Abstract
A significant fraction of hematopoietic stem cells (HSCs) have been shown to be resistant to the effects of cytotoxic agents such as 5-fluorouracil (5-FU), which is thought to eliminate many of the rapidly dividing, more committed progenitors in the bone marrow and to provide a relatively enriched population of the most primitive hematopoietic progenitor cells. Although differences between 5-FU–enriched progenitor populations and those from normal bone marrow have been described, it remained unclear if these differences reflected characteristics of the most primitive stem cells that were revealed by 5-FU, or if there were changes in the stem-cell population itself. Here, we have examined some of the properties of the stem cells in the bone marrow before and after 5-FU treatment and have defined several activation-related changes in the stem-cell population. We found that long-term reconstituting stem cells decrease their expression of the growth factor receptor c-kit by 10-fold and increase their expression of the integrin Mac-1 (CD11b). These changes begin as early as 24 hours after 5-FU treatment and are most pronounced within 2 to 3 days. This activated phenotype of HSCs isolated from 5-FU–treated mice is similar to the phenotype of stem cells found in the fetal liver and to the phenotype of transiently repopulating progenitors in normal bone marrow. We found that cell cycle is induced concomitantly with these physical changes, and within 2 days as many as 29% of the stem-cell population is in the S/G2/M phases of the cell cycle. Furthermore, when examined at a clonal level, we found that 5-FU did not appear to eliminate many of the transient, multipotent progenitors from the bone marrow that were found to be copurified with long-term repopulating, activated stem cells. These results demonstrate the sensitivity of the hematopoietic system to changes in its homeostasis and correlate the expression of several important surface molecules with the activation state of HSCs.
THE MANY TYPES OF mature lymphoid, myeloid, and erythroid cells can trace their origins to a class of common multipotent hematopoietic precursors known as hematopoietic stem cells (HSCs), which at the clonal level can give rise to all the blood cells and platelets.1 HSCs are multipotent progenitors that can self-renew; these, in turn, can be subdivided into long-term reconstituting stem cells with extensive self-renewal capacity, and transiently repopulating HSCs that only self-renew (in mice) for 3 to 6 weeks.2 Unlike many developmental processes, the entire hematopoietic system must continuously replenish itself throughout the life of the animal, suggesting that the stem cells are rapidly proliferating. However, the dynamics of the stem-cell population are somewhat surprising, in that at any given time, the majority of HSCs appear to be in a nondividing or quiescent state,3-5 even though they are ultimately responsible for the daily production of billions of cells. Several lines of evidence support the idea that the stem-cell population is largely quiescent.3,5,6 Originally, it was observed by several groups that a significant fraction of stem cells are resistant to the effects of the cytotoxic agents, including 5-fluororouracil (5-FU) and hydroxyurea.4,7 Since 5-FU is known to kill dividing cells rapidly, and yet spares the vast majority of the long-term reconstituting stem cells, one must conclude that either the stem-cell population is not dividing or that the stem cells must be actively resistant to the effects of 5-FU in other ways. Other evidence in support of the resting stem-cell hypothesis comes from studies using the vital dye rhodamine 123 (Rh123).6 It was shown that the most primitive stem cells retain only low levels of Rh123, which incorporates into mitochondrial membranes.8 Cells that retain high levels of Rh123 are thought to be relatively more active, while those that incorporate only low levels are thought to be resting.8 The most primitive long-term reconstituting stem cells are found in the Rh123low population of bone marrow, consistent with a resting phenotype6,9; however, this interpretation is complicated by the demonstration that Rh123 is sensitive to the effects of the multidrug efflux pump (mdr).10 Low levels of Rh123 may therefore reflect high activity of the mdr gene product, as well as limited mitochondrial activity.10 Regardless of the interpretation of these experiments, when long-term reconstituting stem cells were purified to homogeneity, the percentage of stem cells in the S/G2/M phases of the cell cycle was found to be relatively low (≃4%) compared with the ≥15% of cells with greater than two times the normal amount of DNA in the short-term repopulating and committed progenitor populations, which suggests again that most long-term reconstituting HSCs are not in cycle.2 Unfortunately, measuring the DNA content of cell populations does not distinguish G0 cells from those that are in G1 , and until recently, it remained unclear if HSCs were truly resting or if the population was merely cycling extremely slowly. Evidence presented elsewhere (S. Cheshire and I.L. Weissman, submitted) suggests that all stem cells are in cycle, but that the G1 interval is extended.
Although the adult bone marrow–derived population of stem cells has relatively few cells in the S/G2/M phases of the cell cycle, the equivalent population of long-term reconstituting stem cells isolated from the fetal liver has up to 30% of its cells in the S/G2/M phases of the cell cycle,11 demonstrating that there are at least some times when stem cells have the ability to cycle rapidly. Recently, investigators have sought ways to drive adult bone marrow–derived stem cells out of the resting state and into self-renewing cell divisions to facilitate transplantation, as well as to allow the incorporation of retroviral DNA.1,12-14 Treatment with 5-FU is commonly used to force murine stem cells into cycle, since they must divide in order to replace the ablated hematopoietic system. As an added bonus, 5-FU eliminates the majority of more committed progenitors,4,7,15-17 and thus allows investigators to study the properties of only the most primitive resting stem cells that have survived. These data are supported by the observation that the majority of colony-forming units–spleen (CFU-S) are eliminated with 5-FU treatment,4 although the 5-FU depletion appears most effective on the relatively more advanced population of CFU-S, since day 8 CFU-S are virtually eliminated, while day 12 CFU-S are only partly depleted.4,7 The colony-forming cell (CFC) potential of 5-FU–treated bone marrow is similarly affected, with the vast majority of more advanced CFC being eliminated, while many of the primitive blast CFC18,19 or long-term culture-initiating cells (LTC-IC)16 either survive or are produced as the earliest progeny of stem cells after recovery of the bone marrow from the effects of 5-FU. Several studies have examined the properties of stem cells after 5-FU treatment and have claimed that 5-FU treatment unveils previously uncharacterized populations of resting stem cells.19 20 However, the interpretations of these studies are complicated by the inability to determine if the 5-FU treatment merely enriches a preexisting, resting population of stem cells, or if the treatment induces physical and functional changes in previously described stem-cell populations such that these cells look and behave very differently.
Here, we sought to examine some of the physical and functional changes that occur in the stem-cell population after 5-FU treatment. We found that 5-FU induces the known population of long-term reconstituting stem cells to alter the cell-surface expression of the molecules c-kit and Mac-1, and that these changes correlate with the induction of cell cycle in this stem-cell population. Furthermore, although 5-FU treatment does eliminate many of the committed progenitor populations, it does not eliminate all of the short-term multipotent progenitors. However, with the changes in the expression of Mac-1, the transiently repopulating and long-term reconstituting progenitors could no longer be distinguished using the parameters we investigated. Thus, rather than revealing a cryptic population of resting stem cells, 5-FU appears to induce the long-term reconstituting stem cells to enter an activated state similar to that observed in fetal liver stem cells and in the activated short-term progenitors.
MATERIALS AND METHODS
Mice.All mice were bred and maintained in the Stanford Research Animal Facility. C57BL/Ka-Thy-1.1 mice were used as bone marrow donors and as sources of purified stem cells at 6 to 10 weeks of age. C57BL/Ka-Thy1.1-Ly5.1 mice were used as irradiated recipient animals at 9 to 12 weeks of age.
Antibodies.Monoclonal antibodies against the following surface molecules were used: fluorescein isothiocyanate (FITC)-labeled anti–Thy-1.1 (19XE5), FITC, phycoerytherin (PE)- or biotin-labeled anti–Sca-1 (E13). FITC-labeled anti-CD3 (145-2C11), biotin-labeled anti-B220 (RA3-6B2), PE-labeled anti–Mac-1 (M1/70), FITC-labeled anti-GR1 (8C5) allophycocyanin (APC)-labeled anti-Ly5.1 (A20), and APC-labeled anti-Ly5.2 (AL1-4A2) were used as directly conjugated fluorochromes. Anti-CD4 (GK1.5), antierythroid antigen (Ter119), anti-CD5 (53-7.3), and anti-CD8 (53-6.7) were used as unlabeled primary antibodies. The APC-conjugated antibodies 2B8 and 3C11 were used to detect c-kit expression, although the 2B8 antibody appears to be a more sensitive reagent.21 Secondary reagents included Texas Red–labeled goat antirat IgG, (Caltag, South San Francisco, CA), Texas Red–labeled avidin (Cappel, Durham, NC) and goat antirat or streptavidin magnetic beads (Miltenyi Biotec, Auburn, CA).
Preparation and staining of bone marrow.Bone marrow was obtained by flushing the femurs and tibias with phosphate-buffered saline (PBS) containing 2% calf serum. Erythrocytes were eliminated by lysis with NH4Cl. The remaining cells were filtered through nylon mesh and then stained with the indicated combinations of antibodies. Cells were stained in PBS with 2% calf serum for 15 minutes on ice, washed in PBS with 2% serum, and centrifuged through a serum layer. After the final round of staining and washing, the cells were resuspended in PBS 2% calf serum containing 1 μg/mL propidium iodide.
MACS depletion of lineage-positive cells.For cell sorting, bone marrow was initially depleted of lineage-positive cells using magnetic activated cell sorting (MACS; Miltenyi Biotec, Sunnyvale, CA). Cells were stained with unconjugated or biotinylated antilineage antibodies and then allowed to bind either goat antirat or streptavidin magnetic beads. A 100-μL quantity of beads was used per 108 cells. Cells were then applied to a C-type MACS column and the nonadherent (lineage-negative) cells were stained with the subsequent antibodies as indicated in the Results.
Cell-cycle analysis.The DNA content of cells was determined by lysing the cell populations in 0.1% Triton X-100, 1 mg/mL RNase, and 10 μg/mL propidium iodide in PBS with 1% bovine serum albumin (BSA) and measuring the resulting fluorescence in the PE channel. All cytometry was performed on a dual-laser FACS (Becton Dickenson, Mountain View, CA) as described, and made available through the shared user group at Stanford University.22
Treatment with 5-FU.Mice were treated with single doses of 5-FU (150 mg 5-FU/kg body weight) from a stock solution of 10 mg/mL in PBS. Bone marrow was recovered at the times indicated and stained as described earlier.
Competitive long-term reconstitution assays.Irradiated recipients received 950 rad from an x-ray source operated at 200 kV delivering 85 rad/min. Mice were irradiated in a split dose administered 4 to 5 hours apart. After irradiation, mice were maintained on antibiotic water (neomycin sulfate 1.1 g/L and polymyxin B sulfate 106 U/L) for at least 6 weeks. Progenitor cells sorted from Ly5.2 donors were injected retroorbitally into the irradiated recipients along with 2 × 105 whole bone marrow (WBM) cells from normal Ly5.1 mice as a source of radioprotective cells. For the analysis of reconstitution, mice were bled from the tail and assayed for the presence of Ly5.2-positive cells in each of the lineages. Blood was collected in PBS with 10 mmol/L EDTA and 1% dextran. Erythrocytes were allowed to sediment at 37°C for 45 minutes, and the leukocytes in the supernatant were collected and stained for lineage markers and for Ly5.2. Anti-B220 was used to identify B cells, anti-CD3 to identify T cells, and the combination of anti–Mac-1 and anti–GR-1 to identify myeloid cells.
RESULTS
To characterize the surface phenotype of stem cells after 5-FU treatment in C57BL/Thy-1.1 mice, we divided WBM into populations that expressed either high, low, or undetectable levels of various surface molecules from normal, as well as 5-FU–treated bone marrow, as previously described.23 Since it had been observed that the majority of long-term reconstituting stem cells go through the cell cycle between 3 and 4 days after treatment with 5-FU,13,24 and we wanted to identify any activation related changes that occurred as the cells were beginning to enter cell cycle, we sorted the various populations from the bone marrow 3 days after 5-FU treatment. These sorted populations were then assayed for stem-cell activity in long-term (>16 weeks) competitive repopulation experiments.25 Using this approach, we examined the expression of multiple surface molecules, including B220, Sca-1, Thy1.1, c-kit, and Mac-1 on stem-cell populations before and after treatment with 5-FU. We did not observe any differences in the expression of B220, Sca-1, or Thy1.1 on long-term repopulating stem cells derived from the bone marrow of either normal or 5-FU–treated mice (not shown). As seen previously,26 the long-term multipotent stem cells remained in the B220–negative, Sca-1–positive, and Thy-1.1low fractions of bone marrow (not shown).
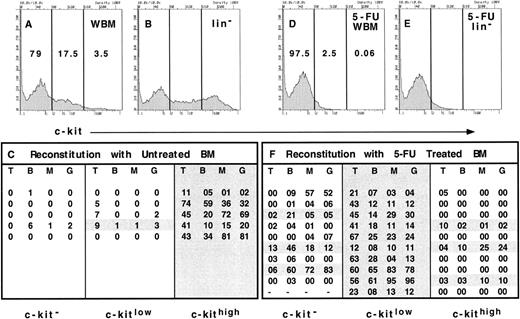
Expression of c-kit on long-term reconstituting stem cells from normal and 5-FU–treated bone marrow. Erythrocyte-depleted WBM from normal mice was stained with anti–c-kit and the lineage cocktail and was sorted into 3 populations as indicated (A, WBM). The relative representation of each population is shown as a percentage within each gate. For comparison, the relative representation of the 3 populations in lineage− bone marrow is shown (B, lin−). WBM cells were sorted based only on the expression of c-kit into a c-kit− population (79% of bone marrow), c-kitlow population (17.5%), and c-kithigh population (3.5%) from Ly5.2-expressing mice. The equivalent of 2 × 105 WBM cells from each population was injected into irradiated Ly5.1 recipients in competition with 2 × 105 Ly5.1 WBM cells. Thus, 2 × 105 × 79% or 1.58 × 105 cells of the c-kit− population was injected per recipient, 0.35 × 105 cells of the c-kitlow population per recipient and 0.07 × 105 cells of the c-kithigh population per recipient. Recipient mice were assayed for donor type reconstitution in each lineage after at least 16 weeks' postreconstitution. The percentage of Ly5.2+ T cells, B cells, and myeloid cells was determined by double staining with anti-Ly5.1 and either anti-CD3, B220, Mac-1, or Gr-1. The percentage of donor-derived cells of each lineage are shown for each recipient of c-kit−, c-kitlow, or c-kithigh cells (C). Each line of each column represents the percent of donor-derived cells of each lineage in an individual recipient. WBM from mice that had received a single dose of 5-FU 3 days prior was also collected and treated as described above (D, 5-FU WBM). The distribution of cells in each gate from the lineage− population of 5-FU–treated bone marrow is shown in E (5-FU lin−). The 3 populations were sorted from WBM of 5-FU–treated Ly5.2 mice and 1.95 × 105 cells of the c-kit− cells, 0.05 × 105 of the c-kitlow cells, and 120 of the c-kithigh cells were injected per irradiated Ly5.1 recipient in competition with 2 × 105 Ly5.1 WBM cells. The long-term reconstitution data are shown as the percent of donor-derived cells in each lineage (F ) and represent the sum of 2 independent experiments with 5 mice in each group.
Expression of c-kit on long-term reconstituting stem cells from normal and 5-FU–treated bone marrow. Erythrocyte-depleted WBM from normal mice was stained with anti–c-kit and the lineage cocktail and was sorted into 3 populations as indicated (A, WBM). The relative representation of each population is shown as a percentage within each gate. For comparison, the relative representation of the 3 populations in lineage− bone marrow is shown (B, lin−). WBM cells were sorted based only on the expression of c-kit into a c-kit− population (79% of bone marrow), c-kitlow population (17.5%), and c-kithigh population (3.5%) from Ly5.2-expressing mice. The equivalent of 2 × 105 WBM cells from each population was injected into irradiated Ly5.1 recipients in competition with 2 × 105 Ly5.1 WBM cells. Thus, 2 × 105 × 79% or 1.58 × 105 cells of the c-kit− population was injected per recipient, 0.35 × 105 cells of the c-kitlow population per recipient and 0.07 × 105 cells of the c-kithigh population per recipient. Recipient mice were assayed for donor type reconstitution in each lineage after at least 16 weeks' postreconstitution. The percentage of Ly5.2+ T cells, B cells, and myeloid cells was determined by double staining with anti-Ly5.1 and either anti-CD3, B220, Mac-1, or Gr-1. The percentage of donor-derived cells of each lineage are shown for each recipient of c-kit−, c-kitlow, or c-kithigh cells (C). Each line of each column represents the percent of donor-derived cells of each lineage in an individual recipient. WBM from mice that had received a single dose of 5-FU 3 days prior was also collected and treated as described above (D, 5-FU WBM). The distribution of cells in each gate from the lineage− population of 5-FU–treated bone marrow is shown in E (5-FU lin−). The 3 populations were sorted from WBM of 5-FU–treated Ly5.2 mice and 1.95 × 105 cells of the c-kit− cells, 0.05 × 105 of the c-kitlow cells, and 120 of the c-kithigh cells were injected per irradiated Ly5.1 recipient in competition with 2 × 105 Ly5.1 WBM cells. The long-term reconstitution data are shown as the percent of donor-derived cells in each lineage (F ) and represent the sum of 2 independent experiments with 5 mice in each group.
However, we did observe changes in the expression of other characteristic stem-cell markers. As can be seen in Fig 1A, bone marrow from untreated adult mice can be divided into populations that express high levels of c-kit (3.5% of WBM), those cells that express low levels of c-kit (17.5% of WBM), and those cells that are c-kit-negative (79% of WBM). As a comparison, the expression of c-kit is shown when lineage-positive cells are eliminated (Fig 1B), demonstrating that the majority of c-kithigh cells are in the lineage-negative population. Each of these populations were sorted from the bone marrow of Ly5.2-expressing donors and the relative number of cells found in 2 × 105 WBM cells was used in a competitive repopulation with 2 × 105 Ly5.1 bone marrow into Ly5.1-irradiated hosts. Thus, individual mice received either 1.58 × 105 c-kit–negative cells, 0.35 × 105 c-kitlow cells, or 0.07 × 105 c-kithigh cells from the Ly5.2 donor in competition with 2 × 105 WBM cells of the Ly5.1 type. This treatment was performed in five mice for each group and the reconstitution of all lineages in peripheral blood was assayed at 16 weeks' postreconstitution. The percentage of donor-derived cells of each lineage is shown in Fig 1C, and the individual mice that showed long-term reconstitution of donor derived cells in all lineages are shaded. We observed that five of five mice were long-term reconstituted in all lineages with c-kithigh cells, while only one mouse was long-term reconstituted with cells that expressed low levels of c-kit. In mice injected with the c-kit–negative population of cells, only one showed multilineage reconstitution; however, no T cells were observed at any time point in this individual and the relative levels of the other lineages were low. Thus, as we have observed previously with this strain of mice,2 27 it is immediately apparent that although only 3.5% of total bone marrow cells are c-kitbright, the vast majority of stem cells are contained within this population of cells.
We then performed the same type of experiment with bone marrow cells from mice that were treated 3 days previously with 150 mg/kg of 5-FU. As seen in Fig 1D, the vast majority of c-kit–positive cells disappear by the third day after 5-FU treatment, leaving only 0.06% of WBM cells in the c-kithigh gate and only 2.5% of cells in the c-kitlow gate. Contrary to what we observed in untreated bone marrow, when the lineage-positive cells were eliminated from the FACS analysis, there was no obvious c-kit–positive population in the 5-FU–treated bone marrow (Fig 1E). This result is consistent with the hypothesis that the majority of the rapidly cycling committed hematopoietic progenitors that express c-kit are killed by 5-FU treatment, leaving only the rare c-kit–positive stem cells. In fact, 0.06% of WBM is similar to the frequency of stem cells initially reported by Spangrude et al.28 As described for the analysis of normal bone marrow, 5-FU–treated WBM was sorted into three populations based on the expression of c-kit and the repopulating ability of each of these populations was tested in competitive reconstitution assays. After 16 weeks, the percentage of donor-derived cells found in mice of each group was analyzed and is shown in Fig 1F. In contrast to what was observed with cells from normal bone marrow, only three of 10 mice were reconstituted in all lineages with the rare c-kithigh cells, whereas 10 of 10 mice were robustly reconstituted with the c-kitlow cells. In addition, three of nine mice that were reconstituted with c-kit–negative cells showed multilineage long-term reconstitution at 16 weeks, while the remaining mice in this group demonstrated varying levels of reconstitution in one or more lineages, including two mice that were reconstituted in both myeloid and B lineages, but had no detectable donor-derived T cells. Although the mice that showed multilineage reconstitution derived from the c-kit–negative and c-kithigh cells had much lower donor-derived T-cell repopulation than that achieved with the c-kitlow cells, it is unclear whether this represents intrinsic differences within these populations, or if the data for the c-kit–negative and c-kithigh cells represent limited dilution clonal responses, while mice in the c-kitlow group were repopulated with more than one stem cell. Thus, although the vast majority of the long-term repopulating stem cells were now contained in the c-kitlow population, a significant fraction of the stem-cell activity could be found in the c-kit–negative population and at least some long-term reconstituting stem cells retained their c-kithigh phenotype. Interestingly, however, the c-kithigh population was relatively enriched for stem cells, since only 120 sorted cells were injected per mouse. The data represent two independent experiments with five mice in each group.
Interestingly, stem cells were found in the c-kitlow population only with the anti–c-kit antibody 2B8. When the antibody 3C11 was used to stain c-kit, all of the long-term reconstituting stem cells were found in the c-kit–negative population (data not shown). It is unclear if this phenomenon correlates with a particular epitope on the c-kit molecule or if the 2B8 antibody simply stains brighter and allows a more sensitive detection of low levels of c-kit expression.21 Regardless, the data demonstrate that the surface expression of c-kit is dramatically decreased on the long-term reconstituting stem-cell population after 5-FU treatment.
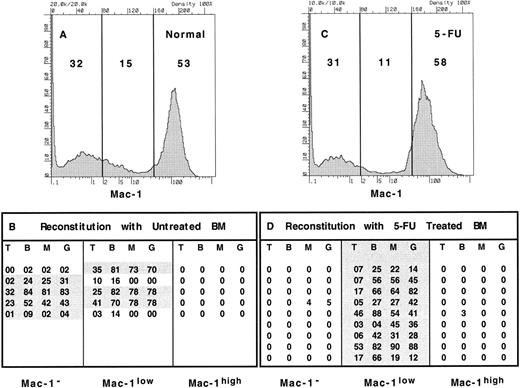
We next performed similar experiments using an antibody that recognized the adhesion molecule Mac-1 or CD11b, since activated populations of stem cells often express low levels of this molecule. Bone marrow stained with anti–Mac-1 could easily be divided into three populations. Normal bone marrow was found to be composed of 53% Mac-1high cells, 15% Mac-1low cells and 32% of Mac-1–negative cells (Fig 2). When these populations were sorted as shown in Fig 2A and used in competitive reconstitution assays, stem-cell activity was observed in both the Mac-1–negative, as well as the Mac-1low populations. Of the five mice that received Mac-1–negative cells, four exhibited long-term reconstitution, while three of five mice that received Mac-1low cells showed long-term reconstitution. Two of the mice that received cells from the Mac-1low population were reconstituted in all lineages for several weeks (not shown), but by 16 weeks had lost myeloid lineage cells, consistent with the observation that a low level of Mac-1 is expressed on short-term multipotent progenitors.2 In agreement with this, we also observed donor-derived T cells in all mice that received Mac-1low cells as early as 4 weeks (not shown), while T cells were not observed until after 6 weeks in mice repopulated with Mac-1–negative cells (not shown). This is consistent with the hypothesis that long-term reconstituting stem cells take several weeks to produce more committed progeny in the bone marrow than can subsequently seed the thymus and mature, while other more committed progenitors can seed the thymus directly and produce mature T cells more rapidly.2
Expression of Mac-1 on long-term reconstituting stem cells from normal and 5-FU–treated bone marrow. Erythrocyte-depleted WBM was collected from normal and 5-FU–treated mice, stained with anti–Mac-1, and sorted into 3 populations as indicated (A, normal; C, 5-FU); the relative representation is shown as a percent within each gate. WBM cells from normal mice were sorted into Mac-1− (32% of bone marrow), Mac-1low (15%), and Mac-1high (53%) populations from Ly5.2-expressing mice. The equivalent of 2 × 105 WBM cells from each population was injected into irradiated Ly5.1 recipients in competition with 2 × 105 Ly5.1 WBM cells. Thus, 0.64 × 105 cells of the Mac-1− population were injected per recipient, 0.3 × 105 cells of the Mac-1low population per recipient, and 1.06 × 105 cells of the c-kithigh population per recipient. The equivalent populations were sorted from 5-FU–treated Ly5.2 mice and 0.62 × 105 cells of the Mac-1− cells, 0.22 × 105 of the Mac-1low cells, and 1.16 × 105 of the Mac-1high cells were injected per irradiated Ly5.1 recipient in competition with 2 × 105 Ly5.1 WBM cells. Recipient mice were assayed for donor-type reconstitution after at least 16 weeks postreconstitution and the percent of donor-derived cells in each lineage was determined for each recipient of Mac-1−, Mac-1low, or Mac-1high cells (B, normal; D, 5-FU). The long-term reconstitution data for mice that received 5-FU–treated bone marrow represents the sum of 2 independent experiments with 5 mice in each group.
Expression of Mac-1 on long-term reconstituting stem cells from normal and 5-FU–treated bone marrow. Erythrocyte-depleted WBM was collected from normal and 5-FU–treated mice, stained with anti–Mac-1, and sorted into 3 populations as indicated (A, normal; C, 5-FU); the relative representation is shown as a percent within each gate. WBM cells from normal mice were sorted into Mac-1− (32% of bone marrow), Mac-1low (15%), and Mac-1high (53%) populations from Ly5.2-expressing mice. The equivalent of 2 × 105 WBM cells from each population was injected into irradiated Ly5.1 recipients in competition with 2 × 105 Ly5.1 WBM cells. Thus, 0.64 × 105 cells of the Mac-1− population were injected per recipient, 0.3 × 105 cells of the Mac-1low population per recipient, and 1.06 × 105 cells of the c-kithigh population per recipient. The equivalent populations were sorted from 5-FU–treated Ly5.2 mice and 0.62 × 105 cells of the Mac-1− cells, 0.22 × 105 of the Mac-1low cells, and 1.16 × 105 of the Mac-1high cells were injected per irradiated Ly5.1 recipient in competition with 2 × 105 Ly5.1 WBM cells. Recipient mice were assayed for donor-type reconstitution after at least 16 weeks postreconstitution and the percent of donor-derived cells in each lineage was determined for each recipient of Mac-1−, Mac-1low, or Mac-1high cells (B, normal; D, 5-FU). The long-term reconstitution data for mice that received 5-FU–treated bone marrow represents the sum of 2 independent experiments with 5 mice in each group.
In contrast to what we observed in normal bone marrow, long-term reconstituting stem cells from day 3 post 5-FU–treated bone marrow were found exclusively within the Mac-1low gate (Fig 2C). Although the Mac-1 staining profile of 5-FU–treated bone marrow and normal marrow appear similar, there is a small, but consistent, decrease in the frequency of Mac-1low cells as seen in this experiment as a decrease from 15% to 11%. The Mac-1–negative population remained steady at 31% and the Mac-1high population was slightly increased to 58% of WBM. In competitive reconstitution assays with the three populations, 10 of 10 mice that received Mac-1low cells were long-term reconstituted in all lineages, while mice that had received either Mac-1high or Mac-1–negative cells were virtually devoid of donor-derived cells at all time points (Fig 2D). These data represent two independent experiments with five mice in each group. Thus, it appears that while long-term repopulating stem cells can be found in both the Mac-1–negative and the Mac-1low populations of normal bone marrow, all stem cells express low levels of Mac-1 after 5-FU treatment.
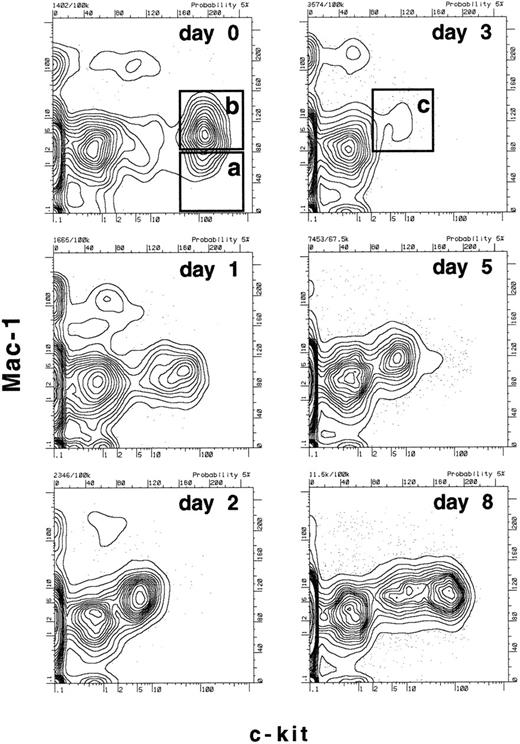
Since we had determined that there were dramatic changes in the expression of c-kit and Mac-1 on functionally defined long-term reconstituting stem cells within the first 3 days after 5-FU treatment, we now wanted to establish a more specific time frame for these changes. Mice were treated with a single dose of 5-FU and bone marrow was harvested 1, 2, 3, 5, or 8 days after treatment and the expression of Mac-1 and c-kit on the progenitor populations was compared with that of untreated mice (day 0). Figure 3 shows the expression of Mac-1 and c-kit on the B220-negative, Sca-1–positive population of bone marrow. As determined functionally in Figs 1 and 2, and consistent with previous data,2 boxes a and b in Fig 3 enclose either predominantly long-term stem cells (box a: Mac-1–negative, c-kithigh) or a mixture of long-term and short-term stem cells (box b: Mac-1low, c-kithigh) before 5-FU treatment. However, as early as 1 day after 5-FU treatment, the surface expression of c-kit begins to decrease and by day 2 and 3 there are almost no c-kitbright cells left. At the same time, the overall c-kit–positive population (including both c-kithigh and c-kitlow cells) begins to express a higher level of Mac-1, such that by days 2 and 3 there are no detectable Mac-1–negative cells in the B220-negative, Sca-1–positive, c-kit–positive population. On day 3, the single boxed area containing only a small population of c-kitlow, Mac-1low cells (Fig 3, box c) encloses the majority of the functionally defined stem-cell population as determined in Figs 1 and 2. By days 5 and 8, the population of c-kithigh cells is returning, and as soon as 18 days after 5-FU treatment, the (presumptive) stem-cell compartment of the bone marrow looks normal (not shown). Thus, although the functional capacities of the populations described in Fig 3 were only tested on days 0 and 3, it appears that the expression of c-kit gradually declines on the stem-cell population over the first few days after 5-FU administration and then begins to recover by day 8. The expression of Mac-1 also changes over this time frame, appearing lowest on the stem-cell population before 5-FU treatment and reaching a peak at day 3; a small number of Mac-1–negative cells appears again at day 8, but their frequency is relatively low in comparison to the greatly expanded Mac-1low population (Fig 3).
Time course of changes in the expression of c-kit and Mac-1 on lineage− Sca-1+ cells after treatment with 5-FU. Mice were treated with a single dose of 5-FU and bone marrow was harvested after the number of days indicated. RBCs were lysed and the remaining cells were stained with anti-B220, Sca-1, Mac-1, and c-kit. The profiles shown are of Mac-1 and c-kit expression on B220−, Sca-1+ gated bone marrow cells at the various time points. In the day 0 time point, box (a) represents where the majority of long-term reconstituting stem cells are found2 (Figs 1 and 2), while box (b) is where a mixture of functionally defined long- and short-term reconstituting stem cells are found2 (Figs 1 and 2). Box (c) in the day 3 time point represents the population defined here that contains the majority of long-term reconstituting stem cells and possibly other progenitors (see Fig 6). Note that on day 3, the populations equivalent to (a) or (b) are undetectable.
Time course of changes in the expression of c-kit and Mac-1 on lineage− Sca-1+ cells after treatment with 5-FU. Mice were treated with a single dose of 5-FU and bone marrow was harvested after the number of days indicated. RBCs were lysed and the remaining cells were stained with anti-B220, Sca-1, Mac-1, and c-kit. The profiles shown are of Mac-1 and c-kit expression on B220−, Sca-1+ gated bone marrow cells at the various time points. In the day 0 time point, box (a) represents where the majority of long-term reconstituting stem cells are found2 (Figs 1 and 2), while box (b) is where a mixture of functionally defined long- and short-term reconstituting stem cells are found2 (Figs 1 and 2). Box (c) in the day 3 time point represents the population defined here that contains the majority of long-term reconstituting stem cells and possibly other progenitors (see Fig 6). Note that on day 3, the populations equivalent to (a) or (b) are undetectable.
We next examined how the onset of cell division related to the changes in surface expression of Mac-1 and c-kit in the stem-cell compartment of 5-FU–treated bone marrow. We sorted the total population of stem cells (including both short- and long-term stem cells) using the criteria of lineage-negative (except Mac-1), Sca-1–positive, Thy-1.1low, and c-kit–positive (including both c-kithigh and c-kitlow cells). Cells were then analyzed for DNA content by propidium iodide staining. As can be seen in Fig 4A, the cellularity of the bone marrow declines precipitously during the first 5 days after treatment. Not surprisingly, the number of cells in the lineage-negative, Sca-1–positive, Thy1.1low, c-kit–positive population that were in the S/G2/M phases of the cell cycle decreased from 15% in untreated mice to approximately 5% within 24 hours after 5-FU treatment (Fig 4B). However, over the next several days, the lineage-negative, Sca-1–positive, c-kit–positive, Thy-1.1low population began to divide rapidly. By day 2, we observed that 29% of the population was in cycle and on day 5 as much as 49% of the population was cycling, although by 10 days posttreatment, this rapid burst of proliferation had abated (Fig 4B). The frequency of cells in S/G2/M in normal bone marrow is shown as the solid bar for comparison (Fig 4B). We also calculated the frequency, as well as the total number of lineage-negative, Sca-1–positive, Thy-1.1low, c-kit–positive cells in the bone marrow at the various time points (Fig 4C). The frequency of the lineage-negative, Sca-1–positive, Thy-1.1low, c-kit–positive population decreased relatively little over the first few days after treatment, even though this population initially included the long-term reconstituting stem cells, as well as the transiently repopulating progenitors2 (Fig 4C). However, there was a dramatic increase in the frequency of this population between 5 and 10 days. Similarly, the absolute number of lineage-negative, Sca-1–positive, Thy-1.1low, c-kit–positive cells decreased over the first 2 days, remained relatively steady until day 5, and then by day 10 the population had significantly expanded (Fig 4D), suggesting that initially some of the cells in this phenotypically defined population were killed by the 5-FU and then at later times the remaining cells in the population began to expand.
Induction of cell cycle in stem-cell–containing populations. Mice were treated with a single dose of 5-FU and bone marrow was harvested 1, 2, 3, 5, and 10 days after treatment. Bone marrow from both femurs and tibias of 5 mice was pooled for each time point, depleted of erythroid cells, and counted (A). To analyze the frequency of progenitor cells (HSC) in the S/G2/M phases of the cell cycle, progenitor populations from each time point were purified by depleting lineage+ cells (excluding Mac-1), by MACS, and staining the remaining cells with anti–Sca-1, c-kit, and Thy-1.1. The lineage−, Sca-1+, Thy-1.1low, c-kit+ population (including both c-kithigh and c-kitlow cells) was then sorted and analyzed for DNA content by propidium iodide uptake (B). At least 7,000 cells at each time point were collected for DNA analysis. For an analysis of progenitor frequency, WBM from each time point was stained with the lineage cocktail (including anti–Gr-1, CD3, CD4, CD8, B220, and Ter119) and with anti–Sca-1, Thy-1.1, and c-kit. The percent of lineage−, Sca-1+, Thy-1.1low, c-kit+ (including c-kithigh and c-kitlow) cells in WBM was determined by integration analysis using FACSDESK22 software (C). The total number of lineage−, Sca-1+, Thy-1.1low, c-kit+ cells in the femurs and tibias of mice at each time point was calculated by multiplying the frequency of the population (C) by the cellularity of the bone marrow (A) and is shown in D.
Induction of cell cycle in stem-cell–containing populations. Mice were treated with a single dose of 5-FU and bone marrow was harvested 1, 2, 3, 5, and 10 days after treatment. Bone marrow from both femurs and tibias of 5 mice was pooled for each time point, depleted of erythroid cells, and counted (A). To analyze the frequency of progenitor cells (HSC) in the S/G2/M phases of the cell cycle, progenitor populations from each time point were purified by depleting lineage+ cells (excluding Mac-1), by MACS, and staining the remaining cells with anti–Sca-1, c-kit, and Thy-1.1. The lineage−, Sca-1+, Thy-1.1low, c-kit+ population (including both c-kithigh and c-kitlow cells) was then sorted and analyzed for DNA content by propidium iodide uptake (B). At least 7,000 cells at each time point were collected for DNA analysis. For an analysis of progenitor frequency, WBM from each time point was stained with the lineage cocktail (including anti–Gr-1, CD3, CD4, CD8, B220, and Ter119) and with anti–Sca-1, Thy-1.1, and c-kit. The percent of lineage−, Sca-1+, Thy-1.1low, c-kit+ (including c-kithigh and c-kitlow) cells in WBM was determined by integration analysis using FACSDESK22 software (C). The total number of lineage−, Sca-1+, Thy-1.1low, c-kit+ cells in the femurs and tibias of mice at each time point was calculated by multiplying the frequency of the population (C) by the cellularity of the bone marrow (A) and is shown in D.
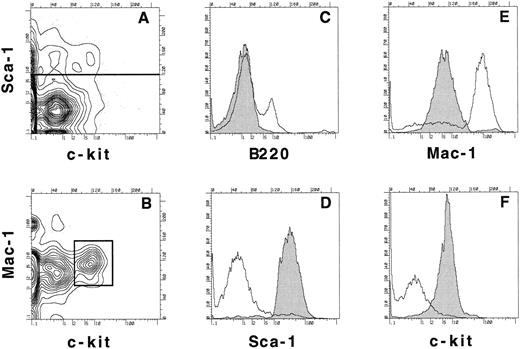
We also wanted to examine the behavior of the stem-cell population at limiting dilution in mice to determine whether only long-term quiescent stem cells survived 5-FU treatment or if other progenitors survived as well. Bone marrow was harvested from Ly5.2-positive mice treated 3 days previously with 5-FU and the B220-negative, Sca-1–positive, Mac-1low, c-kitlow population was purified by cell sorting. In Fig 5A the expression of Sca-1 and c-kit is shown on the B220-negative population and the horizontal line in Fig 5A indicates the demarcation between Sca-1–negative and Sca-1–positive cells. When the Sca-1 gate was applied and the expression of Mac-1 and c-kit was examined (Fig 5B), the B220-negative, Sca-1–positive, Mac-1low, c-kitlow population (Fig 5B box) was found to consist of 0.03% of day 3 post 5-FU WBM. This population was sorted and then resorted according to the same gates to ensure purity. A reanalysis of the expression of B220, Sca-1, Mac-1, and c-kit on the cells collected during the initial sort is shown as the shaded histograms in Fig 5C through F and is compared with their expression on day 3 5-FU WBM (C, open histogram), and B220-negative day 3 bone marrow (D through F, open histograms). Thus, this population does not express detectable levels of B220, has high levels of Sca-1, low but clearly positive levels of Mac-1, and low but positive levels of c-kit. The doubly sorted cells were injected into irradiated Ly5.1 recipients at 15, 45, or 135 cells per mouse in competition with 2 × 105 WBM cells of the Ly5.1 type to ensure the survival of all animals.
Sorting and reanalysis of stem cells from 5-FU–treated bone marrow. Bone marrow was harvested from mice that had been treated 3 days previously with 5-FU. RBCs were lysed and the cells were stained with anti-B220, Sca-1, Mac-1, and c-kit. (A) Expression of Sca-1 and c-kit on B220− bone marrow cells. (B) Mac-1 and c-kit expression on B220−, Sca-1+ gated population as determined in (A). 0.03% of day 3 post 5-FU WBM is contained in the lineage−, Sca-1+, Mac-1low, c-kitlow population as shown in the box. The boxed population was sorted and a reanalysis of the expression of B220, Sca-1, Mac-1, and c-kit (C-F, shaded histograms) was compared with the expression of these same molecules on ungated day 3 post 5-FU WBM (C, open histogram) or on B220− day 3 bone marrow cells (D-F, open histograms).
Sorting and reanalysis of stem cells from 5-FU–treated bone marrow. Bone marrow was harvested from mice that had been treated 3 days previously with 5-FU. RBCs were lysed and the cells were stained with anti-B220, Sca-1, Mac-1, and c-kit. (A) Expression of Sca-1 and c-kit on B220− bone marrow cells. (B) Mac-1 and c-kit expression on B220−, Sca-1+ gated population as determined in (A). 0.03% of day 3 post 5-FU WBM is contained in the lineage−, Sca-1+, Mac-1low, c-kitlow population as shown in the box. The boxed population was sorted and a reanalysis of the expression of B220, Sca-1, Mac-1, and c-kit (C-F, shaded histograms) was compared with the expression of these same molecules on ungated day 3 post 5-FU WBM (C, open histogram) or on B220− day 3 bone marrow cells (D-F, open histograms).
The animals were analyzed for donor-type reconstitution in both lymphoid and myeloid lineages at 4, 16, and 40 weeks' postreconstitution. Mice were considered to be engrafted with long-term reconstituting stem cells if donor-derived cells were observed in all lineages at 40 weeks. Those animals that had donor-derived cells in one or more lineages at 4 or 16 weeks but did not show evidence of donor-derived cells in the myeloid lineages at 40 weeks were considered to be repopulated with transient progenitors. At the 15-cell dose, only four of 10 mice showed evidence of long-term reconstitution (shown as dark shading in Fig 6). However, donor-derived repopulation of various lineages was observed at 4 weeks in five of the mice that had received 15 cells (lightly shaded), but these mice failed to engraft permanently the hematopoietic stem-cell pool as shown by the lack of any donor-derived cells at the 40-week time point. In addition, only one recipient failed to develop any detectable donor-derived cells at the 15-cell dose (no shading). At the 45-cell dose, again only four recipients were long-term reconstituted, while the remaining six were transiently reconstituted. At a dose of 135 cells per recipient, nine of 10 mice were reconstituted at 40 weeks (long-term) and one was only transiently repopulated. Thus, although this phenotypically defined population of cells contains a high frequency of long-term reconstituting stem cells (of 29 cells when calculated from the 15-cell dose according to Smith et al29 ), it also contains an even higher frequency of transiently repopulating progenitors, since many of the short-term repopulating clones will be obscured by those with long-term repopulating functions.
Analysis of long-term reconstitution. Progenitor populations were sorted using the parameters described in Fig 5B (box) from Ly5.2-expressing animals that had been treated with 5-FU 3 days previously. These cells were then subjected to another round of cell sorting using the same parameters to ensure the purity of the population. Either 15, 45, or 135 cells were injected into irradiated Ly5.1-expressing recipients in competition with 2 × 105 bone marrow cells from Ly5.1 donors. Mice were bled at 4, 16, and 40 weeks after reconstitution and analyzed for donor-derived cells in each lineage by staining with anti-Ly5.2 and either anti-CD3 for T cells, anti-B220 for B cells, or anti–Mac-1 or anti–Gr-1 for myeloid cells. The numbers in the table represent the percentage of donor-derived cells in each lineage for each animal at 4, 16, and 40 weeks after reconstitution.
Analysis of long-term reconstitution. Progenitor populations were sorted using the parameters described in Fig 5B (box) from Ly5.2-expressing animals that had been treated with 5-FU 3 days previously. These cells were then subjected to another round of cell sorting using the same parameters to ensure the purity of the population. Either 15, 45, or 135 cells were injected into irradiated Ly5.1-expressing recipients in competition with 2 × 105 bone marrow cells from Ly5.1 donors. Mice were bled at 4, 16, and 40 weeks after reconstitution and analyzed for donor-derived cells in each lineage by staining with anti-Ly5.2 and either anti-CD3 for T cells, anti-B220 for B cells, or anti–Mac-1 or anti–Gr-1 for myeloid cells. The numbers in the table represent the percentage of donor-derived cells in each lineage for each animal at 4, 16, and 40 weeks after reconstitution.
DISCUSSION
Many investigators have used 5-FU to deplete bone marrow of committed progenitors to simplify the purification and subsequent characterization of the most primitive HSCs.18-20,26,30 While 5-FU depletion does appear to enrich for primitive cells,3,4,7 16 it is likely that the homeostatic mechanisms that regulate hematopoiesis will induce both physical and functional changes in the remaining stem-cell population following treatment, making it difficult to compare directly stem cells from normal and 5-FU–treated bone marrow. However, the changes that occur in stem-cell populations during the recovery from 5-FU treatment are likely to reflect relevant physiologic processes that are important during transplantation and chemotherapy procedures. We have examined some of the properties of stem cells isolated 3 days after 5-FU treatment and have found that an induction of the adhesion molecule Mac-1 and the reduction of growth factor receptor c-kit correlate with the onset of cell division in the stem-cell population. Furthermore, we have found that the repopulating potential of purified hematopoietic progenitors that have survived 5-FU treatment is consistent with the survival of both long- and short-term reconstituting cells.
The most direct and dramatic consequence of 5-FU treatment is the loss of the cycling hematopoietic progenitor cells within the bone marrow, followed by the induction of cell cycle in the rare surviving stem cells.13,24 We observed that the lineage-negative, Sca-1–positive, Thy-1.1low, c-kit–positive population of progenitors was rapidly recruited into cell cycle after 5-FU treatment, with up to 29% of these cells being found in the S/G2/M phases of the cell cycle on day 2 post 5-FU and almost 50% by day 5 (Fig 4). Although this contrasts with data showing that cell cycle was still depressed in the phenotypically defined lineage-negative, Sca-1–positive population at day 2, but recovered by day 3 after 5-FU,13 it is entirely consistent with data showing that large numbers of functionally, rather than phenotypically, defined long-term reconstituting stem cells begin to cycle as early as 2 days post 5-FU.24 This rapid initiation of cell cycle in the functionally defined long-term reconstituting stem-cell population after 5-FU treatment may suggest that many of these cells are not truly resting, but are in fact cycling slowly, consistent with others' interpretations.31 In addition, labeling experiments with BRdU have suggested that the long-term reconstituting stem-cell population is in cycle more often than expected, with about half of the long-term reconstituting stem cells incorporating BRdU within 6 days (S. Cheshire and I.L. Weissman, submitted). Furthermore, the fact that cell cycle is initiated as early as 2 days also suggests that the hematopoietic system is sensitive to changes in its steady-state and can rapidly respond to the loss of progenitors.
Although 5-FU has been shown to eliminate the more committed progenitors from stem-cell–containing populations, some cells such as day 12 CFU-S do remain at low frequency,4,7 suggesting that not all of the committed progenitors are eliminated. Our data indicate that both long-term and some short-term reconstituting stem cells are present at day 3 post 5-FU, although at present we cannot distinguish between these two populations, since they appear phenotypically identical (Figs 5 and 6). Additionally, it is not totally clear at this point if the short-term repopulating cells represent daughter cells derived from the few remaining long-term reconstituting stem cells, or if some transiently repopulating cells themselves survive 5-FU treatment. On a numerical basis, it appears that many of the transiently repopulating cells survive, since at days 1 and 2 post 5-FU there is only a slight reduction in the total numbers of cells within the lineage-negative, Sca-1–positive, Mac-1low/−, c-kit–positive population, which should include only a minority of long-term repopulating cells2 (Figs 3 and 4).
Within 10 days after treatment with 5-FU, the phenotypically defined stem-cell population has expanded approximately 10-fold, even though we (T.D.R., unpublished data, July 1994) and others3,17 have observed that the number of transplantable stem cells in the bone marrow does not appear to change over this period. Similarly, the expansion of phenotypically defined populations of stem cells after transplantation or32 growth factor treatment,33 or in aged mice,34 appears to correlate with the reduced efficiency of the population to reconstitute recipient animals. This has been interpreted as a defect in the homing properties of the overall stem-cell population, such that the majority of cells are true long-term reconstituting stem cells, but that they have an altered homing pattern and cannot efficiently migrate back to their proper microenvironment.34 Alternatively, it has been suggested that during the process of stem-cell expansion, many cells with only transiently reconstituting potential are formed that have the same surface phenotype as the long-term reconstituting stem cells.32 Here, we have shown that as early as 3 days after 5-FU treatment, at least half of the cells with a stem-cell phenotype are short-term repopulating cells. It is likely that many of these cells are expanded during the next several days, but do not contribute to the long-term reconstituting stem-cell pool. In addition, it has been observed that retroviral infection of phenotypically defined stem-cell populations isolated 8 days after 5-FU treatment resulted in the infection of mostly transiently repopulating progenitors, as shown by the rapidly declining expression of the reporter gene after reconstitution.13 A similar effect was reported by Silvassey et al,26 who found that progenitor populations purified 6 days after 5-FU treatment were not efficient at reconstituting myeloid cells beyond 10 weeks, suggesting that many of these cells were only transiently repopulating progenitors.
The functional changes that we observed in the stem-cell populations temporally correlated with changes in the expression of several important surface molecules. We were surprised to find that the expression of one of the major growth factor receptors on stem cells, c-kit,35 was reduced at least 10-fold within 2 days after the administration of 5-FU. The low level (or even lack) of surface expression of the c-kit molecule on progenitor populations after treatment with 5-FU has been observed previously,19,20 and has been interpreted as evidence that the most primitive stem-cell populations from untreated mice are also found in the c-kitlow/- population of the bone marrow,19,20 as others have suggested.36 Furthermore, it was suggested that the expression of c-kit was highest in the more committed progenitor populations and that the administration of 5-FU rapidly eliminated these c-kithigh progenitor cells revealing the cryptic c-kitlow population.19 However, in contrast, we saw no evidence of any significant repopulating potential in the c- kitlow/- population of WBM from normal mice (Fig 1C). We also demonstrated that the vast majority of the transplantable long-term reconstituting cells from normal marrow express high levels of c-kit (Fig 1C), consistent with that seen previously,2,27,37 38 and that stem cells are only found in the c-kitlow population after 5-FU treatment (Fig 1F ). Thus, we suggest that 5-FU treatment itself causes stem cells to lose expression of c-kit, rather than revealing a previously uncharacterized population. This is most evident from the analysis of c-kit expression in Fig 3, which shows a gradual loss of c-kit expression on the lineage-negative, Sca-1–positive population over the first 2 days after 5-FU treatment.
The mechanism for the loss of c-kit expression on stem cells after exposure to 5-FU is presently unknown. However, since it has been shown that factors such as interleukin-3 and granulocyte-macrophage colony-stimulating cells can downregulate the expression of c-kit on both mast cells and progenitor cells,39 40 the loss of c-kit may be triggered, in part, by the increased production of hematopoietic cytokines in response to the abrupt loss of most of the hematopoietic precursors in the bone marrow. Alternatively, the ligand for c-kit has been shown to modulate directly the cell-surface expression of c-kit.40 Although the reduction in c-kit expression does correlate with the induction of the cell cycle, it is unlikely that c-kit expression is regulated by the cell cycle, since in normal mice many of the transiently repopulating stem cells from normal bone marrow are in the cell cycle, but still express high levels of c-kit.
The other change that we have observed in the phenotype of long-term repopulating stem cells after 5-FU treatment is the induction of Mac-1 expression. We have observed that in untreated mice, long-term reconstituting stem cells can be found in the Mac-1–negative, as well as the Mac-1low populations. However, after 5-FU treatment, all of the stem-cell activity (both short- and long-term) is found in the Mac-1low population just as these cells are induced to cycle. Mac-1 is similarly expressed on long-term repopulating stem cells in the fetal liver, where virtually all the stem cells are rapidly cycling,11 and in the activated transiently repopulating population found in normal bone marrow.2 Although it was postulated that the low levels of Mac-1 expression on the short-term repopulating cells from bone marrow correlated with a change in the differentiative state of the stem-cell population,2 the induction of Mac-1 on stem cells may actually correlate with their activation state. Since the long-term stem cells from the fetal liver, as well as the transiently repopulating stem cells from normal bone marrow, are enriched for cycling cells, we suggest that the Mac-1 may be a marker of stem cells that are activated to go through cell division, rather than an indicator of commitment to differentiation. In addition, since Mac-1 is an adhesion molecule in the integrin family, it may play a functional role in the redistribution and/or repopulation of the stem-cell compartment.
In this study, we have shown that HSCs undergo several dramatic phenotypic changes as they are induced to cycle after 5-FU treatment, including the induction of Mac-1 and the reduction of c-kit expression. We suggest that these changes are manifestations of activation events that occur as the surviving population of HSCs responds to the loss of cycling hematopoietic progenitors. In addition, we have shown that primitive progenitors other than long-term reconstituting stem cells survive the cytotoxic effects of 5-FU and that these cells contribute to much of the expanded progenitor population during the recovery of the bone marrow. These results clarify the characteristics of both resting and self-renewing populations of stem cells and should help those involved in the transplantation and gene therapy of HSCs.
ACKNOWLEDGMENT
We thank Tim Knack and Jack Sun for help with flow cytometry and Frances Lund for critically reading the manuscript and for helpful suggestions during the course of this work.
Supported in part by National Institutes of Health Grant No. CA-42551 and by a grant to I.L.W. from Systemix/Sandoz. T.D.R. is a recipient of a Helen Hay Whitney Foundation Fellowship.
Address reprint requests to Troy Randall, PhD, c/o Weissman Lab, Department of Pathology, B265 Beckman Center, Stanford Medical School, Stanford, CA 94305.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal