Abstract
We have designed in vitro assays to investigate the possible association between apoptosis and chemotherapeutic sensitivity in acute myeloid leukemias (AMLs). Consistent low levels of spontaneous apoptosis were observed in myeloid cells from normal bone marrow samples, while untreated cells collected from 56 de novo AML patients showed variable apoptosis. Control myeloid cells showed increased apoptosis after in vitro treatments with daunomycin (DNR), cytosine arabinoside (ARA-C), or gamma irradiation (RAD). Most AML samples showed less treatment-associated apoptosis, suggesting that apoptosis responses to therapeutic agents may be frequently attenuated in AML. Certain cytogenetic abnormalities common in AML may affect apoptosis, as acute promyelocytic leukemia (APL) samples with t(15; 17) karyotypes showed consistently low levels of spontaneous and treatment-associated apoptosis. Apoptosis assays may provide unique functional subtyping of AMLs, as other common cytogenetic subsets showed variable apoptosis. Altered function of two well-characterized regulators of apoptosis, BCL-2 and p53, was not entirely responsible for this variability. A genomic p53 mutation was found in only one AML sample. All samples that demonstrated the highest BCL-2–positive cell fractions showed low apoptosis, but reduced apoptosis was seen in both the presence and absence of BCL-2 overexpression. Finally, data from matched diagnosis and relapse sample pairs suggest that neither further reduced apoptosis nor additional BCL-2 overexpression is necessarily associated with disease progression.
A GROWING BODY of evidence suggests that many chemotherapeutic agents kill tumor cells by initiating apoptosis, the highly ordered process in which cells actively self-destruct. Tumors that demonstrate little or no apoptosis are assumed to be relatively resistant to therapeutic intervention. The preliminary studies described here were aimed to determine if simple in vitro assays can be used to determine the variability of spontaneous apoptosis and to measure variable apoptosis responses to relevant therapeutic agents. We approached these experimental questions in acute myelogenous leukemia (AML). AMLs show varied morphologic, cytochemical, immunologic, and cytogenetic characteristics and varied sensitivity to conventional chemotherapeutic regimens. Sixty percent to 70% of patients with de novo AML initially achieve complete remission. However, the majority of these patients relapse and eventually die of the disease. The biologic bases of drug resistance and relapse in AML are not well understood and prognoses are still largely based on descriptive parameters. The biologic and clinical heterogeneity in AML suggested that this would be a fruitful tumor model in which to study apoptosis in association with other descriptors of known prognostic value. The apoptosis studies described here lay the groundwork for further studies aimed to determine whether intrinsic or acquired resistance to apoptosis contributes to therapeutic outcome.
Several lines of evidence indicate that apoptosis plays roles in responses of AML patients to chemotherapy. A small study of AML samples collected from patients undergoing induction chemotherapy suggested that apoptosis is a common but variable response of AML cells to therapeutic agents.1 Other data suggested an association between therapy-induced apoptosis and therapeutic efficacy in AML. For example, the combination of fludarabine, cytosine arabinoside (ARA-C), and granulocyte colony-stimulating factor (G-CSF ) has proven to be a highly tumoricidal regimen in poor-prognosis AML, and combined in vitro treatment with these agents induces apoptosis in fresh AML cells, whereas treatments with any one of them does not.2 Increasing evidence suggests that specific genetic alterations may abrogate apoptotic responses in myeloid tumorigenesis. In acute promyelocytic leukemias (APLs), the antiapoptotic activity of the PML-RARα fusion may be integral to the development of APLs.3 An antiapoptotic activity of the bcr-abl gene product has been similarly implicated in chronic myeloid leukemia (CML).4 Additionally, altered p53 function and high BCL-2 protein expression may contribute to leukemogenesis and drug resistance,5-10 although the mechanisms linking these molecules to drug resistance have not been experimentally addressed in AML.
To investigate whether the sensitivity of AMLs to chemotherapeutic agents depends on the abilities of leukemia cells to respond to therapeutic insult by initiating apoptosis, assays appropriate to the analysis of large numbers of primary tumor-cell samples are needed. Apoptosis is experimentally defined by cytoplasmic and nuclear condensation that can be assessed microscopically,2 and by nonrandom DNA fragmentation that can be measured by agarose gel electrophoresis or by terminal deoxytidyl transferase/deoxy uridine triphosphate (dUTP) end-labeling (TUNEL) assays.1,3 Nucleosomal “DNA ladders” revealed by agarose gel electrophoresis have been observed in many apoptosis models, but a recent study showed that neither morphologic changes nor DNA ladders are necessary components of apoptosis in leukemia cells exposed to therapeutic agents.11 Apoptosis can involve various changes in the cell membrane. In particular models, increased membrane permeability associated with apoptosis can be measured in dye exclusion assays,8 ceramide conversion to sphingosine can be quantitated,12 exposure of phosphatidylserine on the outer membrane leaflet can be detected by increased annexin V binding,13 and dissolution of cell surface structures can be visualized as decreased phalloidin binding to F-actin networks.14
In the preliminary study reported here, we developed flow cytometry assays to quantitate spontaneous and treatment-specific sub-G1 apoptosis in multiparameter, single-cell assays. We chose this experimental approach to allow assessments of apoptosis frequencies, cell-cycle distributions, and associated protein expression patterns specifically in normal myeloid subsets of control bone marrows and in AML samples. We used these assays to address several questions. First, how variable is apoptosis in AML and can abnormal levels of apoptosis be recognized in vitro as compared with apoptosis in normal myeloid cells? Second, do therapeutic agents commonly used to treat AML patients produce apoptosis in vitro? Third, can differential in vitro apoptosis be associated with different clinical features? The results of this preliminary study suggest that in vitro flow cytometry–based apoptosis assays can reproducibly measure spontaneous and treatment-induced apoptosis in normal myeloid and AML cells, that differences between normal and AML cells can be recognized, and that different patterns of AML responses can be distinguished. A statistical assessment of the prognostic value of this variable in vitro apoptosis, as well as the possible independence of apoptosis as a prognostic marker, will require a larger study based on these initial findings.
MATERIALS AND METHODS
Cells.Myeloid cell lines used in these studies were produced from myeloid leukemias at one of several different differentiation stages/French-American-British (FAB) classes. ML-1 cells are considered to be myeloblastic/FAB M1-2; KG1a cells are undifferentiated promyeloblasts/M0; and K562 cells are from a CML patient. These cell lines have different p53 genotypic statuses (ML-1 wild type, KG1a mutant, and K562 mutant), different BCL-2 expression levels (K562-negative, ML1-low, and Kg1a-high), and only K562 cells express BCR-ABL fusion proteins. We confirmed the p53-mutant status of KG1a and K562 cells and wild-type status of ML1 cells in single-chain DNA polymorphism (SSCP) assays (data not shown). None of these cell lines requires growth factors in excess of 10% fetal bovine serum (HyClone, Logan, UT) for exponential growth in RPMI culture medium (GIBCO, Gaithersburg, MD).
Six normal bone marrow samples were obtained from the Children's Cancer Group (CCG) cell repository and the Fred Hutchinson Cancer Research Center (FHCRC) bone marrow donor program. All necessary consents were obtained for the research use of these samples. These samples had been ficoll-purified before freezing and contained approximately 107 cells per sample. Samples thawed with 40% to 99% viability as determined by Trypan blue (Sigma, St Louis, MO) exclusion assays.
Frozen AML cell samples were obtained from the CCG and Southwest Oncology Group (SWOG) cell repositories. These samples had been ficoll-purified before freezing and contained approximately 107 cells per sample. Samples were decoded for cytogenetics (as noted in the figures), immunophenotypes (20/56 CD34−), blast percents (30% to 98%), and clinical characteristics after in vitro analyses were complete.
In vitro treatments.Normal bone marrow and AML cell samples were thawed into Iscove's medium that contained 20% heat-inactivated fetal calf serum (FCS), with both stem-cell factor/c-kit ligand (SCF ) and interleukin-3 (IL-3) (BioSource, Camarillo, CA) at 100 ng/mL. Cells were left undisturbed for 2 days. These conditions promote immature myeloid cell survival and proliferation rather than differentiation (personal communication, F. Smith). After 2 days of culture, cells were fed with fresh growth factor–containing medium with or without daunomycin (DNR) or ARA-C, or were irradiated with gamma radiation (RAD) from a GammaCell Irradiator Model GC1000 (Radiation Machinery Corp, Parsippany, NJ). They were assayed for cell-cycle arrest and apoptosis at 16 to 20 hours posttreatment. Treatment doses and duration were chosen in optimization assays described in the Results. Trypan blue exclusion was used as a measure of cell viability in microscopic assays of all cell samples. Data included here were collected from samples that thawed with 10% to 94% viability (mode, 75%) as determined by Trypan blue exclusion (data not shown). Cells that were dead at thawing were sub–cell-sized, as noted microscopically, and were largely excluded from flow cytometric data analysis by setting a low DNA fluorescence threshold (see Fig 2).
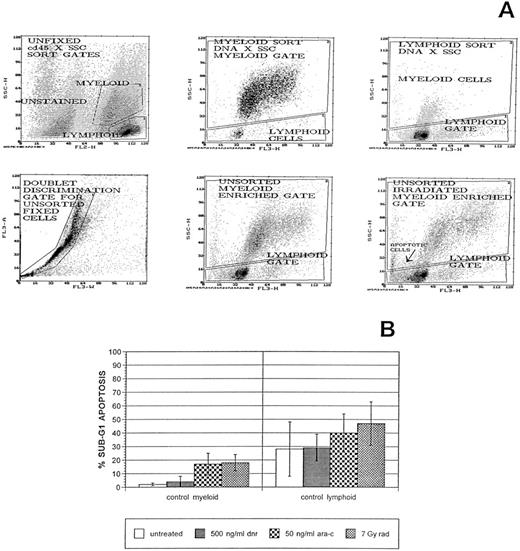
Normal myeloid-enriched cell populations in control bone marrow samples show consistently low apoptosis responses to treatments with therapeutic agents. Primary bone marrow cells were cultured, treated with therapeutic agents, and prepared for flow cytometric analysis as described in Materials and Methods. Immature myeloid cells and monocytes were first recognized and flow-sorted from unfixed, CD45-immunostained bone marrow samples and then ethanol-fixed and stained with 7AAD, a DNA-specific stain. This demonstrated that the same myeloid versus lymphoid enrichments could be obtained by software gating DNA × SSC plots. Doublet discrimination gates were also applied to all flow cytometric analyses of fixed cells. (A) Representative histograms from flow cytometric sorting and follow-up cell-cycle/apoptosis assays of untreated or gamma irradiated cells from one control bone marrow sample. Sub-G1 apoptotic cells in lymphoid and myeloid subpopulations of an irradiated aliquot of this sample are noted. Cell-cycle distributions and apoptosis frequencies were determined by curve fitting using the Multicycle AV DNA analysis program. (B) Sub-G1 apoptosis data from this assay and its replicate and from assays of each of five other, independent bone marrow samples means and SEMs denoted for each treatment condition. Apoptosis frequencies are expressed relative to the cell fraction in G1 + S + G2/M compartments.
Normal myeloid-enriched cell populations in control bone marrow samples show consistently low apoptosis responses to treatments with therapeutic agents. Primary bone marrow cells were cultured, treated with therapeutic agents, and prepared for flow cytometric analysis as described in Materials and Methods. Immature myeloid cells and monocytes were first recognized and flow-sorted from unfixed, CD45-immunostained bone marrow samples and then ethanol-fixed and stained with 7AAD, a DNA-specific stain. This demonstrated that the same myeloid versus lymphoid enrichments could be obtained by software gating DNA × SSC plots. Doublet discrimination gates were also applied to all flow cytometric analyses of fixed cells. (A) Representative histograms from flow cytometric sorting and follow-up cell-cycle/apoptosis assays of untreated or gamma irradiated cells from one control bone marrow sample. Sub-G1 apoptotic cells in lymphoid and myeloid subpopulations of an irradiated aliquot of this sample are noted. Cell-cycle distributions and apoptosis frequencies were determined by curve fitting using the Multicycle AV DNA analysis program. (B) Sub-G1 apoptosis data from this assay and its replicate and from assays of each of five other, independent bone marrow samples means and SEMs denoted for each treatment condition. Apoptosis frequencies are expressed relative to the cell fraction in G1 + S + G2/M compartments.
Flow cytometry assays.Normal myeloid cells in control bone marrows and leukemic myeloid cells in AML samples were analyzed after software gating as described in the Results. Immunodetection of BCL-2 protein was accomplished after 30 to 60 minutes of fixation in 80% ethanol at 4°C and permeabilization in 0.25% Triton-X/phosphate-buffered saline (PBS) for 5 minutes at room temperature. Cells were washed in PBS/SER (PBS containing FBS [GIBCO] to 2%, human serum [Gemini, Calabasas, CA] to 2%, and sodium azide at 0.1%). Cells were subsequently incubated in the dark for 1 hour with mouse anti-human BCL-2 clone 124 antiserum (Dako, Carpinteria, CA) at 500 ng/2 to 5 × 105 cells in PBS/SER. After washing, cells were incubated with flouresceinated (FITC) secondary rabbit anti-mouse IgG antibody (Sigma) at approximately 2 μg per sample in the dark for an additional 30 minutes. The DNA-specific dye, 7-aminoactinomycin D (7AAD), was added at 25 μg/mL after further washing and cells were incubated with the DNA stain for 30 minutes before taking samples to the FacScan (Becton Dickinson, San Jose, CA) flow cytometer for analysis. Typically, in experiments summarized in this report, fluorescence detector 1 (FL1) photomultiplier tube (PMT) voltage was set at 435 with logarithmic amplification to visualize FITC signals, and FL3 PMT voltage was set at 450 with linear amplification to visualize 7AAD signals. Flow data were displayed and analyzed using Winlist (Verity Software House, Topsham, ME) and Multicycle AV (Phoenix Flow Systems, San Diego, CA) data analysis programs. Typically, 10,000 to 50,000 cells were analyzed in the myeloid blast-enriched gate of each sample, compared with 100,000 to 500,000 total bone marrow/peripheral blood cells treated. Cell frequencies in cell-cycle compartments are expressed as percentages of the cell number within the complete cell cycle. Apoptotic cell frequencies are expressed as percentages of the total cell number.
Microscopic assessment of apoptotic morphology.After flow cytometric analysis, aliquots of all stained samples were placed on microscopic slides and the nuclear morphology of these fluorescent nuclei was observed using a Nikon fluorescence microscope (Germany) fitted with a 60× objective. Nuclei were scored as apoptotic if chromatin was notably condensed, marginated, or highly divided, as has been documented for apoptotic cells in other studies.2
RESULTS
Multiparameter, flow cytometry assays were developed to measure differential in vitro cell cycle and apoptosis responses of myeloid cells to therapeutic agents. Preliminary cell viability assays were performed on cells from myeloid cell lines to determine appropriate therapeutic agent doses for in vitro apoptosis assays. ML1 cells were used to represent myeloid tumor cells with wild-type p53 expression, while KG1a cells were used as representatives of myeloid tumor cells with mutant p53 expression. The p53 status of these cells was confirmed in SSCP assays (data not shown). Tenfold ranges of DNR and ARA-C concentrations were used to bracket the reported peak plasma concentrations in AML patients undergoing induction therapy. Radiation doses used were based on in vitro doses reported to affect cell-cycle and apoptosis responses.5 Trypan blue dye exclusion assessments of membrane integrity/viability were made after 24- and 48-hour continuous drug treatments or after one exposure to RAD. Exposure to 9 × 10−7 mol/L (500 ng/mL) DNR, 2 × 10−7 mol/L (50 ng/mL) ARA-C, and 700 rad (7 Gy) irradiation were approximately equitoxic in cells of these lines and consistently affected cell cycle (Fig 1 and data not shown). Samples were treated with these doses and collected at various times after treatment initiation to chose time points at which differential cell-cycle arrest and apoptosis could be best elucidated in flow cytometric analyses. These preliminary assays demonstrated that the most marked changes in cell-cycle distributions and the most consistent differences between ML1 and Kg1a cells were seen when cells were collected at 16 to 18 hours posttreatment (Fig 1B and data not shown).
Myeloid leukemia cell lines demonstrate reproducibility and accuracy of in vitro flow cytometric apoptosis assays. Cells from myeloid leukemia cell lines were cultured in the presence or absence of varying doses of DNR or ARA-C, or were treated with RAD and prepared for flow cytometric analysis as described in Materials and Methods. Cells of the ML1 cell line (wild-type p53, low BCL-2 expression) showed distinct cell-cycle perturbations (A) and reproducible treatment-associated sub-G1 apoptosis (A and B) after treatments with DNR, ARA-C, or RAD. Dose-responsive apoptosis was confirmed in other commonly used apoptosis assays, including Trypan blue staining demonstrations of membrane permeability and microscopic presentations of abnormal fluorescent nuclei (B). In contrast, KG1a cells (mutant p53, high BCL-2 expression) showed significantly less apoptosis in all assays of apoptosis (B). *Doses of each treatment shown to be equitoxic in Trypan blue exclusion assays of ML1 cells. These doses were used for treatments of cells in control bone marrow and AML samples.
Myeloid leukemia cell lines demonstrate reproducibility and accuracy of in vitro flow cytometric apoptosis assays. Cells from myeloid leukemia cell lines were cultured in the presence or absence of varying doses of DNR or ARA-C, or were treated with RAD and prepared for flow cytometric analysis as described in Materials and Methods. Cells of the ML1 cell line (wild-type p53, low BCL-2 expression) showed distinct cell-cycle perturbations (A) and reproducible treatment-associated sub-G1 apoptosis (A and B) after treatments with DNR, ARA-C, or RAD. Dose-responsive apoptosis was confirmed in other commonly used apoptosis assays, including Trypan blue staining demonstrations of membrane permeability and microscopic presentations of abnormal fluorescent nuclei (B). In contrast, KG1a cells (mutant p53, high BCL-2 expression) showed significantly less apoptosis in all assays of apoptosis (B). *Doses of each treatment shown to be equitoxic in Trypan blue exclusion assays of ML1 cells. These doses were used for treatments of cells in control bone marrow and AML samples.
Cells with sub-G1 DNA contents were scored as apoptotic based on previous studies in a wide variety of models.1 7AAD was used as the DNA stain because it is a DNA-specific dye whose use does not require RNase treatments and because its emissa have minimal spectral overlap with FITC fluorescent signals. This allows accurate measurement of weak FITC signals (as from BCL-2 immunostaining in some cell samples). Phycoerythrin (PE)-labeled antibodies can also be used in three-color assays to measure the expression of a second, highly expressed protein (such as the proliferation marker, proliferation-associated cell nuclear antigen [PCNA]; data not shown). DNA histograms collected for untreated cells of the KG1a and ML1 myeloid cell lines had low coefficients of variation (3% to 6%).
Sub-G1 apoptosis frequencies differ in myeloid cell lines with different genetic constitutions.Both G1 and G2/M cell-cycle arrests were noted in ML1 cells after RAD, as expected for wild-type p53-containing cells, while DNR and ARA-C produced distinct cell-cycle effects as shown in Fig 1A. All three treatments consistently produced sub-G1 apoptosis in ML1 cells that was dose-responsive and concordant with other measures of apoptosis, including microscopic assessments of membrane permeability by Trypan blue staining and classic apoptotic morphology in fluorescent nuclei (Fig 1B). Kg1a cells were used to document the ability of these assays to recognize aberrant apoptosis patterns. KG1a cells contain mutant p53, and wild-type p53 function has been shown to be necessary for apoptosis responses in many models.5,6 Kg1a cells did not exhibit significant sub-G1 apoptosis after treatment with the therapeutic agents at the equitoxic doses, although some apoptosis was measured at a higher ARA-C dose. Membrane permeability and apoptotic morphology assays confirmed the reduced sensitivity of cells of this p53-mutant cell line. Cells from the K562 cell line did not demonstrate apoptosis under any condition in these in vitro assays (data not shown), consistent with bcr-abl expression reported for this cell line15 and data supporting an antiapoptotic activity of this fusion protein.4 Therefore, these simple in vitro assays yield highly reproducible results that can measure the effects of previously defined molecular regulators of mammalian cell cycle and apoptosis.
Primary myeloid cells from different normal bone marrow samples show similar arrest and apoptosis responses in vitro.To recognize aberrant patterns of apoptosis in primary AML cell samples, it was necessary to characterize apoptosis in comparable, normal myeloid cells and to determine the reproducibility of normal apoptosis responses. Therefore, we examined the in vitro effects of therapeutic agents on immature myeloid cells and monocytes from normal bone marrows. Hematopoietic stem cells and early myeloid progenitors are rare in unmanipulated blood cell compartments, but committed myeloid precursors and monocytes are relatively abundant.
In pilot studies, CD45 staining of bone marrow cells was performed before fixation and DNA staining. Cd45 is expressed differentially by white blood cells and this differential expression has been used to distinguish cell type subsets in unfixed peripheral blood and bone marrow cell samples. Orthogonal light scatter (SSC) × CD45 gates have been used to enrich subpopulations for immature myeloid cells and monocytes with few contaminating lymphocytes or mature granulocytes.16 This separation is possible due to the intermediate side scatter/intermediate CD45 staining of immature myeloid cells versus the low side scatter/high CD45 staining of lymphoid cells and the high side scatter/minimal CD45 staining of granulocytes. We flow-sorted myeloid- versus lymphoid-enriched subpopulations from unfixed, normal bone marrow cells using SSC × Cd45 gates. Sorted cells were subjected to ethanol fixation and 7AAD staining. Analyses of these fixed cell subpopulations demonstrated that similar myeloid cell enrichments could be obtained by gating SSC × DNA histograms directly, in part because 7AAD is sensitive to DNA conformation differences between myeloid versus lymphoid cells.17 18 Representative data from one of three sorting experiments are presented in the first three histograms of Fig 2A.
In subsequent analyses, doublet discrimination gates were used to exclude cell clumps and SSC × 7AAD DNA stain gates were used to perform cell-cycle and protein expression analyses of myeloid-enriched subpopulations in normal and AML primary cell samples. Representative data from the gated analysis of one fixed control bone marrow is shown in the lower two parameter histograms of Fig 2A. The apoptotic responses of cells in the myeloid-enriched cell subpopulations of six different normal myeloid cell samples were remarkably consistent under each of the different treatment conditions (Fig 2B). First, low but consistent basal apoptosis was noted in untreated bone marrow cells. Cells that were dead when cell samples were thawed were largely excluded from cell-cycle fits as they were considerably smaller and nonfluorescent (data not shown and Fig 2A). In fact, the modal position of sub-G1 peaks decreased with increased duration of apoptosis-inducing treatments (Fig 1 and data not shown). Second, apoptosis increased above basal, untreated levels was consistently noted after ARA-C treatment or RAD. ARA-C also produced variable accumulations of cells in G1 and/or S compartments (G1/S arrests, data not shown), while RAD produced G1 and G2 arrest (Fig 1A). DNR did not produce a consistent increase in apoptosis in the myeloid cell subpopulations, but did produce increases in G2/M-cell frequencies (Fig 1A). The apoptotic responses of lymphoid cells in these control bone marrows were more frequent and more variable (Fig 2B), demonstrating the advantage of the subpopulation separations performed in these flow cytometric analyses.
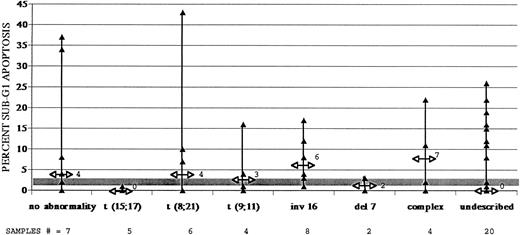
AML samples show widely variable levels of spontaneous, untreated apoptosis, but certain cytogenetic subtypes consistently demonstrate low apoptosis, relative to normal myeloid cells. Apoptosis frequencies are expressed relative to nonapoptotic cell frequencies. Shaded area denotes levels of spontaneous apoptosis measured in normal myeloid cells in six different control bone marrows (also shown in Fig 2). Median apoptosis for each AML subgroup is denoted by arrows on AML sample ranges and is expressed numerically. Samples with simple karyotypic abnormalities, including (15; 17), (8; 21), and (9; 11) translocations, chromosome 16 inversions, and chromosome 7 deletions, were tested, as were samples with many cytogenetic abnormalities and samples with normal or undescribed karyotypes. Sample numbers in each cytogenetic subgroup are denoted.
AML samples show widely variable levels of spontaneous, untreated apoptosis, but certain cytogenetic subtypes consistently demonstrate low apoptosis, relative to normal myeloid cells. Apoptosis frequencies are expressed relative to nonapoptotic cell frequencies. Shaded area denotes levels of spontaneous apoptosis measured in normal myeloid cells in six different control bone marrows (also shown in Fig 2). Median apoptosis for each AML subgroup is denoted by arrows on AML sample ranges and is expressed numerically. Samples with simple karyotypic abnormalities, including (15; 17), (8; 21), and (9; 11) translocations, chromosome 16 inversions, and chromosome 7 deletions, were tested, as were samples with many cytogenetic abnormalities and samples with normal or undescribed karyotypes. Sample numbers in each cytogenetic subgroup are denoted.
AML samples collected at disease onset show widely variable in vitro apoptosis.We next addressed the apoptosis responses of different primary AML cell samples using the same in vitro assays of apoptosis. Several cytogenetic abnormalities common in AML patients are associated with good or poor responses of patients to chemotherapeutic intervention.19 20 The genetic alterations shown by these cytogenetic markers are assumed to be integral to the development of AML in these patients. We examined whether any of these simple karyotypes were specifically associated with unique patterns of in vitro apoptosis. Presentation samples from 56 AML patients were analyzed (Fig 3). Twenty-nine of these samples had one of several, common karyotypic abnormalities found in AMLs. Data from subgroups containing samples with normal cytogenetics or containing samples with uncharacterized cytogenetics were also collected (Fig 3). Control data from myeloid cells in normal bone marrows are depicted as ranges by the shaded area in Fig 3.
Median basal apoptosis values for these 56 AML samples are noted with ranges for each cytogenetic subgroup (Fig 3). The apoptosis ranges of most cytogenetic subgroups overlapped and exceeded the control apoptosis range of normal myeloid cells (shaded area in Fig 3). Two subgroups were notably different from the others and from control myeloid cells in normal bone marrow samples. The five t(15; 17) samples were all characterized by low basal apoptosis, as were the two samples with del(7) abnormalities. Three of four samples with (9; 11) translocations as their only cytogenetic abnormality also had little or no spontaneous apoptosis. Three of 56 untreated AML samples were characterized by very high basal apoptosis frequencies. Two of these had normal karyotypes and one of these had (8; 21) translocations. The high basal apoptosis in only one case was associated with a notably low thawing viability (10%) and, therefore, may be artifactual, despite the fact that most cells dead at thawing were excluded from cell-cycle fits as negligibly fluorescent debris (see Fig 2A).
Relative, treatment-specific apoptosis was determined by subtracting untreated percents apoptosis from treated percents apoptosis (Table 1). In these analyses, only apoptosis at levels above those in untreated samples was scored as positive. Therefore, cells dead at thawing were not included in these assessments. Neither control nor AML myeloid cells responded significantly to DNR treatments with treatment-specific apoptosis, despite the fact that the DNR dose used did elicit apoptosis and G2 arrest in ML1 cells (Fig 1) and did produce G2 arrests in control myeloid cells and in many AML samples (data not shown). AML cells, like normal myeloid cells, tended to show significant apoptosis increases after ARA-C (P = .0001) or irradiation (P = .0001). None of the t(15; 17) samples showed substantial apoptosis increases after ARA-C or irradiation as compared with the responses of myeloid-gated control samples or as compared to the apoptosis responses of other AML samples. Fifty-four of 56 presentation samples responded less to ARA-C than did myeloid cells from normal bone marrows, and 50 of 52 presentation samples tested showed less radiation-specific apoptosis than did control cells. Table 1 shows that the responses of AMLs to ARA-C or irradiation are significantly less than the responses of normal myeloid cells from the six control bone marrows (two-tailed P values = .025 and .017). Certain AML samples, including one of the t(8; 21) samples, demonstrated notably more radiation-specific apoptosis (data not shown) than ARA-C–specific apoptosis, suggesting that these in vitro apoptosis assays can uncover differential sensitivities to specific therapeutic agents.
Comparison of AML Samples and Normal Bone Marrow Myeloid Cells With Respect to the Effects of Treatments on Sub-G1 Apoptosis and BCL-2 Positivity
| Measure . | AML Samples . | Control Myeloid-Enriched Samples From Normal Bone Marrows . | |||||
|---|---|---|---|---|---|---|---|
| . | Treatment . | N . | Mean Change . | 95% CI . | N . | Mean Change . | 95% CI . |
| % G1 apoptosis | DNR | 43 | +0.6 | −0.1-1.4 | 6 | +2.2 | −1.5-5.8 |
| ARA-C | 56 | +2.5 | 1.6-3.3 | 6 | +15.0 | 7.2-22.8 | |
| RAD | 52 | +4.3 | 2.7-5.8 | 6 | +15.2 | 9.2-21.0 | |
| % BCL-2–positive | DNR | 31 | +0.6 | −1.6-2.9 | 5 | +7.6 | 3.8-11.4 |
| ARA-C | 31 | +2.2 | 0.4-7.7 | 4 | +8.8 | 5.1-12.5 | |
| RAD | 31 | +2.2 | −1.7-5.7 | 4 | +13.8 | 5.8-16.7 | |
| Measure . | AML Samples . | Control Myeloid-Enriched Samples From Normal Bone Marrows . | |||||
|---|---|---|---|---|---|---|---|
| . | Treatment . | N . | Mean Change . | 95% CI . | N . | Mean Change . | 95% CI . |
| % G1 apoptosis | DNR | 43 | +0.6 | −0.1-1.4 | 6 | +2.2 | −1.5-5.8 |
| ARA-C | 56 | +2.5 | 1.6-3.3 | 6 | +15.0 | 7.2-22.8 | |
| RAD | 52 | +4.3 | 2.7-5.8 | 6 | +15.2 | 9.2-21.0 | |
| % BCL-2–positive | DNR | 31 | +0.6 | −1.6-2.9 | 5 | +7.6 | 3.8-11.4 |
| ARA-C | 31 | +2.2 | 0.4-7.7 | 4 | +8.8 | 5.1-12.5 | |
| RAD | 31 | +2.2 | −1.7-5.7 | 4 | +13.8 | 5.8-16.7 | |
Sub-G1 apoptosis and BCL-2 positivity data were collected in multiparameter, flow cytometic analyses performed after treatments with therapeutic agents as described in the text. The numbers of samples in each treatment group are noted (N). Mean changes are averages of treated values with untreated values subtracted for each AML and control myeloid cell sample. Confidence intervals (95% CI) are based on 2 sample t-tests, using the approximation of Cochran and Cox to adjust for unequal variances. The mean changes in AML v normal myeloid cell responses to ARA-C and RAD were significantly different (P = .025).
Myeloid cell lines and control bone marrows demonstrate the reproducibility and accuracy of BCL-2 immunostaining. Cells were cultured, treated with therapeutic agents, fixed and immunostained with BCL-2–specific monclonal antibody and flouresceinated secondary antibody as described in Materials and Methods. Cells of the ML1 cell line showed consistently low BCL-2–positive staining fractions, while cells of the KG1a cell line showed consistently higher BCL-2–positive fractions (A). KG1a cells were used as interassay immunostaining standards and data presented in (A) include those collected for KG1a cells in flow cytometric analyses of all control bone marrow and AML sample preparations. Myeloid-enriched subpopulations were gated as described in the Results. Untreated cells in these subpopulations reproducibly demonstrated low BCL-2–positive fractions and increased positivity after treatments with therapeutic agents (B, C). BCL-2 – positive fractions were determined in comparison to fluorescence intensities of cells exposed to isotype-matched nonspecific antibody controls (B). Cells in lymphoid-enriched subpopulations of control bone marrows showed higher BCL-2-positive fractions (C).
Myeloid cell lines and control bone marrows demonstrate the reproducibility and accuracy of BCL-2 immunostaining. Cells were cultured, treated with therapeutic agents, fixed and immunostained with BCL-2–specific monclonal antibody and flouresceinated secondary antibody as described in Materials and Methods. Cells of the ML1 cell line showed consistently low BCL-2–positive staining fractions, while cells of the KG1a cell line showed consistently higher BCL-2–positive fractions (A). KG1a cells were used as interassay immunostaining standards and data presented in (A) include those collected for KG1a cells in flow cytometric analyses of all control bone marrow and AML sample preparations. Myeloid-enriched subpopulations were gated as described in the Results. Untreated cells in these subpopulations reproducibly demonstrated low BCL-2–positive fractions and increased positivity after treatments with therapeutic agents (B, C). BCL-2 – positive fractions were determined in comparison to fluorescence intensities of cells exposed to isotype-matched nonspecific antibody controls (B). Cells in lymphoid-enriched subpopulations of control bone marrows showed higher BCL-2-positive fractions (C).
AML samples collected at disease onset show BCL-2 overexpression.Several mechanisms of apoptosis abrogation, including p53 mutation 5,8 and BCL-2 overexpression,21 have been demonstrated in other models. We performed SSCP analyses of exons 5 to 8 of the p53 gene6 in DNA prepared from all of the AML presentation samples that showed little or no apoptosis in our in vitro assays. Only one of 28 AML samples tested showed evidence of mutant p53 sequence in these assays (data not shown). Thus, low in vitro apoptosis in presentation AML samples was not commonly associated with p53 mutations in our sample set. BCL-2 overexpression had been noted in AML samples in previous studies.9 10 We, therefore, addressed whether in vitro apoptosis variability was associated with variable BCL-2 expression. We used multiparameter flow cytometric analyses to quantitate BCL-2 protein expression in the same cells in which DNA content was ascertained.
Immunostaining and DNA staining procedures were optimized in repeated assays of KG1a cells that had been previously shown by Western blot to have relatively high BCL-2 expression22 (data not shown). BCL-2–positive staining fractions and DNA cell-cycle distributions were assessed in untreated and therapeutic agent-treated KG1a cells. The specificity of BCL-2 antibody binding was determined relative to that of an IgG subtype-matched control antibody. A large fraction of KG1a cells consistently showed positive immunostaining, while consistent low immunoreactivity of ML1 cells was shown in the same assays (Fig 4A). KG1a samples were included as BCL-2–positive immunostaining standards in all subsequent analyses of primary myeloid cells. The KG1a data in Fig 4A are cumulative from 21 different assays, including all those in which primary cells were analyzed.
Six normal bone marrows were ethanol-fixed, BCL-2–immunostained, and exposed to 7AAD to stain DNA. No evidence of cell-cycle stage-specific regulation of BCL-2 expression was noted in myeloid cells (Fig 4B), consistent with a previous report.22 Untreated, normal myeloid cells showed consistently low frequencies of BCL-2–positive cells while untreated, normal lymphoid cells showed higher, more variable immunostaining, also consistent with previous reports.23 BCL-2–positive fractions were increased in normal myeloid cells by all treatments, especially irradiation (Fig 4B and C and Table 1). Apoptotic cells were consistently BCL-2–negative (Fig 4B and data not shown).
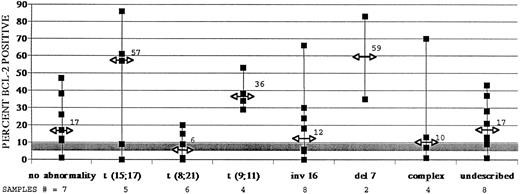
Forty-three AML samples were similarly analyzed. Thirty-four untreated AML samples showed higher BCL-2–positive fractions than did control myeloid cells (Fig 5 and Table 2). Again, median values are noted in Fig 5, along with data ranges for each cytogenetic subgroup. These data confirm the regularity with which BCL-2 overexpression occurs in AML. However, most AML samples did not show a further increase in BCL-2 expression after treatments with either DNR or irradiation, whereas an increase was noted in control myeloid cells (Table 1). Only ARA-C consistently induced increases in BCL-2 positivity in AML samples (P = .013).
The majority of adult AML samples show greater than normal myeloid cell levels of BCL-2 protein expression, as measured by immunostaining and flow cytometry. Data derived from 43 adult AML samples are displayed. Percents BCL-2–positive cells were determined after indirect immunofluorescent staining of myeloid cells with an anti–BCL-2 monoclonal antibody as described in Fig 4. Data, including AML ranges and median values and control myeloid cell ranges, are presented as in Fig 3.
The majority of adult AML samples show greater than normal myeloid cell levels of BCL-2 protein expression, as measured by immunostaining and flow cytometry. Data derived from 43 adult AML samples are displayed. Percents BCL-2–positive cells were determined after indirect immunofluorescent staining of myeloid cells with an anti–BCL-2 monoclonal antibody as described in Fig 4. Data, including AML ranges and median values and control myeloid cell ranges, are presented as in Fig 3.
Comparison of Untreated Sub-G1 Apoptosis Percents With Percents BCL-2–Positive Cells in Data Collected From 43 Adult AML Samples
| Apoptosis . | BCL-2 . | ||
|---|---|---|---|
| . | 0%-5% . | >5% . | Total . |
| 0%-5% | 3 | 20 | 23 |
| >5% | 6 | 14 | 20 |
| Total | 9 | 34 | 43 |
| Apoptosis . | BCL-2 . | ||
|---|---|---|---|
| . | 0%-5% . | >5% . | Total . |
| 0%-5% | 3 | 20 | 23 |
| >5% | 6 | 14 | 20 |
| Total | 9 | 34 | 43 |
Data were dichotomized at 5%. Based on the Fisher's exact test, no significant association between apoptosis and BCL-2 positivity was found in analyses of AML presentation samples.
Reduced basal apoptosis frequencies were often, but not always, noted in samples with elevated BCL-2–positive fractions (Table 2). Twenty-three of 43 adult AML presentation samples showed relatively low levels of basal apoptosis (0% to 5%) and showed relatively high BCL-2–positive cell fractions (> 5%). However, apoptosis frequencies were not consistently correlated with BCL-2–positive fractions, suggesting that other mechanisms are also involved in the regulation of apoptosis in AML.
Neither reduced apoptosis nor increased BCL-2 expression are necessarily associated with relapse in AML.A large fraction of AML patients who achieve complete remission ultimately relapse. This disease progression is associated with acquired tumor-cell drug resistance and tumor regrowth. We used paired tumor-cell samples collected from AML patients at presentation, and then at relapse, to address several questions. First, are diagnosis samples from patients who eventually relapsed characterized by markedly low in vitro apoptosis, suggesting that low apoptosis, as measured in these assays, predicts relapse? Second, do relapse samples from these patients show further decreased apoptosis responses to therapeutic agents in these in vitro assays, suggesting that apoptosis signaling pathways are altered during AML disease progression?
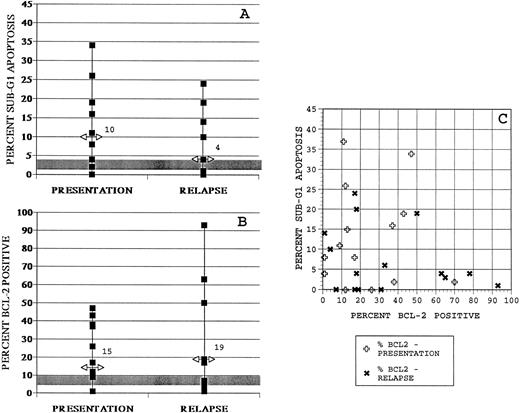
Fourteen presentation/relapse sample pairs were examined in assays of spontaneous apoptosis (Fig 6A). Seven of these presentation samples were characterized by normal karyotypes, while seven of them were uncharacterized. Data from the presentation samples of these matched pairs are included in Figs 3 and 5 in normal versus uncharacterized cytogenetic subgroups. A wide range of basal apoptosis frequencies was observed in assays of the paired AML presentation samples, with a median value (10%) exceeding control ranges of basal apoptosis. Only four untreated presentation samples showed low basal apoptosis compared with control frequencies observed in similar assays of myeloid cells in normal bone marrow samples. Several relapse samples showed reduced apoptosis relative to the relevant presentation sample such that the median apoptosis in this group was 4%. However, relapse data were not statistically different from presentation data, and neither subgroup showed apoptosis significantly lower than control. Treatment-associated apoptosis data also showed no consistent differences between presentation and relapse subgroups (data not shown). Thus, low basal apoptosis in these assays is not a universal feature of AML samples collected from patients who went on to relapse or even from patients at relapse.
Samples collected from adult AML patients at relapse do not consistently show further apoptosis reduction or increased BCL-2 positivity as compared with samples collected from the same patients at diagnosis. (A) Median values and ranges of apoptosis frequencies for untreated cells in diagnosis (presentation) and relapse samples collected from 14 adult AML patients. (B) Median values and ranges of BCL-2–positive fractions for untreated cells in presentation and relapse samples collected from 14 adult AML patients. As in Figs 3 and 5, control bone marrow myeloid data are represented in shaded bars. (C) Comparison of apoptosis and BCL-2 positivity data for each of these samples.
Samples collected from adult AML patients at relapse do not consistently show further apoptosis reduction or increased BCL-2 positivity as compared with samples collected from the same patients at diagnosis. (A) Median values and ranges of apoptosis frequencies for untreated cells in diagnosis (presentation) and relapse samples collected from 14 adult AML patients. (B) Median values and ranges of BCL-2–positive fractions for untreated cells in presentation and relapse samples collected from 14 adult AML patients. As in Figs 3 and 5, control bone marrow myeloid data are represented in shaded bars. (C) Comparison of apoptosis and BCL-2 positivity data for each of these samples.
To address whether BCL-2 protein expression commonly changes during the progression of AML to relapse, the 14 presentation/relapse sample pairs were also examined for BCL-2 expression. A wide range of positive staining frequencies was observed (Fig 6B). The majority of presentation and relapse samples showed greater than control BCL-2–positive frequencies as had been noted in analyses of cytogenetic subgroups of AML. The BCL-2–positive fraction in eight relapse samples was higher (median, 19%) than that of their presentation sample counterparts (median, 15%) and both medians exceeded control BCL-2–positive ranges (Fig 4C). However, BCL-2–positive fractions in relapse versus presentation samples were not statistically different in this study of 14 sample pairs. As in the larger set of 56 AML samples collected at diagnosis, the highest BCL-2–positive fractions were associated with low apoptosis (and these were in relapse samples), but low apoptosis did not require high BCL-2 expression (Fig 6C).
DISCUSSION
This report describes preliminary analyses of AML samples using flow cytometric assays to measure the apoptosis and BCL-2 expression responses of primary cells to in vitro treatments with therapeutic agents. The long-range goal of these studies is to develop assays that functionally characterize AMLs at diagnosis and thereby anticipate their clinical responses to currently used therapeutic agents. One previous study had shown that autonomous, in vitro proliferation of AML cells was associated with poor clinical outcome,24 but few functional analyses of primary AML samples have been performed. Such in vitro analyses of AML might provide new insights into the evolution of drug-resistant tumors and suggest novel approaches to controlling tumor growth. The data presented here demonstrate that in vitro analyses of cell-cycle arrest and apoptosis in primary AML cell samples are simple and reproducible and can measure therapeutic agent-specific apoptosis. We found relatively low apoptosis in the majority of AML samples analyzed in this study. Apoptosis abrogation was noted in AML samples with the highest BCL-2–positive cell fractions, but other samples showed that apoptosis abrogation can exist in the absence of BCL-2 overexpression or of p53 mutation. We found no significant association of apoptosis frequencies or BCL-2–positive fractions with FAB class, white blood cell count, or blast fractions. These results suggest that in vitro assays of apoptosis may provide new information about differential AML cell function and drug sensitivity.
The multiparametric, flow cytometry apoptosis assay is reproducible and sensitive to different modulators and effectors of apoptosis.We designed multiparameter, flow cytometric assays that can distinguish less mature myeloid cells by their light scatter and DNA characteristics, quantitate apoptosis and cell-cycle arrest in DNA histograms, and measure protein expression of one or two proteins that, like BCL-2, are known or suspected to affect cell-cycle arrest and/or apoptosis. DNA contents of single cells are visualized by stoichiometric binding of 7AAD after ethanol fixation and detergent permeabilization. Apoptotic cells can be distinguished from viable cells and from subcellular debris due to their sub-G1 DNA content, and cell-cycle distributions of G1, S, and G2/M cells can be simultaneously determined. Since 7AAD emits in the far-red wavelength range, levels and cell-cycle patterns of protein expression of a weakly FITC-labeled protein can be accurately measured without signal compensation, which is necessary with propidium iodide, or two marker proteins can be assayed using PE- and FITC-labeled antibodies. Repeated assays of apoptosis and BCL-2 immunostaining in cells of the KG1a versus ML1 cell lines showed that these assays can accurately measure the abrogating effects of p53 mutation and BCL-2 overexpression on apoptosis.
Assays of cells from six different normal bone marrows showed that distinct, normal response patterns can be established for immature myeloid cells and lymphoid cells. Moreover, these patterns are distinct from those noted in the majority of the AML samples analyzed in this study. Whether there are any rare populations of cells within normal bone marrow that have patterns of cell-cycle arrest and apoptosis identical to those seen here in leukemic samples could not be formally determined in these studies.
We exposed AML cells to DNR and ARA-C in our assays because both agents are commonly used to treat AML. We also investigated the effects of RAD on in vitro apoptosis because total-body irradiation is used to ablate leukemia-containing bone marrows before bone marrow transplant for AML. In addition, these three agents are known to produce different types of DNA damage and have been associated in other models with different effects on the cell cycle. Therefore, we expected this battery of agents to be useful in uncovering alterations in different apoptosis-signaling pathways. The small number of AML samples assayed in this preliminary study limits the conclusions that can be drawn from these analyses. However, our data suggest that AML cells show variable spontaneous apoptosis, that AML cells generally differ from normal myeloid cells in demonstrating reduced treatment-induced apoptosis, and that differences in apoptosis frequencies may exist between different categories of AML.
Particular AMLs demonstrate low spontaneous apoptosis, while the majority of AMLs demonstrate low treatment-specific apoptosis.Reduced apoptosis frequencies were recognized in many of the primary cell samples collected from AML patients at diagnosis when they were compared with control apoptosis frequencies. Data from approximately half of the 56 primary cell samples provided evidence that many AMLs are characterized by low spontaneous apoptosis relative to control myeloid cells in normal bone marrows. Attenuated therapeutic agent-specific inductions of apoptosis were noted in the majority of AML samples treated with ARA-C or RAD. Two cytogenetic subgroups, t(15; 17) and del(7), showed consistently low apoptosis under all conditions. Decreased levels of spontaneous and agent-specific apoptosis were also noted in several samples collected from AML patients at relapse as compared with samples collected from the same patients at presentation. This suggests that regulation of apoptosis pathways may be altered during the genesis of AML, may characterize subgroups with particular genetic alterations, and may be associated with the survival and regrowth of some drug-resistant tumors after chemotherapy. The fact that particular AML samples demonstrated heightened sensitivity to one therapeutic agent versus another suggests that these apoptosis assays might be further developed to ascertain differential chemotherapeutic agent sensitivities and help to tailor clinical treatment strategies.
Cells from only three AML samples and from the ML-1 cell line showed DNR-specific increases in apoptosis frequencies (Fig 1 and data not shown). A large number of adult AMLs are characterized by expression of MDR, which facilitates efficient efflux of drugs, such that toxicities of these drugs, including DNR, are reduced in MDR+ patients.25 However, we found no evidence that DNR-specific levels of cell-cycle arrest or apoptosis were correlated with MDR staining and/or dye efflux in the adult AML samples for which these data were available to us (data not shown). In addition, the same dose of DNR (500 ng/mL) affected cells in many AML samples, because 17 of 43 adult AML samples showed DNR-specific G2/M arrests in the same assays (data not shown). Perhaps DNR concentrations and/or treatment times optimal for apoptosis induction are different than those that elicit cell-cycle arrest. Another group measured responses of primary AML samples to DNR in TdT/TUNEL apoptosis assays26 and found that the DNR doses and the duration of DNR treatment at which different AML samples demonstrated measurable responses were quite variable. Clearly, more experiments will be necessary to develop assays that can accurately measure differential DNR-associated apoptosis in primary AML cell samples. Apoptosis assays optimized to measure DNR-generated apoptosis will be important in further examining the relevance of low apoptosis in APL, as APL patients are most effectively treated with high-dose anthracyclines or with retinoic acid and are relatively less sensitive to ARA-C.20,27 28 APL samples were relatively insensitive to ARA-C and radiation in our apoptosis assays, but we are unable to assess the relative sensitivity to DNR of this cytogenetic subgroup.
Low apoptosis in in vitro analyses of AML samples is not necessarily associated with BCL-2 overexpression.An earlier study had shown that less mature myeloblasts, metamyelocytes, myelocytes, and CD34+ cells in bone marrow samples stained positively with anti–BCL-2 antibodies, as did splenic lymphocytes, while mature myeloid cells were negative for BCL-2 staining.22 Another study demonstrated that monocytes were weakly positive for BCL-2 immunostaining as compared with T cells.23 Our findings are consistent with these in that normal cells in lymphocyte gates immunostained more strongly than cells in neutrophil gates or cells in the immature myeloid/monocyte gate. High BCL-2 expression had previously been associated with attenuated apoptosis after treatment with therapeutic agents in a comparison of six mouse myeloid leukemia cell lines with varying BCL-2 expression levels.29 BCL-2 antisense RNAs had been shown to specifically increase apoptosis in human AML cell lines and primary cell samples.21 Two other studies had used flow cytometric assays to analyze BCL-2 protein expression in AML primary cell samples and found that BCL-2 was commonly overexpressed.9,10 The larger study9 found a range of 0% to 95% BCL-2–positive cells per sample in 82 samples of newly diagnosed adult AML, with 28 samples expressing measurable BCL-2 in 20% or more of cells. Neither apoptosis frequencies nor the effects of relevant therapeutic agents were assessed in these two studies.
Our analyses found high BCL-2–positive fractions in many primary cell samples collected at diagnosis from adult AML patients with diverse clinical and cytogenetic features. Data from AML presentation/relapse sample pairs show that further increases in BCL-2 positivity are not necessary for relapse. AML presentation samples with the highest BCL-2–positive fractions generally had the lowest in vitro apoptosis in these flow cytometric assays. The fact that lymphoid-enriched subpopulations of normal bone marrows demonstrated higher apoptosis frequencies and higher BCL-2–positive fractions than did myeloid-enriched subpopulations in the same marrows demonstrates that relatively high BCL-2 expression is not sufficient to abrogate apoptosis responses in lymphoid cells. On the other hand, many AML samples with low apoptosis frequencies did not show evidence of BCL-2 overexpression nor of p53 mutations. These data imply that neither of these well-characterized mechanisms of apoptosis abrogation are necessary for the reduced apoptosis seen in many AML samples. Thus, functional assays of apoptosis may demonstrate altered apoptosis pathways unrecognized in assays of the expression of any known molecular regulator of apoptosis.
Apoptotic cells were consistently BCL-2–negative in our analyses. Cells may have apoptosed because they had lower levels of BCL-2, BCL-2 expression may have been downregulated by apoptosis-inducing agents, or BCL-2 protein may have been specifically degraded by apoptosis-associated proteases. The fact that BCL-2–positive fractions were increased by treatments of some AML samples indicates that BCL-2 downregulation is not a necessary consequence of treatment with therapeutic agents. Many AML samples showed greater than normal inductions of BCL-2 by ARA-C, but less than normal BCL-2 increases after irradiation. This suggests that BCL-2 expression can be differentially modulated by these agents and that protein expression measurements in multiparameter apoptosis assays might be useful in further elucidating differential responses to chemotherapeutic agents.
In vitro apoptosis and arrest assays may provide insights into biologic processes determining clinical outcomes in AML.Low levels of apoptosis after chemotherapeutic exposures are assumed to be necessarily associated with clinical drug resistance. In our study, relatively low spontaneous apoptosis characterized both “bad prognostic” del(7) and “good prognostic” t(15:17) AML samples and low treatment-specific apoptosis characterized the majority of AML samples in all of the subgroups analyzed. These findings suggest that any prognostic value that in vitro apoptosis measures have will be independent of karyotypic predictors. We have also found no association of in vitro apoptosis with other predictive parameters, including FAB class, white blood cell count, or blast fraction.
Previous studies provided evidence that BCL-2 overexpression is associated with clinical drug resistance in AMLs.9 10 As with apoptosis, we have no indication that BCL-2 expression is necessarily associated with other commonly used predictors of therapeutic outcome. The fact that AML samples that showed low apoptosis in our study did not always show high BCL-2 expression suggests that these may be independent parameters in AML. Multiparameter characterizations of a large collection of primary AML cell samples might, for example, show that patients with low apoptosis and high BCL-2 in in vitro assays have poorer clinical responses than do patients who show only low apoptosis or high BCL-2 expression. In this preliminary study, we are unable to statistically assess whether apoptosis and/or BCL-2 expression is significantly correlated with therapeutic outcomes in AML patients. A full evaluation of the prognostic import of in vitro multiparameter cell-cycle arrest and apoptosis analyses will be conducted in a larger study specifically aimed at addressing these issues.
ACKNOWLEDGMENT
We gratefully acknowledge the invaluable assistance and advice of Drs Irwin Bernstein and Franklin Smith associated with the CCG, Drs Cheryl Wilman and Kenneth Kopecky associated with the SWOG, and Drs Jerald Radich and Elaine Ostrander of the FHCRC. Essential materials were received from the SWOG cell repository and from the CCG cell repository. The expert technical support of Nola Butz was indispensable to the completion of these experiments.
Supported in part by Grant No. IRG-185 from the American Cancer Society and Grant No. 6011-96 from the Leukemia Society of America (D.E.B.), by Grant No. RO-1CA58954 (M.G.) awarded by the National Cancer Institute of the National Institutes of Health, and by funds from the Center for Radiation Research (M.G.), by Grants No. CA 18029 and CA 18105 (F.R.A.), by DHHS CA32102 supporting the Southwest Oncology Group Leukemia Biology Program, and in part by grant CA13539 from the National Cancer Institute.
Address reprint requests to Deborah E. Banker, PhD, Fred Hutchinson Cancer Research Center, 1124 Columbia St, Seattle, WA 98104.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal