Abstract
The plasma protein transcobalamin II (TCII) binds and delivers cobalamin (Cbl; vitamin B12) to all cells, which internalize the TCII/Cbl complex by receptor-mediated endocytosis. Congenital deficiency of TCII results in intracellular Cbl deficiency, one effect of which is to disrupt DNA synthesis, leading to megaloblastic anemia. We report here an in vitro culture system in which cell growth is dependent on delivery of Cbl to cells by TCII. Recombinant human holo-TCII was shown to support in dose-dependent manner the growth of the human erythroleukemic cell line K562 and the murine lymphoma cell line BW5147. Free Cbl also supported cell growth; however, at 100- to 1,000-fold higher concentrations than those effective in the presence of apo-TCII. To determine if cellular depletion of Cbl could be achieved by interfering with interactions between TCII/Cbl and its cell-surface receptor, several monoclonal antibodies raised against human TCII were studied. Three antibodies, found to compete for the same binding site on TCII, proved to be effective inhibitors of TCII/Cbl-dependent cell growth. Our results suggest that monoclonal anti-TCII antibodies that block the function of this protein may prove useful in antitumor therapies.
CELLULAR UPTAKE of cobalamin (Cbl; vitamin B12) is mediated by the plasma protein transcobalamin II (TCII),1 although several other Cbl-binding proteins aid in its absorption from the diet.2 The Cbl-TCII complex binds to specific high-affinity receptors on the surface of cells and is internalized by endocytosis.3 Human TCII has been characterized as a nonglycosylated 43-kD serum protein.4 The complementary DNA (cDNA) for human TCII has now been cloned5 and functional recombinant protein produced by SF9 insect cells using the baculovirus expression system.6
In humans, two essential metabolic pathways require Cbl as cofactors. One involves the methylation of homocysteine in the de novo synthesis of methionine, and is catalyzed by methionine synthase, and the other rearranges methylmalonyl CoA to succinyl CoA, and is catalyzed by methylmalonyl CoA mutase.7 Cbl deficiency leads to the impairment of these pathways, which results in megaloblastic anemia, methylmalonic aciduria, and CNS abnormalities.8,9 Cbl deficiency may be brought on by a lack of dietary Cbl, intestinal malabsorption due to a lack of intrinsic factor, failure of cellular uptake due to the absence or improper functioning of TCII,10 or errors of intracellular Cbl metabolism.11 In cases of impaired cellular uptake of Cbl, the problems associated with Cbl deficiency may be overcome through administration of doses of Cbl greatly in excess of physiological levels, whereas in cases of aberrant intracellular Cbl metabolism this therapy offers little relief.11
Although proliferating cells have been shown to take up more Cbl than nonproliferating cells in vitro,12 it has been difficult to establish in vitro growth conditions dependent on Cbl, since growth media contain generally high levels of Cbl and folic acid. Here we describe a proliferation assay and its dependence on Cbl. We have used this assay to characterize the biologic properties of recombinant human TCII (rhTCII) in a system in which leukemic cells require exogenous TCII-Cbl for survival and proliferation. Furthermore, we have used this system to characterize the ability of murine anti-human TCII monoclonal antibodies to neutralize this TCII-dependent cell growth. This study was also initiated to further assess the role of inhibitors of the uptake of Cbl and TCII in suppressing the growth and survival of malignant cells.
MATERIALS AND METHODS
The mouse lymphoma cell line BW5147 was from ATCC (Rockville, MD) and the human erythroleukemic cell line K562 from NRC (Ottawa, Canada). RPMI 1640 culture medium and RPMI 1640 culture medium deficient in cobalamin and folic acid were obtained from Stem Cell Technologies (Vancouver, Canada). Fetal calf serum (FCS) was from GIBCO (Grand Island, NY). Microfine precipitated silica (QUSO) was a gift from Degussa Corp (Ridgefield, NJ). Cyanocobalamin, folic acid (pteroylglutamic acid), 5-methyltetrahydrofolic acid, and DL-homocysteine were obtained from Sigma Chemical (St Louis, MO).
rhTCII.Recombinant protein was produced by infection of SF9 cells with baculovirus containing human TCII cDNA and purified as described previously.6 Recombinant human holo-TCII was dialyzed against several changes of phosphate/saline buffer (20 mmol/L NaPO4/1 mol/L NaCl, pH 8.5) for 3 days to remove the excess free Cbl. The Cbl concentration of recombinant human holo-TCII after dialysis was determined by measuring the optical density at 360 nm using the millimolar extinction coefficient of 34.6 as previously described.4 Aliquots of dialyzed recombinant human holo-TCII were stored in the buffer with 50% glycerol at −20°C.
Cell culture.BW5147 and K562 cells were maintained in complete RPMI 1640 medium supplemented with 10% FCS. Cells were grown in 60- × 15-mm culture dishes (Fisher Scientific, Nepean, Canada) in a humidified atmosphere (5% CO2 , 95% air) at 37°C. Cells used in the Cbl/TCII bioassay were grown to late log phase then washed three times in phosphate-buffered saline (PBS) before resuspension in Cbl-deficient bioassay medium.
Cbl/TCII bioassay.To measure TCII/Cbl-dependent cell growth, RPMI 1640 deficient in Cbl and folic acid was used (medium I) and FCS was pretreated with QUSO to reduce interference of endogenous bovine TCII/Cbl in the bioassay. In brief, 30 mg of QUSO was added per 1 mL of FCS, well mixed, and removed by centrifugation as described previously.13 In some experiments, RPMI 1640 deficient in Cbl with the folic acid replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine was used (medium II) Washed cells were plated out in 96-well microtiter plates (Nunc InterMed, Roskilde, Denmark) at 2,000 to 5,000 cells per well in 100 μL of either medium I or medium II supplemented with 10% QUSO-treated FCS. Following 5 to 7 days in culture with various concentrations of holo-TCII, CN-Cbl, apo-TCII, and/or anti-TCII monoclonal antibodies, cell viability was assessed by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) reduction, as described previously.14 15 Affinity-purified antibodies, to be tested in bioassays for growth inhibition, were dialyzed against RPMI 1640 deficient in Cbl and folic acid.
Cell counts.Cells were washed and transferred into 2-mL culture dishes in 1 mL of the indicated assay medium at 1 to 10 × 104 cells/mL and were split into fresh medium approximately every 6 days. At various times of culture, a 10-μL aliquot of the cell suspension was removed and mixed with an equal volume of eosin for determination of cell numbers. Viable cells were counted using a Neubauer Haemocytometer (Fischer Scientific, Nepean, Canada) and light microscopy.
Staining of apototic cells.A 10-μL quantity of a 1 μg/mL stock solution of acridine orange in PBS was added to 105 cells in 100 μL PBS. The mixture was incubated for 10 minutes at room temperature, washed once with PBS, and viewed under the fluorescent microscope.
Anti-human TCII monoclonal antibodies.The anti-human TCII antibodies used in this study have been described and characterized previously.16 Eight hybridoma cell lines producing monoclonal antibodies specific for human TCII were expanded as ascites fluid in pristane-primed Balb/C mice. Antibodies were purified from ascites fluid using a protein G affinity column (Pharmacia, Uppsala, Sweden). High stringency wash conditions applied consisted of three consecutive wash steps, 10 column volumes each. Buffer 1 consisted of 0.1 mol/L Tris-HCl pH 8.0, 1.0 mol/L NaCl, 0.1% Triton X-100; buffer 2 consisted of 0.1 mol/L Tris-HCl pH 8.0, 0.1 mol/L NaCl, 1.0% Triton X-100; and buffer 3 consisted of 0.1 mol/L Tris-HCl pH 8.0, 0.1% Triton X-100. Aliquots of purified antibodies were biotinylated as described previously.17 Antibody isotyping was performed on tissue culture–conditioned medium obtained from the different hybridoma supernatants, using the BioRad kit (Mississauga, Canada).
Competition sandwich–enzyme-linked immunosorbent assay analysis of monoclonal antibody binding to TCII.Enzyme-linked immunosorbent assay (ELISA) trays (Falcon; Becton Dickinson Labware, Oxnard, CA) were coated with 50 μL of each of the anti-TCII monoclonal antibodies at 10 μg/mL in PBS by incubation overnight at 4°C; plates were then blocked with PBS containing 0.5% skim milk powder. Recombinant human apo- or holo-TCII at 100 ng/mL (in milk/PBS) was then added and incubated for 2 hours. To assess competitive binding of anti-TCII antibodies, 50 μL of biotinylated anti-TCII monoclonal antibody was added at 10 μg/mL (in milk/PBS) and incubated for 2 hours before washing the wells nine times using milk/PBS then adding streptavidin-peroxidase (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Bound peroxidase was assayed by H2O2⋅ -mediated oxidation of 2,2′-azinodi-(3-ethylbenzthiazoline sulfonic acid) (Sigma Chemical), the reaction product being detected at 414 nm using a Bio-tek ELISA reader (Burlington, VT).
RESULTS
Effect of depletion of Cbl, folic acid, and TCII on cell growth.We compared the growth of K562 cells in complete RPMI and in RPMI lacking Cbl and folic acid (medium I), the medium in both cases being supplemented with 10% FCS. Figure 1A shows that K562 cells proliferate at a constant rate (doubling time, 14 to 20 hours) in complete RPMI, but fail to proliferate when cultured in RPMI lacking Cbl and folic acid, and only maintain their numbers until day 7, after which the numbers decline. Diluting cells with fresh Cbl and folic acid–free medium on day 10 restored the viability of cells a little before numbers declined once again. Medium containing 10 μmol/L folic acid but lacking Cbl supported cell proliferation at levels comparable to those observed in complete RPMI, whereas medium lacking folic acid but supplemented with 3.7 nmol/L CN-Cbl did not support proliferation of K562 cells. Moreover, many dead cells were observed in cultures lacking folic acid and Cbl.
Human cells fail to proliferate in Cbl/folic acid–deficient medium. (A) K562 cells were seeded at 1.2 × 105 cells/well (in 1-mL volumes) and cultured in RPMI (○), RPMI lacking Cbl and folic acid (▵), RPMI lacking Cbl supplemented with 10 μmol/L folic acid (□), RPMI lacking folic acid supplemented with 3.7 nmol/L CN-Cbl (▴). All media were supplemented with 10% FCS. QUSO treatment of FCS does not affect cell proliferation. (B) K562 cells were seeded at 1 × 104 cells/well and cultured in RPMI supplemented with 10% FCS (○) or 10% QUSO-treated FCS (•). At various times of culture, an aliquot (10 μL) of cells was removed for determination of cell numbers. Results are expressed as the mean ± SEM of a single cell number determination from three individual cultures.
Human cells fail to proliferate in Cbl/folic acid–deficient medium. (A) K562 cells were seeded at 1.2 × 105 cells/well (in 1-mL volumes) and cultured in RPMI (○), RPMI lacking Cbl and folic acid (▵), RPMI lacking Cbl supplemented with 10 μmol/L folic acid (□), RPMI lacking folic acid supplemented with 3.7 nmol/L CN-Cbl (▴). All media were supplemented with 10% FCS. QUSO treatment of FCS does not affect cell proliferation. (B) K562 cells were seeded at 1 × 104 cells/well and cultured in RPMI supplemented with 10% FCS (○) or 10% QUSO-treated FCS (•). At various times of culture, an aliquot (10 μL) of cells was removed for determination of cell numbers. Results are expressed as the mean ± SEM of a single cell number determination from three individual cultures.
To test if the removal of TCII from FCS using QUSO negatively affected the proliferation of K562 cells cultured in complete RPMI, the growth of cells in complete RPMI supplemented with either 10% FCS or 10% QUSO-treated FCS was compared. We observed that after an initial 1- to 2-day lag in growth, K562 cells in media supplemented with 10% QUSO-treated FCS proliferated at essentially the same rate as cells in media supplemented with 10% FCS (Fig 1B). This indicates that treatment of FCS with QUSO did not result in the removal of serum components important for the in vitro growth of cells in complete RPMI, which contains high levels of Cbl (3.7 nmol/L) and folic acid (2.3 μmol/L).
Cell growth in Cbl-deficient media.We compared cell growth in medium I, supplemented either with QUSO-treated FCS or untreated FCS, to test whether use of QUSO-treated FCS will allow the measurement of CN-Cbl– supported cell growth in this medium. Following 7 days of culture in media lacking Cbl and folic acid, addition of CN-Cbl resulted in a dose-dependent enhancement of the viability and proliferation of BW5147 cells when QUSO-treated FCS was used (Fig 2A). Maximal cell growth was achieved with 250 nmol/L CN-Cbl, but significant enhancement of cell growth above background required as little as 30 pmol/L CN-Cbl. Control experiments showed only marginal enhancement of cell growth over the same range of CN-Cbl concentrations in the presence of normal FCS. QUSO treatment of FCS also reduced the background of proliferation by sevenfold in the absence of CN-Cbl. CN-Cbl enhancement of K562 cell viability and proliferation was less dependent on pretreatment of FCS with QUSO than was the case with BW5147 cells. Nevertheless, a more pronounced dose-response curve was observed when CN-Cbl was added to cells grown in QUSO-treated FCS (Fig 2B). With QUSO-treated FCS, maximal stimulation of cell growth required 250 nmol/L CN-Cbl, and small effects on cell growth were seen with 60 pmol/L CN-Cbl. For similar effects on cell growth in the presence of normal FCS, higher concentrations of CN-Cbl were required.
QUSO treatment of FCS permits measurement of Cbl-dependent growth of mouse and human cells. Cells were seeded at 5,000 cells/well (in 0.1-mL volumes) and cultured in RPMI lacking Cbl and folic acid (medium I), supplemented with 10% FCS (○) or 10% QUSO-treated FCS (•). Serial dilutions of CN-Cbl were added to cultures and viability of murine BW5147 cells (A) and human K562 cells (B) was assessed, after 7 days in culture, using the MTT reduction assay. Results are expressed as the mean ± SEM of three replicate data points. Background represents cell viability in the absence of CN-Cbl for FCS (○) and QUSO-treated FCS (•).
QUSO treatment of FCS permits measurement of Cbl-dependent growth of mouse and human cells. Cells were seeded at 5,000 cells/well (in 0.1-mL volumes) and cultured in RPMI lacking Cbl and folic acid (medium I), supplemented with 10% FCS (○) or 10% QUSO-treated FCS (•). Serial dilutions of CN-Cbl were added to cultures and viability of murine BW5147 cells (A) and human K562 cells (B) was assessed, after 7 days in culture, using the MTT reduction assay. Results are expressed as the mean ± SEM of three replicate data points. Background represents cell viability in the absence of CN-Cbl for FCS (○) and QUSO-treated FCS (•).
We next examined the growth potential of BW5147 and K562 cells in RPMI medium that lacked Cbl and where the folic acid was replaced with the substrates for the Me-Cbl–dependent enzyme methionine synthase, homocysteine (1 μmol/L) and 5-methyltetrahydrofolic acid (1 μmol/L) (medium II). Once again, we used 10% FCS that had been pretreated with QUSO to reduce interference from endogenous bovine TCII. Concentrations of free CN-Cbl above 0.1 nmol/L were required for significant increases in cell growth and viability above background with both BW5147 and K562 cells in vitro (Fig 3). The addition of recombinant human apo-TCII (25 ng/mL) to these cultures resulted in cell growth at approximately 1,000-fold lower CN-Cbl concentrations. Recombinant holo-TCII exhibited a dose-dependent enhancement of the viability and proliferation of both BW5147 cells (Fig 4A) and K562 cells (Fig 4B). Maximal proliferation was observed with 100 ng/mL (2.3 nmol/L) holo-TCII, which increased the signal with BW5147 cells sixfold above background levels. In the case of K562 cells, holo-TCII increased the signal twofold to threefold above background. These results indicate that in appropriate medium, cell growth and viability in vitro is dependent on the delivery of CN-Cbl to the cell by the natural route via uptake of holo-TCII.
Recombinant apo-TCII reduces the concentration of Cbl required to support in vitro cell growth. Cells were seeded at 2,000 cells/well (in 0.1-mL volumes), cultured in RPMI lacking Cbl, where the folic acid was replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine (medium II) and then supplemented with 10% QUSO-treated FCS. Serial dilutions of CN-Cbl were added to cultures. After 5 days in culture, viability of BW5147 cells (A) and K562 cells (B) was assessed using the MTT reduction assay. (○), No apo-TCII; (•), 25 ng/mL apo-TCII. Results are expressed as the mean ± SEM of 3 replicate data points. Background represents cell viability in the absence of Cbl and apo-TCII (○) or absence of Cbl and presence of 25 ng/mL apo-TCII (•).
Recombinant apo-TCII reduces the concentration of Cbl required to support in vitro cell growth. Cells were seeded at 2,000 cells/well (in 0.1-mL volumes), cultured in RPMI lacking Cbl, where the folic acid was replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine (medium II) and then supplemented with 10% QUSO-treated FCS. Serial dilutions of CN-Cbl were added to cultures. After 5 days in culture, viability of BW5147 cells (A) and K562 cells (B) was assessed using the MTT reduction assay. (○), No apo-TCII; (•), 25 ng/mL apo-TCII. Results are expressed as the mean ± SEM of 3 replicate data points. Background represents cell viability in the absence of Cbl and apo-TCII (○) or absence of Cbl and presence of 25 ng/mL apo-TCII (•).
rhTCII supports the growth of human and mouse cells. Cells were seeded at 2,000 cells/well (in 0.1-mL volumes), cultured in RPMI lacking Cbl where the folic acid was replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine (medium II), and then supplemented with 10% QUSO-treated FCS. Serial dilutions of recombinant TCII were added to cultures. Viability of murine BW5147 cells (A) and human K562 cells (B) was assessed after 5 days using the MTT reduction assay. Results are expressed as the mean ± SEM of six replicate data points. Background represents cell viability in the absence of holo-TCII (•).
rhTCII supports the growth of human and mouse cells. Cells were seeded at 2,000 cells/well (in 0.1-mL volumes), cultured in RPMI lacking Cbl where the folic acid was replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine (medium II), and then supplemented with 10% QUSO-treated FCS. Serial dilutions of recombinant TCII were added to cultures. Viability of murine BW5147 cells (A) and human K562 cells (B) was assessed after 5 days using the MTT reduction assay. Results are expressed as the mean ± SEM of six replicate data points. Background represents cell viability in the absence of holo-TCII (•).
Effect of anti-TCII monoclonal antibodies on in vitro cell proliferation.Monoclonal antibodies raised against human TCII had been characterized into three distinct types based on their properties. Type I inhibits the binding of TCII to its receptor; type II blocks binding of Cbl to TCII; type III blocks neither, but still can be used to immunoprecipitate TCII.16 We tested three of the type I and five of the type II anti-TCII antibodies for their ability to inhibit holo-TCII–dependent proliferation of murine and human leukemic cells. The growth of BW5147 cells and K562 cells in medium I supplemented with 100 ng/mL recombinant human holo-TCII was analyzed after 7 days in culture in the presence of serial dilutions of affinity-purified murine anti-TCII monoclonal antibodies. Figure 5 shows that three monoclonal antibodies, 2-2, 3-11, and 4-7 (classified previously as type I), exhibit strongest ability to block cell growth. The type II monoclonal antibody 2-6 had intermediate inhibitory activity and the other four type II monoclonal antibodies, 5-18, 3-5, 3-9, and 1-9, exhibited little or no inhibitory activity at the antibody concentrations used. Growth of both cell lines tested, BW5147 and K562, was inhibited by the antibodies with slightly different dose-response characteristics. The three monoclonal antibodies that exhibit the strongest neutralizing activity completely inhibited proliferation of BW5147 cells at concentrations as low as 0.02 μg/mL (Fig 5A), whereas higher antibody concentrations were required for similar inhibitory effects on K562 cells (Fig 5B). Dead cells were apparent in K562 cell cultures grown in presence of monoclonal antibody 2-2. Examination of cells from those cultures by staining with acridine orange showed typical apoptotic bodies (data not shown).
Anti-TCII antibodies inhibit in vitro cell growth to varying degrees. Cells were seeded at 5,000 cells/well (in 0.1-mL volumes) and cultured in RPMI lacking Cbl and folic acid (medium I), supplemented with 10% QUSO-treated FCS and 100 ng/mL recombinant human holo-TCII. Serial dilutions of anti-TCII monoclonal antibodies (mAbs) were added to the wells and viability of BW5147 cells (A) and K562 cells (B) assessed after 7 days in culture using the MTT reduction assay. Results are expressed as the mean ± SEM of triplicate determinations. mAb 3-9 (○), mAb 2-6 (•), mAb 4-7 (▪), mAb 3-11 (▴), mAb 1-9 (□), mAb 5-18 (▵), mAb 2-2 (▾), and mAb 3-5 (▿). Control cultures were grown in presence of TCII (100 ng/mL) and absence of antibody (○). Background represents cell viability in the absence of holo-TCII (□).
Anti-TCII antibodies inhibit in vitro cell growth to varying degrees. Cells were seeded at 5,000 cells/well (in 0.1-mL volumes) and cultured in RPMI lacking Cbl and folic acid (medium I), supplemented with 10% QUSO-treated FCS and 100 ng/mL recombinant human holo-TCII. Serial dilutions of anti-TCII monoclonal antibodies (mAbs) were added to the wells and viability of BW5147 cells (A) and K562 cells (B) assessed after 7 days in culture using the MTT reduction assay. Results are expressed as the mean ± SEM of triplicate determinations. mAb 3-9 (○), mAb 2-6 (•), mAb 4-7 (▪), mAb 3-11 (▴), mAb 1-9 (□), mAb 5-18 (▵), mAb 2-2 (▾), and mAb 3-5 (▿). Control cultures were grown in presence of TCII (100 ng/mL) and absence of antibody (○). Background represents cell viability in the absence of holo-TCII (□).
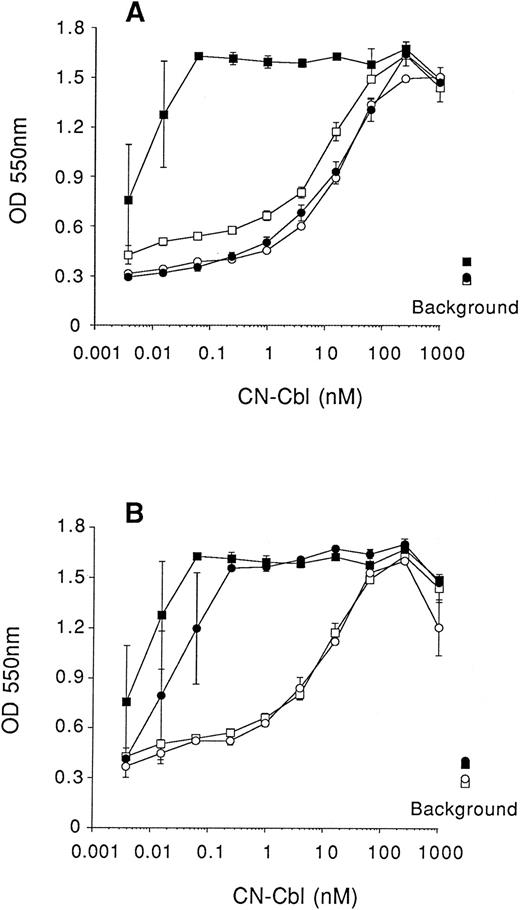
As an additional control experiment we measured, in a 5-day assay, the effect of one type I and one type II monoclonal antibody on the proliferation of K562 cells in the presence of free CN-Cbl, with or without apo-TCII in medium II. A single concentration (1 μg/mL) of either monoclonal antibody 2-2 (type I) or monoclonal antibody 5-18 (type II) was added to a series of culture wells containing titrated amounts of free CN-Cbl in the absence and presence of 25 ng/mL apo-TCII. Both the type I (Fig 6A) and type II (Fig 6B) monoclonal antibody had no inhibitory effect on the proliferation of K562 cells supported by free CN-Cbl, whereas the type I monoclonal antibody but not the type II monoclonal antibody abrogated the enhancement of proliferation usually seen with apo-TCII. These results confirmed that the type I monoclonal antibodies were inhibitory and the type II monoclonal antibodies were not. These results also indicate that the proliferation of K562 cells observed with CN-Cbl is due to uptake of free Cbl and eliminate the possibility that the uptake was dependent on TCII synthesized by the cultured cells themselves.
Inhibition of cell growth induced by anti-TCII antibody is abrogated by free Cbl. Cells were seeded at 2,000 cells/well (in 0.1-mL volumes), cultured in RPMI lacking Cbl in which the folic acid was replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine (medium II), and then supplemented with 10 % QUSO-treated FCS. Serial dilutions of CN-Cbl were added to cultures and cell growth measured, using the MTT reduction assay, after 5 days of culture. (□), No antibody, no recombinant human apo-TCII; (▪), no antibody, 25 ng/mL apo-TCII; (○), antibody (1 μg/mL), no apo-TCII; (•), antibody (1 μg/mL), 25 ng/mL apo-TCII. (A) mAb 2-2 was added where indicated (•,○); (B) mAb 5-18 was added where indicated (○,•). Results are expressed as the mean ± SEM of three replicate data points. Background represents cell viability in the absence of Cbl and apo-TCII with (○) and without (□) antibody added, or absence of Cbl and presence of 25 ng/mL apo-TCII with (•) and without (▪) antibody added.
Inhibition of cell growth induced by anti-TCII antibody is abrogated by free Cbl. Cells were seeded at 2,000 cells/well (in 0.1-mL volumes), cultured in RPMI lacking Cbl in which the folic acid was replaced with 1 μmol/L 5-methyltetrahydrofolic acid and 1 μmol/L homocysteine (medium II), and then supplemented with 10 % QUSO-treated FCS. Serial dilutions of CN-Cbl were added to cultures and cell growth measured, using the MTT reduction assay, after 5 days of culture. (□), No antibody, no recombinant human apo-TCII; (▪), no antibody, 25 ng/mL apo-TCII; (○), antibody (1 μg/mL), no apo-TCII; (•), antibody (1 μg/mL), 25 ng/mL apo-TCII. (A) mAb 2-2 was added where indicated (•,○); (B) mAb 5-18 was added where indicated (○,•). Results are expressed as the mean ± SEM of three replicate data points. Background represents cell viability in the absence of Cbl and apo-TCII with (○) and without (□) antibody added, or absence of Cbl and presence of 25 ng/mL apo-TCII with (•) and without (▪) antibody added.
Antibody capture sandwich–ELISA.To characterize differences in epitope recognition by the eight monoclonal antibodies, we compared the ability of pairs of anti-TCII monoclonal antibodies, on the one hand to capture recombinant human apo- and holo-TCII, and on the other to bind to captured TCII. Each of the eight anti-TCII monoclonal antibodies was used to individually coat wells of an ELISA tray. The bound antibodies were then used to capture recombinant human apo- or holo-TCII. The captured TCII was then detected using each of the eight biotinylated preparations of the monoclonal antibodies individually. In cases where the biotinylated antibody used for detection binds to an epitope overlapping with the plate-bound antibody used to capture the TCII, a greatly reduced signal is expected. Table 1 shows the results obtained in summary form. These results demonstrate that the eight anti-TCII monoclonal antibodies captured both apo- and holo-TCII efficiently and that the antibodies could be divided into three groups on the basis of competition for epitopes. Thus, three of the type I antibodies, 3-11 (IgG2a), 2-2 (IgG2a), and 4-7 (IgG2b), compete for binding to one epitope. Two of the type II monoclonal antibodies, 1-9 (IgG2a) and 5-18 (IgG2a), bind to a second epitope, whereas the other three type II monoclonal antibodies, 3-9 (IgG1), 3-5 (IgG2b), and 2-6 (IgG1), bind to a third epitope. When tested in our assay, there was no evidence that any of the antibodies reacted specifically with either apo- or holo-TCII, although monoclonal antibodies 1-9 and 5-18 appeared to preferentially capture holo-TCII over apo-TCII. This is in contrast with previous results that indicate that the type I monoclonal antibodies preferentially recognize holo-TCII and the type II monoclonal antibodies bind to a site on TCII close to the Cbl-binding site and as a consequence interfere with Cbl binding.16
Detection of rhTCII in a Sandwich ELISA by Pairs of Anti-TCII Monoclonal Antibodies
| Monoclonal Antibody . | Capturing Antibody . | |||||||
|---|---|---|---|---|---|---|---|---|
| . | 3-11 . | 1-9 . | 3-9 . | 5-18 . | 2-2 . | 2-6 . | 3-5 . | 4-7 . |
| holo-TCII | ||||||||
| 3-11 | − | + | + | + | − | + | + | − |
| 1-9 | +++ | − | ++ | − | +++ | +++ | ++++ | ++++ |
| 3-9 | +++++ | +++++ | − | +++++ | ++++ | − | − | +++++ |
| 5-18 | ++ | − | + | − | ++ | +++ | +++ | +++ |
| 2-2 | − | ++ | + | + | − | + | + | − |
| 2-6 | +++++ | +++++ | − | ++++ | ++++ | − | − | +++++ |
| 3-5 | ++ | ++++ | − | +++ | ++ | − | − | ++++ |
| 4-7 | − | +++++ | ++++ | ++++ | − | +++++ | +++++ | − |
| apo-TCII | ||||||||
| 3-11 | − | + | + | − | − | + | + | − |
| 1-9 | +++ | − | ++ | − | ++ | ++ | +++ | +++ |
| 3-9 | +++++ | ++ | − | + | ++++ | − | − | +++++ |
| 5-18 | ++ | − | ++ | − | ++ | ++ | ++ | ++ |
| 2-2 | − | + | + | − | − | + | + | − |
| 2-6 | +++++ | ++ | − | + | ++++ | − | − | +++++ |
| 3-5 | +++ | ++ | + | − | ++ | − | − | +++ |
| 4-7 | − | ++ | ++++ | + | − | +++++ | +++++ | − |
| Monoclonal Antibody . | Capturing Antibody . | |||||||
|---|---|---|---|---|---|---|---|---|
| . | 3-11 . | 1-9 . | 3-9 . | 5-18 . | 2-2 . | 2-6 . | 3-5 . | 4-7 . |
| holo-TCII | ||||||||
| 3-11 | − | + | + | + | − | + | + | − |
| 1-9 | +++ | − | ++ | − | +++ | +++ | ++++ | ++++ |
| 3-9 | +++++ | +++++ | − | +++++ | ++++ | − | − | +++++ |
| 5-18 | ++ | − | + | − | ++ | +++ | +++ | +++ |
| 2-2 | − | ++ | + | + | − | + | + | − |
| 2-6 | +++++ | +++++ | − | ++++ | ++++ | − | − | +++++ |
| 3-5 | ++ | ++++ | − | +++ | ++ | − | − | ++++ |
| 4-7 | − | +++++ | ++++ | ++++ | − | +++++ | +++++ | − |
| apo-TCII | ||||||||
| 3-11 | − | + | + | − | − | + | + | − |
| 1-9 | +++ | − | ++ | − | ++ | ++ | +++ | +++ |
| 3-9 | +++++ | ++ | − | + | ++++ | − | − | +++++ |
| 5-18 | ++ | − | ++ | − | ++ | ++ | ++ | ++ |
| 2-2 | − | + | + | − | − | + | + | − |
| 2-6 | +++++ | ++ | − | + | ++++ | − | − | +++++ |
| 3-5 | +++ | ++ | + | − | ++ | − | − | +++ |
| 4-7 | − | ++ | ++++ | + | − | +++++ | +++++ | − |
Pairs of anti-TCII monoclonal antibodies were tested for binding to recombinant human holo-TCII and recombinant human apo-TCII. Data are expressed in +/− format, where + represents approximately 0.3 OD units and − represents background OD.
DISCUSSION
Cbl largely exerts its effects on cell growth by acting as a cofactor for methionine synthase.4 This enzyme catalyzes the transfer of a methyl group from methyltetrahydrofolic acid to homocysteine to produce methionine and regenerates tetrahydrofolic acid, a metabolite essential for providing single carbon units for DNA synthesis. If this reaction does not occur, single carbon units remain trapped as methyltetrahydrofolic acid; this is known as the methylfolate trap.18 Thus, Cbl deficiency indirectly affects DNA synthesis. We now have established a cell culture system in which we are able to demonstrate the growth dependence of murine and human leukemic cells on exogenously supplied holo-TCII or free CN-Cbl. This required the use of medium that lacked Cbl and folic acid and was supplemented with FBS from which endogenous bovine TCII was removed by treatment with QUSO. Folic acid was able to almost completely restore the proliferation of K562 cells, even though Cbl was not present in the growth medium (Fig 1A). To demonstrate support of cell growth by CN-Cbl, it was necessary to pretreat the FCS with QUSO (Fig 2). Addition of rhTCII, the Cbl transport protein, to cell cultures growing in presence of free CN-Cbl, reduced the concentration of free CN-Cbl required for sustained cell growth by approximately three logs, confirming earlier observations made by others using purified human apo-TCII.19-21 Furthermore, supplementation of the bioassay medium with low concentrations of homocysteine and 5-methyltetrahydrofolic acid resulted in considerable improvement of the reproducibility and sensitivity of the Cbl-dependent bioassay.
Previously it has been demonstrated that murine leukemic cells require TCII and/or Cbl for optimal growth in vitro.19-21 Although these studies were performed with essentially the same growth medium as the present study, there were some important differences. The earlier studies were performed using “serum-free” media in which the FBS (containing protein-bound Cbl) was replaced by bovine serum albumin. However, bovine serum albumin has been shown to contain low but significant Cbl-binding capacity.22 Furthermore, these earlier studies used TCII that was purified from human serum.4 Thus, our study is the first demonstration of in vitro cell growth that is dependent on rhTCII and compares its effects on the growth of murine and human leukemic cell lines.
In the absence of TCII, effects of CN-Cbl on the growth of both murine BW5147 cells and human K562 cells were observed with concentrations of free CN-Cbl as low as 60 pmol/L. Optimal cell growth required concentrations of free CN-Cbl of 200 nmol/L, whereas in the presence of recombinant human apo-TCII (25 ng/mL), maximal cell growth was achieved with as little as 0.2 nmol/L CN-Cbl. These results differ from the previous findings with the cell line L1210, which showed that cell growth was optimal with 4,000 pmol/L free aqua-Cbl or 2 pmol/L TCII-bound Cbl20 and with the cell line P388D, which showed that cell growth was optimal with 37 pmol/L free CN-Cbl or 0.37 pmol/L TCII-bound Cbl.21 These differences may be explained by the use of different cell lines, different media composition, and the use of partially purified human TCII rather than rhTCII. Nevertheless, all of these findings are in basic agreement in that the concentration of Cbl required for support of cell growth is reduced 100- to 1,000-fold in the presence of TCII. Our results also suggest, albeit indirectly, that receptors recognizing human TCII-Cbl exist on the surface of both the murine and human leukemic cell lines used. Indeed, TCII receptors have been demonstrated on K562 cells previously.22
The eight anti-TCII monoclonal antibodies we used in our study could be divided into three groups recognizing three different nonoverlapping epitopes, based on their cross-competition for binding to recombinant TCII (Table 1). The antibodies that were the most efficient in neutralizing cell proliferation belonged to one of these groups. We suggest that these neutralizing antibodies bind to a site on TCII that is also recognized by the cell-surface receptor for TCII, thereby blocking binding of Cbl-TCII to the TCII cell-surface receptor. This is in agreement with a study by Carmel and Linker-Israeli23 that described a monoclonal antibody against human TCII that blocked uptake of Cbl-TCII by K562 cells. The anti-TCII monoclonal antibodies used in the present study had been characterized previously for effects on Cbl-TCII uptake by K562 cells where it was shown that both type I (receptor-blocking) and type II (Cbl-blocking) antibodies could block uptake of Cbl-TCII.16 This is in contrast to the results of the present study, which show that only type I antibodies can neutralize cell proliferation, although at higher concentrations, certain type II antibodies did show some neutralizing effects (Fig 5). These differences between the results of the two studies likely reflect differences in the manner the experiments were performed.
The possibility that the antibodies that failed to inhibit cell proliferation were characterized by a lower affinity for TCII can be ruled out, since all the monoclonal antibodies demonstrated comparable potency in immunoprecipitating 57Co-Cbl-TCII from human serum.16 This difference in abilities of anti-TCII monoclonal antibodies to inhibit cell proliferation, despite their similar capacities for precipitating TCII, is further confirmation that the antibodies recognize different epitopes on TCII. Interestingly, monoclonal antibody 2-6, which inhibits growth, and monoclonal antibody 3-9, which does not, both compete for binding to the same epitope on TCII. Clearly, there are some fine differences in functional capacity to neutralize TCII, even between antibodies that cross-compete for similar epitopes. Our observation that monoclonal antibody 2-2, which strongly neutralized TCII-dependent cell growth, failed to inhibit the growth of K562 cells in the presence of free CN-Cbl indicated that the growth of these cells was not dependent on their synthesis of TCII. Furthermore, this suggests that the inhibitory action of these monoclonal antibodies on growth may be reversed by addition of high concentrations of free Cbl. This is important in terms of the possible clinical use of the anti-TCII antibodies in treating cancer, as pharmacologic doses of Cbl have been safely used in the clinic to overcome the effects of genetic deficiency of TCII.11 Although the dose-response curves for inhibition of TCII-dependent cell growth of BW5147 and K562 cells were identical, the inhibition by TCII-specific monoclonal antibodies was more complete with BW5147 cells. This suggests that BW5147 may be more sensitive to alterations in growth and viability following limitation of Cbl uptake, perhaps reflecting a faster intracellular turnover of Cbl. Indeed, this is consistent with the results shown in Fig 4, which indicate that the viability of BW5147 cells is stimulated to a greater extent by holo-TCII than is the case with K562 cells.
It appears from our results that the inhibition of increase in cell numbers by anti-TCII monoclonal antibodies likely reflects a reduction in the proliferation rate and an increase in the rate of cell death. The established role of Cbl in DNA synthesis24 suggests that Cbl depletion is likely to result both in reduced cell proliferation and cell death, as inhibition of DNA synthesis in lymphohematopoietic cells generally results in apoptotic death.25 This demonstration that anti-TCII monoclonal antibodies can inhibit cell proliferation supports the theory that Cbl antagonists may be useful as inhibitors of cell proliferation. The fact that their effects should be rapidly reversed by administration of pharmacologic doses of Cbl may make them particularly useful in cancer therapy.
ACKNOWLEDGMENT
We thank Michael Williams, Helen Merkens, Kirsten Tisdall, and Shelly Duquette for excellent technical assistance; Dr Roy Walker (NRC) for valuable discussions; and Dr Clive Woodhouse (Receptagen Ltd) for valuable discussions and critical reading of the manuscript.
Supported by Receptagen Ltd and Grant No. R01-DK28561-14 from the National Institutes of Health (to S.P.R.).
Address reprint requests to Hermann J. Ziltener, PhD, The Biomedical Research Centre, 2222 Health Sciences Mall, The University of British Columbia, Vancouver, BC, Canada V6T 1Z3.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal