Abstract
Recombinations between c-myc and immunoglobulin (Ig) sequences that typically occur in pristane-induced mouse plasmacytomas were detected in secondary lymphoid tissues from normal mice, chiefly in the gut-associated lymphoid tissue. Based on the analysis of recombination sequences as clonotypic markers, migration of c-myc recombination-positive cells was observed between Peyer's patches and into the intestine. Treatment of plasmacytoma-susceptible BALB/cAn mice with pristane induced proliferation and migration of these cells into mesenteric lymph node, spleen, and oil granuloma within 7 days. Plasmacytoma-resistant strains of mice (DBA/2N, C3H/HeJ, C57BL/6) differed in that (1) they harbored fewer clones (Ig/c-myc recombinations were detected in 33% of resistant mice versus 91% of BALB/cAn mice after pristane treatment); (2) Ig/c-myc-positive cells were rarely detected in the oil granuloma, and (3) c-myc recombined predominantly with the Ig α locus in BALB/cAn mice (72%), but with the Igμ locus in DBA/2N and in C57BL/6 (67%). The results demonstrate that normal mice generate a large number of lymphocytes with aberrant c-myc in intestinal tissues without developing tumors.
DEREGULATION of the transcription of c-myc is consistently found in pristane-induced BALB/cAn plasmacytomas. This mutation may be the crucial change in the oncogenesis of plasma cells, even though it is probably not sufficient by itself to transform plasma cells to neoplastic states (reviewed in Potter and Wiener1 ). Most frequently, transcriptional deregulation is caused by recombination with immunoglobulin (Ig) loci that replace the regulatory c-myc exon 1 by Ig heavy chain sequences (reviewed in Potter and Wiener1 and Mushinski2 ).
The tissue site in which the Ig/c-myc-recombined cells originate is not known. We previously described Ig/c-myc recombinations in oil granuloma (OG) tissues 30 days after the injection of pristane and long before the development of plasmacytomas.3-5 The mesenteric, pristane-induced OG is an inflammatory tissue that recruits phagocytic cells. These cells are capable of producing millimolar concentrations of genotoxic oxygen species.6 Therefore, it has been argued that the DNA damage caused by these substances could result in illegitimate recombinations. Alternatively, Ig/c-myc recombinations could be generated in lymphoid tissues during Ig isotype switching.
Most inbred strains of mice are resistant to plasmacytoma induction by pristane.1 Plasmacytoma resistant DBA/2N mice do harbor Ig/c-myc-rearranged cells in the OG,3 though at a lower frequency than susceptible BALB/cAn mice. In addition, cell clones with these recombinations were smaller in size in DBA/2N mice than clones found in BALB/cAn mice.3
Here we use nested polymerase chain reactions (PCR) to detect Ig/c-myc recombinations in different lymphoid tissues from both normal and pristane-treated mice that are susceptible or resistant to plasmacytoma development.
MATERIALS AND METHODS
Mice.The inbred strains used in this study: DBA/2N, C3H/HeJ, C57BL/6, and BALB/cAnPt, were bred and maintained in a conventional breeding colony protected by quarantine barrier under NCI contract no. NOI-CB-21075 at PerImmune, Rockville, MD. In addition, two BALB/cAn.DBA/2N congenic (C.D2) strains, C.D2-Idh1-Pep-3 and C.D2-Igh1c,7 were studied. C.D2-Idh1-Pep-3 carries a region of DBA/2N chromosome 1 on BALB/cAn background. The C.D2-Igh1c congenic was constructed by introgressively backcrossing the Ig heavy chain locus of DBA/2N onto BALB/cAn. The DBA/2N Ig complex locus was monitored by using restriction fragment differences in the V-regions. To confirm that this mouse carries the Igα switch region from DBA/2N, parts of that region were PCR amplified and were found to be identical in DBA/2N and C.D2-Igh1c mice but different from BALB/cAn. C.D2-Idh1-Pep-3 is susceptible to plasmacytoma induction; susceptibility data on C.D2-Igh1c are not available to date.
DNA preparation.We prepared DNA from a variety of different lymphatic tissues that included fetal liver, bone marrow, mesenteric lymph node, three to seven Peyer's patches (PPs), gut, omentum, spleen, mesentery, and mesenteric OG. The small intestine and colon, free of mesentery and PPs, was dissected into sectors of approximately 2 to 3 cm in length. Tissues were snap frozen in liquid nitrogen, ground, and lysed in 50 mmol/L Tris (pH 7.5), 50 mmol/L EDTA, 100 mmol/L NaCl, 160 μg/mL Proteinase K. One phenol and one chloroform extraction was followed by ethanol precipitation and rehydration in 100 mmol/L Tris (pH 8.0), 10 mmol/L EDTA.
PCR amplification.The use of different PCR primers that amplify recombinations from both aberrant chromosomes has been described.4 5 The repetitiveness of both the Igα and Igμ switch regions and their sequence similarity permitted us to design consensus oligonucleotides that anneal at multiple locations in both switch regions. Because PCR amplification with these primers yields Ig/c-myc products with an Ig portion of usually less than 500 bp, and products up to 2.5 kbp are generated, we were able to use one c-myc nested primer pair only that anneals at the end of the c-myc breakpoint cluster region. The sequences are: Ig1, 5′-AGC-TCA-TTC-CAG-CTC-AGC-TCA-GCC-T-3′; Ig2, 5′-AGC-TCA-GCT-CAG-CCT-ARC-CCA-GCT-C-3′; myc1, 5′-AGG - GAT - ACC - CGC - GGA - TCC - CAA - GTA - GGA - ATG - TGA -GG-3′; myc2, 5′-CCA-AGT-CAA-CGA-ATC-GGT-CAC-ATC-CCT-GTC-CCA-AT-3′. The PCR reaction mixture contained a final concentration of 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 2.5 mmol/L MgCl2 , gelatin at 0.1 mg/mL, each primer at 0.5 μmol/L, each dNTP at 200 μmol/L, and 500 ng genomic DNA in a volume of 50 μL. Cycling conditions involved a 5-minute initial denaturation at 95°C with the reaction subsequently being held at 80°C for the addition of 1.25 U of Taq polymerase. This was followed by 40 cycles of denaturation (15 seconds at 95°C), annealing (15 seconds at 65°C), and extension (between 30 seconds and 2 minutes at 72°C). For nested PCR, 1 μL reaction mixture from round one was used as a template for an additional round of the same 40 cycles. The main artifacts generated with this technique were Igα/Igμ tail-to-tail recombinations that were amplified with the Ig consensus primers only. Tissues were analyzed in triplicate. Approximately 65% of clonotypic Ig/c-myc recombinations could be amplified at least twice in independent PCR reactions using DNA template from the same tissue. All amplified products were size fractionated by agarose gel electrophoresis, purified (GeneClean from Bio 101, LaJolla, CA), cloned into pBluescript vector (Stratagene, LaJolla, CA) and sequenced using the Sequenase kit (USB, Cleveland, OH) or sequenced directly using Taq polymerase (Promega, Madison, WI).
RESULTS
Ig/c-myc recombinations in normal mice.In the untreated plasmacytoma-susceptible strains BALB/cAn and C.D2-Idh1-Pep-3, roughly half of the mice bore detectable Ig/c-myc recombinations in at least one tissue analyzed (Tables 1 and 2). The highest frequency was detected in the gut-associated lymphoid tissues (GALT): 37% of PPs and 27% of small and large intestinal samples were positive, while infrequent recombinations were found in mesenteric node and spleen. Consistently negative were mesentery, bone marrow, and fetal liver. By contrast, in the plasmacytoma-resistant strain DBA/2N, Ig/c-myc recombinations occurred in only 2 of 10 mice.
Frequency of Ig/c-myc Recombinations in Lymphoid Tissues of Inbred Strains of Mice
| Strain . | Susceptible to Plasmacytoma Induction . | No. of Mice Carrying Ig/c-myc . | Tissue-Specific Distribution . | Switch Region* . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | PP . | Mes/OG . | MLN . | Sp . | Gut . | Sα . | recSα . | Sμ . | ?† . |
| Normal mice | |||||||||||
| BALB/cAn | + | 7/12‡ | 3/9 | 0/8 | 1/8 | 1/8 | 8/30ρ | 8 | 1 | 3 | 0 |
| C.D2-Idh1-Pep3 | + | 6/10 | 4/10 | 0/10 | 3/10 | 0/10 | ND | 2 | 0 | 5 | 0 |
| DBA/2N | − | 2/10 | 0/10 | 0/10 | 1/10 | 1/10 | 0/10 | 0 | 0 | 1 | 1 |
| Day 7 post pristane | |||||||||||
| BALB/cAn | + | 20/22 | 9/181-155 | 9/18 | 3/19 | 10/22 | 19/30 | 27 | 3 | 10 | 2 |
| C.D2-Igh1c | ? | 7/10 | 7/10 | 1/10 | 3/10 | 0/10 | ND | 6 | 3 | 2 | 0 |
| DBA/2N | − | 7/21 | 6/18 | 0/18 | 0/18 | 0/21 | 1/30 | 1 | 1 | 5 | 0 |
| C57BL/6 | − | 3/14 | 1/11 | 0/12 | 0/12 | 0/14 | 3/30 | 0 | 0 | 2 | 1 |
| C3H/HeJ | − | 5/11 | 3/8 | 1/8 | 1/8 | 0/11 | 7/30 | 6 | 1 | 1 | 0 |
| Strain . | Susceptible to Plasmacytoma Induction . | No. of Mice Carrying Ig/c-myc . | Tissue-Specific Distribution . | Switch Region* . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | PP . | Mes/OG . | MLN . | Sp . | Gut . | Sα . | recSα . | Sμ . | ?† . |
| Normal mice | |||||||||||
| BALB/cAn | + | 7/12‡ | 3/9 | 0/8 | 1/8 | 1/8 | 8/30ρ | 8 | 1 | 3 | 0 |
| C.D2-Idh1-Pep3 | + | 6/10 | 4/10 | 0/10 | 3/10 | 0/10 | ND | 2 | 0 | 5 | 0 |
| DBA/2N | − | 2/10 | 0/10 | 0/10 | 1/10 | 1/10 | 0/10 | 0 | 0 | 1 | 1 |
| Day 7 post pristane | |||||||||||
| BALB/cAn | + | 20/22 | 9/181-155 | 9/18 | 3/19 | 10/22 | 19/30 | 27 | 3 | 10 | 2 |
| C.D2-Igh1c | ? | 7/10 | 7/10 | 1/10 | 3/10 | 0/10 | ND | 6 | 3 | 2 | 0 |
| DBA/2N | − | 7/21 | 6/18 | 0/18 | 0/18 | 0/21 | 1/30 | 1 | 1 | 5 | 0 |
| C57BL/6 | − | 3/14 | 1/11 | 0/12 | 0/12 | 0/14 | 3/30 | 0 | 0 | 2 | 1 |
| C3H/HeJ | − | 5/11 | 3/8 | 1/8 | 1/8 | 0/11 | 7/30 | 6 | 1 | 1 | 0 |
Abbreviations: PP, Peyer's patches; Mes/OG, mesentery/mesenteric OG; MLN, mesenteric lymph node; Sp, spleen; ND, not done.
The Ig locus that is fused to c-myc is shown. The PCR technique used detects recombinations between c-myc and Sα or Sμ. Rec Sα: an additional rearrangement between Sα and another Ig switch region was amplified adjacent to the Ig/c-myc recombination. The difference between the number of positive tissues and the number of recombinations is caused by the amplification of identical Ig/c-myc products in different tissues and by the detection of more than a single clone in some cases.
In four cases, the sequence that was joined to c-myc could not be aligned to germline.
Numbers represent positive mice or tissues per total number tested.
ρ In these mice, the gut was dissected into 10 different pieces.
One c-myc recombination found in the Peyer's patches was detected in the omentum as well. DNA was prepared from 10 omenta. The study also included eight bone marrows and six fetal livers that were found to be negative.
Sequences of Recombinations Between c-myc and Ig Loci
| BALB/cAn, day 0 . | C.D2-Idh1-Pep-3, day 0 . |
|---|---|
| GCCCAGCTAAGCCCAAACTA GTTCCGTAACAGCTGCTACC NO α | TCACCCCAGCTCAGCTCAGC TTGAAGGCTGGATTTCCTTT PP μ |
| AGCTCAGCTCAGCTCAGCTC GGCTAGCGCAGTGAGGAGAA SP μ | CTCATCCCAGCTCATTCCAG CTCGAGCTGTTTGAAGGCTG PP α |
| TTTAGCTCAGCTCAGCCTAG GGTGGTCTTTCCCTGTGTTC PP α | GCTCACCCTAGCTCAGCTCA GCCGGTTGGACATTCTTGCT NO μ |
| TCCCAGCTCATTCCAGGTCA TGGAGAAGGGATTACCTTTT PP α | GCTCACCCCAGCTCAGCTCA GTTCCCGAGGTTACTATGGG NO μ |
| CCCAGCTCAGCTCACCCCAC CTTCTGACTTACCAGTCTCT PP μ | GTTCAACCCACCAGCTCACA GAGAGGGCATTTAAATTTCA NO α |
| AGTTTAGCTAGTCCAGTCCA CTTCTGACTTACCAGTCTCT GU α | AGCCCAGCTCACCCCAGCTC AGTTGGGGTAGGCTGGGGTA PP μ |
| CCAGCTTACCCCAACTCACC TTGCGTTTGGGAGCGAGAAG GU μ/α | CCCCATCTCAGCTACTCCAG AGTAAGCACAGATCTGGTGG PP μ |
| CTAGCTTACCCCAACTCACC TTGCGTTTGGGAGCGAGAAG GU μ | DBA/2N, day 0 |
| TCCATTCTAGCCTAGCCCAG ACCAGTCTCTGAGAGGGCAT GU α | ACTCCAGCCCAGCTCAGCTC CATTTCTGACAGCCTGGGAC SP μ |
| CCTAGCCCAGACCATGCCAG TTGACACTTTTCTCAAGAGT GU α | AGCCCAGCCCAACTCACCCA TCTGCCTCTGCCCGCGATCA NO ? |
| CACACCAGCCCAGTCCAGCC TAGTTGGGGTAGGCTGGGGT GU α | |
| TCACACCAGCCAGCCCAGCC TCTGGTGGTCTTTCCCTGTG GU α | |
| BALB/cAn, day 7 | C.D2-Igh1c, day 7 |
| TCTCAGCCAAGCTTAGCTCA GACACGGAGGTCGTCCCGCC OG α | AGTATTAGGGACCAGTCCTT AGGGGCTTTCTTTCCGAGCC PP γ2B /α |
| AGCCTAGCTCAGCTCAGCTC ATCTGCGAGCCAGGACAGGA OG μ | ATCCCAGCTCATTCCAGCTC ATCCTGAGGTCTTTGGAGAA LN α |
| GGGAGTATTAGGGACCAGTC CTTCTGACTTACCAGTCTCT OG γ2B /α | CCTATTCCAGCCTAGCTTAG TTTTCTCAAGAGTAGTTGGG LN α |
| CCCAGTTCAGCTCACCCCAG CTTGTCAAGATGACAGAGGA SP μ | CCAGCTCATTCCAGCTCAGC CAGGACTCCCCAGGCTCCGG PP α |
| ACCCAGCTCAGCTCAGCTCA GCTTTCTTACATTAATTGAT PP/LN μ | TCACCCCAGCTCACCCCAGC TGTTATTTCTGCGTCTTGAA PP μ |
| TCAGATCAGCTCACCCCAAC ACAGCCTGGGACCGACACGG SP μ | CAGCCCATCCCAGATCAATC TGGAAACCCCAGTAAGTACA LN ?/α |
| CAGCCCAGCCCAACCTATTC CAGCCTGGGACCGACACGGA SP α | TCACCCCAGCTCAGCCCAGA CGCCCACTCTCCCCAACCCT PP μ |
| CAGCCTAGCTCAGCTCATCC TAGATCTGAGTCGGGGTAGA OG α | CTCAGCCTAACCCAGCTCAC AAACCCCAGTAAGCACAGAT PP α |
| CTAGCTAAGCCCAGCTCAGC TACCAGTCTCTGAGAGGGCA PP ? | TCAGTTTATTCCAGTCTAGT TAGCTTCTGACTTACCAGTC PP ?/α |
| TCATTCATATCCAGAGCATC GCTACATTAATTGATATGTG PP α | ATTCCAGCTCAGCACAACCC GTTGGCTAGCGCAGTGAGGA OG α |
| TATTCCATCTCATTCCAGCT CAGTCTTTCTTCCATTCCTG OG/SP α | TCCATCCAGCCTTGCTCACT TTTGCTACATTAATTGATAT PP α |
| TCAGCTCAGCCTAGCCCAGC TCTGCGTCTTGAATGTAGCG PP α | C57BL/6, day 7 |
| CTAGCTCAGCCCATTCCAGC TCTGAGTCGGGGTAGAGCGA SP α | AGCTCAGCTCAGCTCACCCC AGCTTCTGACTTACCAGTCT PP μ |
| ATCTCATTCCAGCTCAGCTC TCTGAGTCGGGGTAGAGCGA SP α | ACACCAGCCAGCCCAGCTCA TTTGTCTATTTGGGGACAGT GU ? |
| CCAGCCCAGCCTTTTCCAGG CTTTTAAGAAGTTGCTATTT PP α | CCCCAGCTCAGCTCAGCTCA CTCCGTAGCTTCTGACTTAC GU μ |
| CAATCTGGTTCAGCCCAGAC CAGCCTGGGACCGACACGGA OG ε/α | DBA/2N, day 7 |
| GCTCATTCCAGCTCAGCTCA CAGTCTCTGAGAGGGCATTT SP α | CATCCCAGCTTACCCCAGCT CAGCCTGGGACCGACACGGA PP μ |
| ATTCCAGCTCAGCACAACCC GTTGGCTAGCGCAGTGAGGA PP α | GCTCAGTCCAGCTCAGCCCA GCTTTTGACACTTTTCTCAA PP μ |
| GCCTAACCCAGCTAACACTA TGTTACATTAATTGATATGT OG α | CTCATATCAGCTCAGAACAG TTCTGACTTAACAGTCTCTG PP μ |
| CCAGTGTAGGCAGTAGAGTT ACGGTGACCCGGCCGGTTGG OG μ | ATAGCTGAGCTCACCCCAGC TAGGCTGGGGTAGATCTGAG PP μ |
| CCAGCCTAGCTCAGCTCAAA AGGCTGGGGTAGATCTGAGT PP α | TAGCCCAGCTCACACTATGT TACATTAATTGATATGTGTC PP ?/α |
| AGCTCAGCTCACCCCAGCTC AGAAAGCCCTTGGAATCCTG PP μ | GTCCAGCTCAGCCCAGCTCC ACAGAGGAAAGGGGAAGGGA PP μ |
| CAGCTCACACCAGCCCAGCC CAAGAGTAGTTGGGGTAGGC PP α | GCTCATCCCAGCTCATTCCA CTGACGCTGACCCGGCCGGT GU α |
| CACTCCAGCTCAGCTCAGCC CAGTCTCTGAGAGGGCATTT LN μ | C3H/HeJ, day 7 |
| AGCTCAGCCTAGCCCAGCTC CCTTTGGGCGTTGGAAACCC OG α | TCCAGCTCATCCCAGCCTGG TTTTGACACTTTTCTCAAGA PP α |
| CCTATTCCAGCCTAGCTCAG CTTTCTGCGTCTTGAATGTA SP α | AGCTCACCCCAACTCGGCTC GTAGCGGCCGGTTAGGACAG LN μ |
| CATCCTAGCTCATTCCAGCT CCAGTCTCTGAGAGGGCATT GU α | GTTTATCCTAGTCCATCGCA GCTTTGCTACATTAATTGAT PP α |
| CCTATTCCAGCCTAGCTCAG CTTGGAATCCTGAGGTCTTT GU α | GCCTATCCAGCCTAGCCCAG TATGTGTCCTTTGAGGGGTC GU α |
| TGTCCAGCTCTGCTCAGCCC ATATGTGTCCTTTGAGGGGT GU α | AGCCCAGCCCAGCCCTGGTT GGGCATTTAAATTTCAGCTT GU ?/α |
| AGCTCGCACCAGCCCAGCCC GTGTTCTTTCTGCGTCTTGA GU α | CAGCCTAGCTCTGCTCATCA GCCTCTGCCCGCGATCAGCT GU α |
| TCAGCTCAGCTCAGCTCAGC TCTGAGTCGGGGTAGAGCGA GU α | CTCTCCTCTCCTCTCCTCTC CTACTTGGGGACAGTGTTCT GU α |
| AGCTCACCCCAGCTCAGCTC AGTTGGGGTAGGCTGGGGTA GU μ | AGCCTATTCCAGCCTAGCTA ATGGGCTGACGTGACCCGGC PP/OG α |
| CCCCAGCTCAGCTCACCCCA GGGTCAAACCGGGAGGTCGC GU μ | |
| TATCCCAGCTCAGCACATCC TGGCTAGCGCAGTGAGGAGA GU α | |
| TCCTCTCCTCTCCTCTCCTT CCAGTCTCTGAGAGGGCATT GU α | |
| AACACAGCCCAGCCCAGCTC CCTTTGAGGGGTCAAACCGG GU ? | |
| ATTTTAGAAACGCTCAGAGA AGCTTGGTGCATTTCTGACA GU μ | |
| TCCCAGCTCATTCCAGGTCA TTGGAGAAGGGATTACCTTT SP α | |
| CTCCTAGCTGTTAGGTCTGG TCCTGAAAAGAGCTCCTCGA GU γ2B /α | |
| TAGCACACACCAGCCCAGCC CAGTGAGGAGAAGCAAAATT GU α | |
| CCCAGCCCAACCTATTCCAG CCCTCGGCGGGGAGAGGGAA GU α | |
| TCCAGCTCAGCCCAGCCTAA CCCGCGATCAGCTCTCCTGA LN/SP α |
| BALB/cAn, day 0 . | C.D2-Idh1-Pep-3, day 0 . |
|---|---|
| GCCCAGCTAAGCCCAAACTA GTTCCGTAACAGCTGCTACC NO α | TCACCCCAGCTCAGCTCAGC TTGAAGGCTGGATTTCCTTT PP μ |
| AGCTCAGCTCAGCTCAGCTC GGCTAGCGCAGTGAGGAGAA SP μ | CTCATCCCAGCTCATTCCAG CTCGAGCTGTTTGAAGGCTG PP α |
| TTTAGCTCAGCTCAGCCTAG GGTGGTCTTTCCCTGTGTTC PP α | GCTCACCCTAGCTCAGCTCA GCCGGTTGGACATTCTTGCT NO μ |
| TCCCAGCTCATTCCAGGTCA TGGAGAAGGGATTACCTTTT PP α | GCTCACCCCAGCTCAGCTCA GTTCCCGAGGTTACTATGGG NO μ |
| CCCAGCTCAGCTCACCCCAC CTTCTGACTTACCAGTCTCT PP μ | GTTCAACCCACCAGCTCACA GAGAGGGCATTTAAATTTCA NO α |
| AGTTTAGCTAGTCCAGTCCA CTTCTGACTTACCAGTCTCT GU α | AGCCCAGCTCACCCCAGCTC AGTTGGGGTAGGCTGGGGTA PP μ |
| CCAGCTTACCCCAACTCACC TTGCGTTTGGGAGCGAGAAG GU μ/α | CCCCATCTCAGCTACTCCAG AGTAAGCACAGATCTGGTGG PP μ |
| CTAGCTTACCCCAACTCACC TTGCGTTTGGGAGCGAGAAG GU μ | DBA/2N, day 0 |
| TCCATTCTAGCCTAGCCCAG ACCAGTCTCTGAGAGGGCAT GU α | ACTCCAGCCCAGCTCAGCTC CATTTCTGACAGCCTGGGAC SP μ |
| CCTAGCCCAGACCATGCCAG TTGACACTTTTCTCAAGAGT GU α | AGCCCAGCCCAACTCACCCA TCTGCCTCTGCCCGCGATCA NO ? |
| CACACCAGCCCAGTCCAGCC TAGTTGGGGTAGGCTGGGGT GU α | |
| TCACACCAGCCAGCCCAGCC TCTGGTGGTCTTTCCCTGTG GU α | |
| BALB/cAn, day 7 | C.D2-Igh1c, day 7 |
| TCTCAGCCAAGCTTAGCTCA GACACGGAGGTCGTCCCGCC OG α | AGTATTAGGGACCAGTCCTT AGGGGCTTTCTTTCCGAGCC PP γ2B /α |
| AGCCTAGCTCAGCTCAGCTC ATCTGCGAGCCAGGACAGGA OG μ | ATCCCAGCTCATTCCAGCTC ATCCTGAGGTCTTTGGAGAA LN α |
| GGGAGTATTAGGGACCAGTC CTTCTGACTTACCAGTCTCT OG γ2B /α | CCTATTCCAGCCTAGCTTAG TTTTCTCAAGAGTAGTTGGG LN α |
| CCCAGTTCAGCTCACCCCAG CTTGTCAAGATGACAGAGGA SP μ | CCAGCTCATTCCAGCTCAGC CAGGACTCCCCAGGCTCCGG PP α |
| ACCCAGCTCAGCTCAGCTCA GCTTTCTTACATTAATTGAT PP/LN μ | TCACCCCAGCTCACCCCAGC TGTTATTTCTGCGTCTTGAA PP μ |
| TCAGATCAGCTCACCCCAAC ACAGCCTGGGACCGACACGG SP μ | CAGCCCATCCCAGATCAATC TGGAAACCCCAGTAAGTACA LN ?/α |
| CAGCCCAGCCCAACCTATTC CAGCCTGGGACCGACACGGA SP α | TCACCCCAGCTCAGCCCAGA CGCCCACTCTCCCCAACCCT PP μ |
| CAGCCTAGCTCAGCTCATCC TAGATCTGAGTCGGGGTAGA OG α | CTCAGCCTAACCCAGCTCAC AAACCCCAGTAAGCACAGAT PP α |
| CTAGCTAAGCCCAGCTCAGC TACCAGTCTCTGAGAGGGCA PP ? | TCAGTTTATTCCAGTCTAGT TAGCTTCTGACTTACCAGTC PP ?/α |
| TCATTCATATCCAGAGCATC GCTACATTAATTGATATGTG PP α | ATTCCAGCTCAGCACAACCC GTTGGCTAGCGCAGTGAGGA OG α |
| TATTCCATCTCATTCCAGCT CAGTCTTTCTTCCATTCCTG OG/SP α | TCCATCCAGCCTTGCTCACT TTTGCTACATTAATTGATAT PP α |
| TCAGCTCAGCCTAGCCCAGC TCTGCGTCTTGAATGTAGCG PP α | C57BL/6, day 7 |
| CTAGCTCAGCCCATTCCAGC TCTGAGTCGGGGTAGAGCGA SP α | AGCTCAGCTCAGCTCACCCC AGCTTCTGACTTACCAGTCT PP μ |
| ATCTCATTCCAGCTCAGCTC TCTGAGTCGGGGTAGAGCGA SP α | ACACCAGCCAGCCCAGCTCA TTTGTCTATTTGGGGACAGT GU ? |
| CCAGCCCAGCCTTTTCCAGG CTTTTAAGAAGTTGCTATTT PP α | CCCCAGCTCAGCTCAGCTCA CTCCGTAGCTTCTGACTTAC GU μ |
| CAATCTGGTTCAGCCCAGAC CAGCCTGGGACCGACACGGA OG ε/α | DBA/2N, day 7 |
| GCTCATTCCAGCTCAGCTCA CAGTCTCTGAGAGGGCATTT SP α | CATCCCAGCTTACCCCAGCT CAGCCTGGGACCGACACGGA PP μ |
| ATTCCAGCTCAGCACAACCC GTTGGCTAGCGCAGTGAGGA PP α | GCTCAGTCCAGCTCAGCCCA GCTTTTGACACTTTTCTCAA PP μ |
| GCCTAACCCAGCTAACACTA TGTTACATTAATTGATATGT OG α | CTCATATCAGCTCAGAACAG TTCTGACTTAACAGTCTCTG PP μ |
| CCAGTGTAGGCAGTAGAGTT ACGGTGACCCGGCCGGTTGG OG μ | ATAGCTGAGCTCACCCCAGC TAGGCTGGGGTAGATCTGAG PP μ |
| CCAGCCTAGCTCAGCTCAAA AGGCTGGGGTAGATCTGAGT PP α | TAGCCCAGCTCACACTATGT TACATTAATTGATATGTGTC PP ?/α |
| AGCTCAGCTCACCCCAGCTC AGAAAGCCCTTGGAATCCTG PP μ | GTCCAGCTCAGCCCAGCTCC ACAGAGGAAAGGGGAAGGGA PP μ |
| CAGCTCACACCAGCCCAGCC CAAGAGTAGTTGGGGTAGGC PP α | GCTCATCCCAGCTCATTCCA CTGACGCTGACCCGGCCGGT GU α |
| CACTCCAGCTCAGCTCAGCC CAGTCTCTGAGAGGGCATTT LN μ | C3H/HeJ, day 7 |
| AGCTCAGCCTAGCCCAGCTC CCTTTGGGCGTTGGAAACCC OG α | TCCAGCTCATCCCAGCCTGG TTTTGACACTTTTCTCAAGA PP α |
| CCTATTCCAGCCTAGCTCAG CTTTCTGCGTCTTGAATGTA SP α | AGCTCACCCCAACTCGGCTC GTAGCGGCCGGTTAGGACAG LN μ |
| CATCCTAGCTCATTCCAGCT CCAGTCTCTGAGAGGGCATT GU α | GTTTATCCTAGTCCATCGCA GCTTTGCTACATTAATTGAT PP α |
| CCTATTCCAGCCTAGCTCAG CTTGGAATCCTGAGGTCTTT GU α | GCCTATCCAGCCTAGCCCAG TATGTGTCCTTTGAGGGGTC GU α |
| TGTCCAGCTCTGCTCAGCCC ATATGTGTCCTTTGAGGGGT GU α | AGCCCAGCCCAGCCCTGGTT GGGCATTTAAATTTCAGCTT GU ?/α |
| AGCTCGCACCAGCCCAGCCC GTGTTCTTTCTGCGTCTTGA GU α | CAGCCTAGCTCTGCTCATCA GCCTCTGCCCGCGATCAGCT GU α |
| TCAGCTCAGCTCAGCTCAGC TCTGAGTCGGGGTAGAGCGA GU α | CTCTCCTCTCCTCTCCTCTC CTACTTGGGGACAGTGTTCT GU α |
| AGCTCACCCCAGCTCAGCTC AGTTGGGGTAGGCTGGGGTA GU μ | AGCCTATTCCAGCCTAGCTA ATGGGCTGACGTGACCCGGC PP/OG α |
| CCCCAGCTCAGCTCACCCCA GGGTCAAACCGGGAGGTCGC GU μ | |
| TATCCCAGCTCAGCACATCC TGGCTAGCGCAGTGAGGAGA GU α | |
| TCCTCTCCTCTCCTCTCCTT CCAGTCTCTGAGAGGGCATT GU α | |
| AACACAGCCCAGCCCAGCTC CCTTTGAGGGGTCAAACCGG GU ? | |
| ATTTTAGAAACGCTCAGAGA AGCTTGGTGCATTTCTGACA GU μ | |
| TCCCAGCTCATTCCAGGTCA TTGGAGAAGGGATTACCTTT SP α | |
| CTCCTAGCTGTTAGGTCTGG TCCTGAAAAGAGCTCCTCGA GU γ2B /α | |
| TAGCACACACCAGCCCAGCC CAGTGAGGAGAAGCAAAATT GU α | |
| CCCAGCCCAACCTATTCCAG CCCTCGGCGGGGAGAGGGAA GU α | |
| TCCAGCTCAGCCCAGCCTAA CCCGCGATCAGCTCTCCTGA LN/SP α |
Shown are 20 bp of Ig sequences followed by 20 bp of c-myc. Also given are the tissues where these recombinations were detected and the Ig locus that is involved in the rearrangement.
Abbreviations: PP, Peyer's patches; SP, spleen; GU, gut; OG, mesenteric oil granuloma; ?, the sequence that was joined to c-myc could not be aligned to germline.
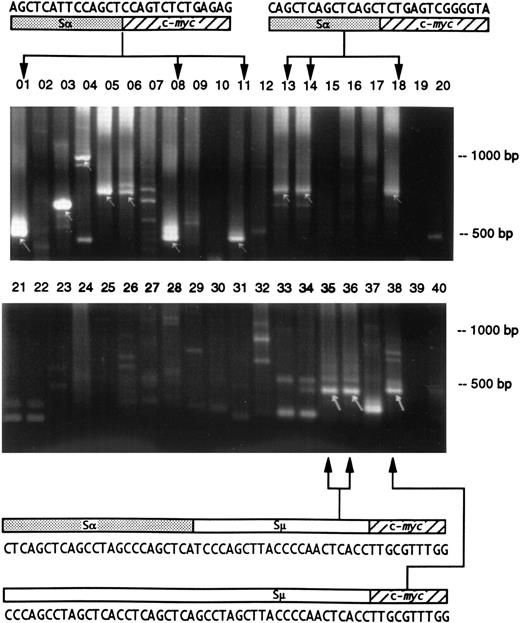
Studies of segments of the gut tissue showed that cells with identical and unique Ig/c-myc junction sequences were found in distant and separate sectors (Fig 1 and Table 3). In addition, two separate PPs had clones with identical Ig/c-myc junctions (not shown). Because recombination breakpoints and junctions are unique, proliferation and migration of sister cells with Ig/c-myc recombinations must have occurred. Interestingly, one mouse carried two clones of cells with an identical Igμ/c-myc junction; one of the clones, however, had undergone an additional switch recombination between Igμ 3′ of the fusion with c-myc and Igα, while the other had only Igμ sequence (Fig 1). This indicates that Igμ/c-myc hybrids can be a substrate for a subsequent Ig isotype switch recombinational event.
Detection of Ig/c-myc recombinations in different regions of the intestine in two BALB/cAn mice. The gut was sectioned into 10 fragments and the DNA preparations were analyzed by duplicate PCR amplifications and electrophoresed on 2% agarose gels. The presence of Ig/c-myc recombinations are indicated by arrows. The upper gel shows samples from a mouse 7 days after pristane treatment; identical products were obtained in lanes 01 (duodenum), 08 (proximal ileum), and 11 (middle ileum) and in 13, 14 (middle ileum), and 18 (distal ileum) indicating a common origin of these cells. In the second, untreated mouse (lower gel), clones from distal (lanes 35 and 36) and terminal ileum (lane 38) are related by virtue of their identical Igμ/c-myc recombination sequence. The upper sequence shows 24 bp of switchα (GenBank “MUSIALPHA” #2293-2270) followed by switchμ (“MUSIGCD07” #5550-5529) and c-myc. In the lower recombination, the same 18 bp of switchμ is preceded by a sequence that has a switchμ-typical pattern of pentamers, but that could not be aligned to germline. It originates most likely from the part of switchμ that has not yet been sequenced. The two recombinations indicate that the Igμ/c-myc rearrangement was followed by additional recombinations.
Detection of Ig/c-myc recombinations in different regions of the intestine in two BALB/cAn mice. The gut was sectioned into 10 fragments and the DNA preparations were analyzed by duplicate PCR amplifications and electrophoresed on 2% agarose gels. The presence of Ig/c-myc recombinations are indicated by arrows. The upper gel shows samples from a mouse 7 days after pristane treatment; identical products were obtained in lanes 01 (duodenum), 08 (proximal ileum), and 11 (middle ileum) and in 13, 14 (middle ileum), and 18 (distal ileum) indicating a common origin of these cells. In the second, untreated mouse (lower gel), clones from distal (lanes 35 and 36) and terminal ileum (lane 38) are related by virtue of their identical Igμ/c-myc recombination sequence. The upper sequence shows 24 bp of switchα (GenBank “MUSIALPHA” #2293-2270) followed by switchμ (“MUSIGCD07” #5550-5529) and c-myc. In the lower recombination, the same 18 bp of switchμ is preceded by a sequence that has a switchμ-typical pattern of pentamers, but that could not be aligned to germline. It originates most likely from the part of switchμ that has not yet been sequenced. The two recombinations indicate that the Igμ/c-myc rearrangement was followed by additional recombinations.
Detection of Clones With Identical Ig/c-mycHybrid Sequences in Different Regions of the Intestine
| Strain . | Pristane3-150 . | Intestinal Distribution3-151 . | |||
|---|---|---|---|---|---|
| . | . | Duodenum . | Jejunum . | Ileum . | Colon . |
| BALB/cAn3-152 | − | − | x − | − − − − − x | − |
| BALB/cAn | − | − | − − | − − − − x x | − |
| BALB/cAn | + | x | − − | x − x − − − | − |
| BALB/cAn | + | − | − − | − − − x − x | − |
| BALB/cAn | + | x | − − | − − − x x − | − |
| C3H/HeJ | + | − | − − | − − − x x x | − |
| C3H/HeJ | + | − | − x | x − − − − − | − |
| C57BL/6 | + | x | − − | − − − − x − | − |
| Strain . | Pristane3-150 . | Intestinal Distribution3-151 . | |||
|---|---|---|---|---|---|
| . | . | Duodenum . | Jejunum . | Ileum . | Colon . |
| BALB/cAn3-152 | − | − | x − | − − − − − x | − |
| BALB/cAn | − | − | − − | − − − − x x | − |
| BALB/cAn | + | x | − − | x − x − − − | − |
| BALB/cAn | + | − | − − | − − − x − x | − |
| BALB/cAn | + | x | − − | − − − x x − | − |
| C3H/HeJ | + | − | − − | − − − x x x | − |
| C3H/HeJ | + | − | − x | x − − − − − | − |
| C57BL/6 | + | x | − − | − − − − x − | − |
The PCR amplification of such a recombination is marked by a cross. Tissues that were negative for that particular clone could harbor cells with an unrelated Ig/c-myc sequence. Note that identical Ig/c-myc sequences were amplified from noncontiguous intestinal samples in several cases. Therefore, a cross-contamination during the dissection appears to be unlikely.
Before (−) or 7 days after (+) the injection of pristane.
DNA preparations were made from the entire duodenum, two pieces of the jejunum, six pieces of the ileum, and from the entire colon.
Each line represents a single clone of cells with identical Ig/c-myc sequences that was detected in different intestinal DNA preparations.
Tissue distribution of cells with Ig/c-myc recombinations after pristane treatment in BALB/cAn mice.The treatment of BALB/cAn mice with pristane 7 days before investigation increased the number of detectable Ig/c-myc recombinations in the following ways (Table 1). Firstly, the frequency of Ig/c-myc-positive cell clones increased so that nearly all mice (20 of 22) were found positive. Secondly, Ig/c-myc junctions were found in the developing mesenteric OG suggesting that cells carrying Ig/c-myc recombinations had migrated into that tissue. The mobility of cells carrying Ig/c-myc recombinations is further evidenced by the amplification of identical recombination sequences from (1) PPs and mesenteric lymph node in one BALB/cAn mouse; (2) PPs and omentum in another; (3) mesenteric node and spleen in a third; and (4) spleen and mesenteric OG in a fourth mouse (Fig 2). Nearly half of the spleens examined (10 of 22) contained a clone with Ig/c-myc recombinations.
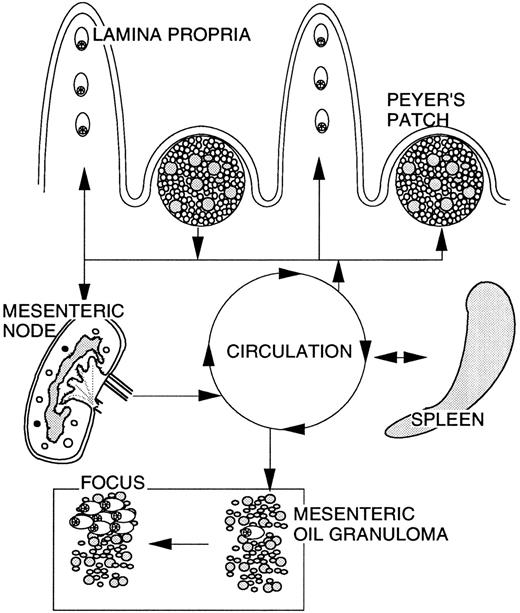
Hypothetical scheme of the migratory pathways of cells with Ig/c-myc recombinations in BALB/cAn mice. In untreated mice, cells carrying these recombinations are mainly found in the GALT and migrate between PPs and into the intestine where differentiation into plasma cells occurs. Following treatment with pristane, cells traffic between PPs, mesenteric lymph node, spleen, and into the developing OG. The environment of the granuloma causes an expansion of cells with Ig/c-myc recombinations and differentiation into plasma cells leading to the development of plasmacytic foci.1 This pristane-induced migration was mainly observed in BALB/cAn, whereas C57BL/6 and C3H/HeJ mice showed migration only within the GALT. The trafficking scheme is based on the detection of clonotypic c-myc recombination sequences in different organs.
Hypothetical scheme of the migratory pathways of cells with Ig/c-myc recombinations in BALB/cAn mice. In untreated mice, cells carrying these recombinations are mainly found in the GALT and migrate between PPs and into the intestine where differentiation into plasma cells occurs. Following treatment with pristane, cells traffic between PPs, mesenteric lymph node, spleen, and into the developing OG. The environment of the granuloma causes an expansion of cells with Ig/c-myc recombinations and differentiation into plasma cells leading to the development of plasmacytic foci.1 This pristane-induced migration was mainly observed in BALB/cAn, whereas C57BL/6 and C3H/HeJ mice showed migration only within the GALT. The trafficking scheme is based on the detection of clonotypic c-myc recombination sequences in different organs.
Ig/c-myc recombinations in plasmacytoma-resistant mice.Most inbred strains of mice do not develop plasmacytomas after treatment with pristane.1 To study whether differences in the occurrence of cells carrying Ig/c-myc recombinations coincide with the phenotype of susceptibility, we examined a variety of different mouse strains (Table 1). Three characteristics were found to be different in mice resistant to plasmacytoma induction. First, the number of cell clones with Ig/c-myc recombinations was lower: c-myc recombinations were found in 15 of 46 resistant mice versus 20 of 22 BALB/cAn mice. Second, the tissue distribution showed pronounced differences in resistant mice: no recombinations were detected in the developing OG with the exception of one C3H/HeJ mouse, and spleen tissue from all resistant mice examined lacked detectable Ig/c-myc recombination-positive cell clones after treatment with pristane. Finally, susceptible strains of mice (BALB/cAn and C.D2-Idh1-Pep-3) preferentially use Igα as the recombination partner for c-myc (41 of 61, Table 1). In contrast, in two resistant mouse strains, DBA/2N and C57BL/6, Igμ is more frequently involved in Ig/c-myc rearrangements (8 of 12). Indeed, only two fusions with Igα were detected in these resistant animals. However, the preference for Igμ may not be a general feature of all resistant strains, since C3H/HeJ mice resembled BALB/cAn in the usage of the Igα locus. Four Ig sequences could not be aligned to a known germline locus.
Differences in the involvement of Igμ versus Igα in fusions with c-myc could be caused by trans-acting factors or by sequence differences in the Ig locus. To distinguish these, congenic mice with the DBA/2N Ig heavy chain locus on the genetic BALB/cAn background (C.D2-Igh1c) were analyzed. The pattern of switch regions juxtaposed to c-myc in C.D2-Igh1c resembled that of BALB/cAn mice (9 Igα v 2 Igμ, Table 1). Therefore, the Igμ/c-myc preference in DBA/2N was not caused by allele-specific Ig sequences.
DISCUSSION
The dependence of trans-chromosomal recombinations on exogenous genotoxins generated during the host response to pristane has been a matter of debate.6 Most of our previous studies focused on mesenteric OG tissues 30 days after the injection of pristane when a well developed OG was in place. Here we report for the first time the detection of Ig/c-myc recombinations in lymphoid tissues from untreated mice. Recombinations in untreated mice were detected in spleen, PPs, gut, and in the mesenteric lymph node. Therefore, Ig/c-myc recombinations can be generated in secondary lymphatic organs without prior exposure to pristane.
Dilution experiments have shown that between 100 and 1,000 cells with recombinations have to be present in a tissue to be detected by PCR.3,5 This high frequency of recombination-positive cells in the PPs and in other gut tissues suggests that the mucosa-associated lymphoid tissue may be a site where Ig/c-myc rearrangements occur, and where cells bearing these recombinations proliferate. Ig/c-myc recombination-positive lymphocytes appear to migrate between PPs and into the lamina propria of the gut as evidenced by the detection of related cells in different parts of the GALT. The high turnover of B lymphocytes in the GALT together with the commitment of these cells to recombining the Igα region8 9 may be the basis for initiating plasmacytoma precursor cells. Neither mesentery nor omentum appeared to be primary locations for generating Ig/c-myc rearrangements, as no recombinations were detected in either tissue of normal mice.
In response to treatment with pristane, recombination-positive cells were found in locations that follow the migratory pathway of PP lymphocytes leading through the intestinal wall and mesenteric lymphatics into the mesenteric lymph node and further through the thoracic duct into the venous circulation.10 A large number of these cells appear to settle in the developing mesenteric OG in plasmacytoma-susceptible mice. Further evidence that cells with Ig/c-myc recombinations migrate was provided by the finding that identical recombination sequences were amplified in PPs and mesenteric lymph node, PPs and omentum, mesenteric node and spleen, and in spleen and OG (Fig 2, Table 2).
The distribution of Ig/c-myc-carrying cells shortly after treatment with pristane was different in mice that are resistant to pristane-induced plasmacytomagenesis: (1) in 13 of 15 positive mice, Ig/c-myc recombined clones were confined to the GALT; (2) c-myc recombination-positive cells were found in the OG in only one of the 38 resistant mice; (3) the frequency of Ig/c-myc recombinations did not increase in the spleens.
Another finding that distinguishes susceptible from resistant mice is the use of different switch regions in the Ig/c-myc junctions. Plasmacytoma cells typically have the Igα switch region joined to c-myc. The presence of enhancers downstream of the Igα locus can lead to transcriptional upregulation of the truncated c-myc gene that is joined to Igα.11 It is possible that this enhancer is less active in Igμ/c-myc recombinations where it is more than 100 kbp further from the c-myc sequence. The low frequency of c-myc recombinations with the Igα locus in plasmacytoma-resistant strains (with the exception of C3H/HeJ mice) could be caused by two mechanisms. In one, the rate of generating Igα/c-myc recombinations could be different between the inbred strains. However, a high proportion of Ig/c-myc recombinations involving the Igα region was observed in the C.D2-Igh1c mice that carry the DBA/2N Ig heavy chain locus. This finding indicates that the structure of the DBA/2N Igα switch region does not inhibit Ig/c-myc recombinations, but does not rule out the possibility that allelic trans-acting factors that render specific switch regions accessible for a recombination with c-myc could be important genetic factors in generating these rearrangements. Alternatively, BALB/cAn, but not DBA/2N cells, may tolerate abnormal c-myc expression caused by the proximity of the Igα 3′ enhancer in Igα/c-myc recombinations.
The differences in the occurrence of cells with Ig/c-myc recombinations could comprise a major determinant of the plasmacytoma susceptibility phenotype. The specifics of c-myc deregulation in BALB/cAn mice may lead to a more pronounced expansion of these cells than in resistant strains and could enlarge the pool of cells available for acquiring secondary genetic changes necessary for neoplastic transformation. The competence to establish c-myc recombination-positive clones of significant size in the OG might be particularly important in that these cells are possibly subjected to genotoxic conditions.6
The results presented in this study show that cells with Ig/c-myc recombinations are present at remarkably high frequencies in the GALT of all inbred strains of mice examined. This suggests that the Ig/c-myc recombination is generated nonrandomly and raises the possibility that cells carrying these recombinations may serve a physiological function in the mucosal immune system. B lymphocytes that constitutively express MYC would not be expected to exit the active cell cycle and could respond with a burst of proliferation in the presence of a supporting environment. Therefore, they could provide a first measure against a microbial challenge. Cessation of environmental support would cause the MYC-overexpressing cells to die of apoptosis12 returning immune function to conventional lymphocytes.
ACKNOWLEDGMENT
The authors thank J. Williams for continuous editorial help and Drs J.F. Mushinski and F. Wiener for reading the manuscript and making valuable suggestions.
Address reprint requests to Jürgen R. Müller, MD, National Institutes of Health, Bldg 37, Room 2B-09, 37 Convent Dr, Bethesda, MD 20892-4255.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal