChE activity was correlated with proliferation arrest. To test the biochemical properties of AChE in proliferating as compared with differentiating cells, we examined the stability of detergent-treated catalytically active AChE from nuclear fractions of control, TPO-, and PMA-treated cells. In nuclei from either control or TPO-treated cells, 1% Triton X-100 decreased the initial activity by 20%, as compared with 45% inactivation in nuclei from PMA-treated cells (Fig 5, left). However, when incubated for 72 hours at 37°C in the presence of 0.1% Triton, control and TPO cultures lost 66% and 58% of their initial nuclear AChE activity, respectively, while AChE in PMA-treated cells was only reduced by 35% under these conditions (Fig 5, right). Thus the enzyme associated with nuclei of PMA-treated cells was considerably more stable with time, yet more susceptible to Triton inactivation than the enzyme from control or TPO-treated cells. Finally, enzyme stability at 37°C was tested on nuclear preparations that were previously subjected to inactivation with 1% Triton X-100. Seventy-two hours post-Triton inactivation, all treated cells lost approximately 90% of their initial activity as measured at 0 time in the presence of 1% Triton (data not shown). Thus at high concentrations of detergent AChE, molecules of human megakaryoblasts drastically lose their activity. This detergent sensitivity may further explain why AChE was never detected in human MK by conventional techniques.
Acetylcholinesterase activity declines with differentiation of human bone marrow MK.To examine if AChE expression in vivo was differentiation-related, we also searched for AChE activity in normal human bone marrow MK. Both fresh BM smears and TPO-expanded MK populations from normal BM cells were subjected to cytochemical staining of AChE activity for 48 hours, followed by immunofluorescent GPIIb/IIIa (CD41) staining. Conspicuous AChE activity staining was associated with the nuclei of small (<10 μm in diameter) CD41-positive megakaryocytes and was significantly reduced in the large and differentiated MK of both fresh normal BM smears and TPO-expanded BM MKs (Fig 6A through D). At the early phase of MK development, AChE staining was most prominent around the nucleus, whereas GPIIb/IIIa labeling was evenly distributed throughout the cytoplasm (compare Fig 6A with 6B). AChE staining in young MK from ITP patients was similarly intense, with the most pronounced labeling around the nuclear domain (Fig 6E and F ). A considerably lower incidence of staining was observed in very large MK with exceedingly lower levels in normal BM MKs as compared with ITP (Fig 6G and H). To test for putative correlations between AChE activity and human MK maturation, we subjected BM smears from normal, TPO-expanded, and ITP patients to quantitative evaluation of AChE stain by assessing three staining levels; intense and moderately intense (++), weak (+), and no stain (−) in small (<10 μm), medium (10 to 25 μm), and large (>25 μm) MK (Table 2). When CD41+ cells were classified by size alone, we detected 50% and 30% lower proportions of small (<10 μm) cells in ITP and normal BM MK, respectively, as compared with TPO-expanded MK (P < .0001, χ2 test), reflecting TPO enhanced early megakaryocytopoiesis. In addition, the proportion of intermediate, but not large MK in ITP, was 25% higher than that of normal BM (data not shown). A comparison of AChE activity in normal, TPO-expanded and ITP MKs demonstrated that while more than 93% of the small cells were prominently stained for AChE in both TPO-expanded and ITP BM MK, only 68% were intensely stained in normal BM MK (P < .0001, χ2 test, Table 2). This association was further observed in intermediate cells. Thirty-seven percent and 50% of these were intensely stained in TPO-expanded and ITP BM MK, respectively, while only 13% were prominently stained in normal BM MK (P < .0001, χ2 test, Table 2). These observations suggest that the effect of TPO on the level of AChE in both small and intermediate MK is mimicked by the pathological changes of BM in ITP patients. However, in large MK, a significant difference in AChE staining was observed in ITP BM as compared with either normal BM MK or TPO-expanded MK. While approximately 18% of the large normal or TPO-expanded MK possessed some AChE activity, 38% of the large ITP MK sustained AChE activity (P < .0001, χ2 test, Table 2). Therefore, the association between the loss of AChE activity and the enlargement of MK cell size appeared to be disrupted under hematopoietic changes induced in ITP.
Cytochemical Staining of AChE in MK From Normal and ITP BM
| Cytochemical AChE Activity . | MK Size . | ||
|---|---|---|---|
| . | Small <10 μm . | Intermediate 10-25 μm . | Large >25 μm . |
| Normal (TPO-stimulated) | |||
| ++ | 93.3 | 37.5 | 2.8 |
| + | 5.7 | 42.7 | 14.3 |
| − | 1.0 | 19.8 | 82.9 |
| Normal BM smear | |||
| ++ | 68.2 | 12.8 | 2.2 |
| + | 26.3 | 42.3 | 16.2 |
| − | 5.5 | 44.9 | 81.6 |
| ITP | |||
| ++ | 98.3 | 50.5 | 5.2 |
| + | 0 | 34.0 | 32.5 |
| − | 1.7 | 15.5 | 62.3 |
| Cytochemical AChE Activity . | MK Size . | ||
|---|---|---|---|
| . | Small <10 μm . | Intermediate 10-25 μm . | Large >25 μm . |
| Normal (TPO-stimulated) | |||
| ++ | 93.3 | 37.5 | 2.8 |
| + | 5.7 | 42.7 | 14.3 |
| − | 1.0 | 19.8 | 82.9 |
| Normal BM smear | |||
| ++ | 68.2 | 12.8 | 2.2 |
| + | 26.3 | 42.3 | 16.2 |
| − | 5.5 | 44.9 | 81.6 |
| ITP | |||
| ++ | 98.3 | 50.5 | 5.2 |
| + | 0 | 34.0 | 32.5 |
| − | 1.7 | 15.5 | 62.3 |
More than 400 MK were counted in each group and the cells were classified according to their size into small, intermediate, and large categories as in Fig 6. Arbitrary degrees of AChE staining determined under ×1,000 magnification were defined as ++ (intensely and moderately stained), + (weakly stained), or − (not stained at all). Data are presented as the percentage of cells counted in each category. The presented analysis was performed on 1 of 3 normal or ITP patients and 1 of 5 TPO-expanded normal BM cultures.
DISCUSSION
Highly sensitive molecular biology approaches coupled with cytochemical and biochemical AChE analyses showed that human DAMI, CHRF-288-11, and MEGO1 megakaryoblasts and primary normal human BM MK express the ACHE gene and that AChE activity is closely associated with the megakaryocyte nucleus. ACHE transcription and ACHEmRNA splicing were downregulated by phorbol ester-induced differentiation of megakaryoblasts and in mature normal human MK. These findings are consistent with the lack of AChE activity from mature human MK. The reduction in AChE was, however, attenuated in the ITP BM, where MK enlargement proceeds at an abnormally high rate.
Refinement of the in situ hybridization analyses to provide subcellular localization showed ACHEmRNA molecules around the nucleus of small cells and surrounding the nuclear lobes of differentiated polyploid DAMI cells, with a denser signal in small cells. This indicated primary transcriptional and/or posttranscriptional control for this gene, operating at similar levels in different MK nuclear lobes. Nuclear association of ACHEmRNA was also found in mouse megakaryocytes.17 In muscle, ACHEmRNA molecules also accumulate next to the subset of subsynaptic nuclei, representing 1:200 of total nuclei, and are practically excluded from the sarcolemma.30 In contrast, we found ACHEmRNA molecules to be localized throughout the perikaryon of spinal cord motoneurons,26 demonstrating tissue specificity for the mode of subcellular distribution of ACHEmRNAs. Protein synthesis inhibitors shift mRNA molecules from neuronal cell bodies to dendrites,31 suggesting association between the subcellular distribution of mRNA and neuronal translational control. However, MK ACHEmRNA levels corresponded with the levels of the AChE protein, which makes translational control unlikely in these cells.
The 50 to 100 fluorescent dots detected by in situ hybridization correlated well with the number of mRNA molecules per cell as determined by the semiquantitative RT-PCR. Also, measuring ATCh hydrolysis showed corresponding values of 130 active AChE subunits in each DAMI cell. Estimating a 50- to 80- minute long translational round for the 2.5 Kb ACHEmRNA species, each ACHEmRNA transcript can be translated up to six times within the G1 productive phase (circa 10 hours) of a single DAMI cell cycle (circa 24 to 30 hours).18 Assuming that half of the produced enzyme molecules would be eliminated with time (based on the estimated 10- to 15-hour life for the active enzyme [reviewed in Soreq and Zakut8]), this allows for threefold higher numbers of protein molecules as compared with ACHEmRNA chains. Our detection of a 1:1 molar ratio between mRNA and intracellular protein suggests that two thirds of the AChE produced in MK (probably the cytoplasmic fraction) is secreted or destroyed, which is another reason for the previous difficulties in detecting this enzyme in human MK.
Using RT-PCR amplification, we demonstrated both synaptic and erythrocyte-characteristic ACHEmRNA transcripts in DAMI cells. This strengthens the hypothesis that the common precursor cell of both megakaryocytic and erythropoietic lineages11-13 expresses the ACHE gene in all mammals. Based on our current data, it is possible that the transition of this bipotential precursor cell into developing MK or erythroblasts involves species-specific differential modulation of ACHE gene expression. The selective 100-fold decrease in ACHEmRNA levels following PMA treatment of human DAMI cells may reflect such differentiation-related modulation, which could be caused by decrements in transcription rate, splicing efficiency or ACHEmRNA stability. The reduction in the mRNA for the GATA I transcription factor under PMA treatment of DAMI cells32 may point toward regulation at the transcription level for ACHE, as well. The mRNA for the SF2/ASF factor, which prevents skipping of tissue-specific exons during the splicing process22 was also decreased following PMA treatment, suggesting that splicing decisions are also involved. Nuclear run-on and pulse labeling experiments should be performed to test the third option, that of ACHEmRNA stability. In any event, the similar decreases in GATA I, SF2/ASF, and ACHEmRNA support the notion that decreases in AChE gene expression may be associated with human MK development and maturation. Untreated DAMI cells, the majority of which displayed 2N-4N ploidy, were calculated to carry 100 ± 25 enzyme molecules/cell (depending on the method of measurments). Under PMA treatment, approximately 25% of the cells acquired 8N ploidy and the specific activity of AChE was reduced, on average, by 30%. These numbers, and the significantly decreased area stained for AChE activity in relatively large DAMI cells, suggests that these cells lose most of their AChE activity during the shift between 4N to 8N.
The nuclear-associated AChE activity in cells under proliferation arrest due to PMA treatment appeared to be more stable and more sensitive to 1% Triton X-100 than the parallel enzyme fraction in either control or TPO-treated cells. This suggests further classification of DAMI cells AChE into three distinct subtypes: (1) cytoplasmic, secretory enzyme fraction, similar in all cells; (2) detergent-resistant enzyme of low stability associated with the nuclear membrane of proliferating cells; (3) detergent-sensitive, yet stable enzyme associated with the nuclear membrane of differentiated cells. The cytoplasmic enzyme form may be the source of AChE activity measured in intact human platelets by improved radiometric methods.33 Our results demonstrate that the stable enzyme form mainly appears in differentiated, PMA-treated cultures, where it constitutes approximately 60% of the nuclear-associated enzyme. The vulnerability of the enzymatic activity of this AChE form to Triton X-100 extraction further explains why it was undetectable in conventionally fixed cells, and was not reported before.
We compared BM specimens from ITP patients with enhanced megakaryocytopoiesis to normal BM smears. In ITP MK, the small immature cells are subject to stimulated endomitosis resulting in higher DNA content and size of these cells. The increased proportion of AChE positive small cells in ITP as compared with normal BM may reflect AChE involvement in proliferation processess. ITP enhancement of proliferation and differentiation of MK is probably operated by a combination of several cytokines including TPO and interleukin-3 (IL-3). Therefore, the less pronounced decline of AChE in MK from ITP patients as compared with normal megakaryocytopoiesis in human BM may be due to the acceleration of proliferation and maturation of MK progenitors. According to this proposal, AChE would be detectable in early maturing MK (like DAMI or ITP), but not during normal megakaryocytopoiesis. This distinction could perhaps be useful for identification purposes.
The limited durability of the nuclear-associated AChE in nondifferentiated MK may be due to incomplete posttranslational processing. AChE acquires its three glycosydic chains34 posttranslationally, while being transported through the rough endoplasmic reticulum into the Golgi apparatus. A conspicuously unstable, intracellular fraction of this enzyme was reported to account for up to 80% of the newly formed AChE in chick myotubes.35 Similarly, the nuclear-associated AChE in immature human MK may contain an unstable, incompletely processed form of the nascent protein. Recombinant human AChE produced in Escherichia coli, which is not glycosylated, was indeed found to be unstable at 37°C.36 The possibility of nonglycosylated forms may provide a tentative explanation for the vulnerability of AChE in immature human MK.
The role of AChE in hematopoietic cells,37,38 has long presented an enigma. As acetylcholine is largely limited to the nervous system, it is unlikely that hematopoietic AChE functions in hydrolyzing acetylcholine. The role of AChE in early hematopoietic precursor cells may rather be related to its subcellular distribution at the nuclear surface. MK AChE was reported to reside in two distinct cellular compartments: structural localization studies demonstrated activity in the perinuclear Golgi complexes of rat MK precursors,39 as well as in tubes and cisterns of the smooth membranes characteristic of cat, rat, and mouse MK in different stages of cytoplasmic maturation.40 The unique nuclear localization of AChE in human megakaryoblasts, therefore, supports the previous suggestion that it is involved in regulating the early MK proliferation phase.9 To perform such a function, a signal transduction mechanism involving a yet unknown nuclear-associated protein(s) should operate. ACHE DNA transfections into progenitor and differentiated cells could shed more light on this possibility. Moreover, in vitro experiments with antisense ACHE oligonucleotides on human as compared with mouse primary megakaryoblastic cultures should demonstrate the distinct roles of this enzyme in the proliferation and/or differentiation of MK from both species.
While AChE production during megakaryocytopoiesis is a common attribute to mice, rat, cat, and men,11-13 the mode of expression of the ACHE gene during MK development seems species-specific. ACHEmRNA and active enzyme levels decrease with MK development and were mainly observed in immature normal human BM MK, whereas the mouse, rat, and especially cat ACHE genes, conversely increase their expression with normal MK differentiation.11,17,28 However, these distinctions cannot be explained by the functional differences between human and other mammalian MKs, as the level of AChE in mature MK and platelets does not correlate with platelet numbers, volume, and survival in the different species.12 41-43
In conclusion, our data demonstrate that the ACHE gene is expressed in human megakaryocytes, shows species-specific differences in the subcellular localization and the developmental patterns of expression of this gene in megakaryocytes, yet leave the function of AChE in MK differentiation and maturation unresolved.
ACKNOWLEDGMENT
We are grateful to Dr A. Gewirtz (Philadelphia, PA) for critically reviewing this manuscript, M. Pick for the excellent technical assistance, and to A. Blumental-Perry for her contribution. We also thank Drs S. Greenberg, S. Lieberman, and K. Preissner for DAMI, CHRF-288-22, and MEG01 cells and Dr K. Kaushansky for TPO.
Supported by the Israel Ministry of Health (to V.D. and A.E.) and by the German-Israel Foundation (to V.D. and A.E. and to H.S).
Address reprint requests to Hermona Soreq, PhD, Department of Biological Chemistry, The Life Sciences Institute, the Hebrew University of Jerusalem, Jerusalem, Israel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal