Abstract
Fas belongs to the family of type-1 membrane proteins that transduce apoptotic signals. In the present studies, we characterized signaling during Fas-induced apoptosis in RPMI-8226 and IM-9 multiple myeloma (MM) derived cell lines as well as patient plasma cell leukemia cells. Treatment with anti-Fas (7C11) monoclonal antibody (MoAb) induced apoptosis, evidenced by internucleosomal DNA fragmentation and propidium iodide staining, and was associated with increased expression of c-jun early response gene. We also show that anti-Fas MoAb treatment is associated with activation of stress-activated protein kinase (SAPK) and p38 mitogen-activated protein kinase (MAPK); however, no detectable increase in extracellular signal-regulated kinases (ERK1 and ERK2) activity was observed. Because interleukin-6 (IL-6) is a growth factor for MM cells and inhibits apoptosis induced by dexamethasone and serum starvation, we examined whether IL-6 affects anti-Fas MoAb-induced apoptosis and activation of SAPK or p38 MAPK in MM cells. Culture of MM cells with IL-6 before treatment with anti-Fas MoAb significantly reduced both DNA fragmentation and activation of SAPK, without altering induction of p38 MAPK activity. These results therefore suggest that anti-Fas MoAb-induced apoptosis in MM cells is associated with activation of SAPK, and that IL-6 may both inhibit apoptosis and modulate SAPK activity.
MULTIPLE MYELOMA (MM) is characterized by the presence of malignant plasma cells predominantly localized in bone marrow (BM). The role of interleukin-6 (IL-6) as an autocrine and paracrine growth factor for MM cells is well established.1-7 Preliminary studies of IL-6 signal transduction in MM cell lines and MM patient cells suggest that the Ras dependent mitogen-activated protein kinase (MAPK) cascade mediates growth of MM cells.8 Moreover, abnormalities of cell cycle regulatory proteins, ie, RB and p16, may also play a role in IL-6 mediated MM cell growth.9 10
IL-6 has also been shown to be a survival factor for MM cells, since it inhibits apoptosis of MM cell lines and patient cells induced by serum starvation and dexamethasone.11,12 However, the signaling cascades involved in initiating programmed cell death in MM cells, evidenced by nuclear chromatin condensation and DNA fragmentation,13-19 have not been fully characterized. Specifically, triggering via Fas (APO-1/CD95) Ag, a transmembrane molecule belonging to the protein superfamily of tumor necrosis factor (TNF )/nerve growth receptors, can induce apoptosis in a variety of cell types.20-24 Recent studies have shown that Fas Ag is variably expressed on some purified plasma cell leukemia and MM cells as well as derived cell lines, and that crosslinking of Fas Ag induces apoptosis.25-27 Proposed mechanisms of resistance of MM cells to Fas-induced apoptosis include lack of cell surface expression of Fas Ag and high Bcl-2 expression in spite of Fas Ag expression. The sensitivity of purified MM cells and resistance of MM BM mononuclear cells (BMMCs) to Fas-triggered apoptosis, coupled with the ability of human serum to block Fas-induced apoptosis, suggest the presence of a soluble inhibitory factor.25 Although IL-6 has been shown to inhibit decadron-induced apoptosis in MM cells, at least in part by upregulating bcl-x1 expression,24,25 28 the effect of IL-6 on anti-Fas MoAb induced apoptosis and its mechanism of action has not been extensively characterized.
Signaling cascades involved in apoptosis are now being delineated. Specifically, previous studies in our laboratory have demonstrated that DNA damaging agents, ie, 1-β-D-arabinofuranosylcytosine (ara-c) and ultraviolet (UV) light, induce DNA-fragmentation and induction of c-jun gene in myeloid leukemic cells.29-31 This finding suggests that activation of specific transcription factors in response to genotoxic stress may play a role in the regulation of programmed cell death. In addition, studies have demonstrated that c-Jun is phosphorylated at two serine residues (Ser63 and Ser73 ) in its amino-terminal transactivation domain by stress-activated protein kinase (SAPK) or c-Jun amino-terminal kinase (JNK), a member of the MAPK-related family of p54/46 serine/threonine kinases.32-34 Moreover, we and others have shown that stress stimuli, ie, ionizing radiation, TNF, sphingomyelinase and UV light activate SAPK.33,35-37 Finally, recent studies have shown that extracellular signal-regulated kinase (ERK), SAPK, and p38 MAPK all play an important role in regulating cell survival or apoptosis.38 39 To date, however, the role of these kinases in Fas-triggered apoptosis of MM cells has not been delineated.
In the present studies, we examined whether SAPK, p38 MAPK, ERK1, and ERK2 are activated during Fas-induced apoptosis of MM cells. Our studies show that triggering via Fas MoAb induces DNA fragmentation, c-jun expression, and activation of SAPK and p38 MAPK in MM cells without inducing ERK1 or ERK2. Because IL-6 is a growth factor for MM cells and is known to inhibit dexamethasone-induced apoptosis in MM cells,1-7,11 12 we also studied the effect of IL-6 pretreatment of MM cells on Fas-induced DNA fragmentation as well as SAPK and p38 MAPK activation. Pretreatment of cells with IL-6 significantly inhibits both DNA fragmentation and SAPK activity induced via Fas, without altering p38 MAPK. Our studies therefore suggest that IL-6 can modulate Fas-triggered apoptosis and SAPK activation in MM cells.
MATERIALS AND METHODS
Cell Culture
The human MM cell lines RPMI-8226 and IM9 (ATCC, Rockville, MD) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma, St Louis, MO), 100 U/mL penicillin (pen), 100 mg/mL streptomycin (strep) and 2 mmol/L L-glutamine (GIBCO, Grand Island, NY). Mononuclear cells were isolated from the peripheral blood of a patient with plasma cell leukemia (PCL) by Ficoll-Hypaque density gradient centrifugation and incubated with HB7 (anti-CD38) monoclonal antibody (MoAb)-biotin-streptavidin and 2H4 (anti-CD45RA) MoAb-fluorescein isothiocyanate on ice. Tumor cells (96 ± 2% CD38+CD45RA−) were isolated using an Epics C Cell Sorter (Coulter Electronics, Hialeah, FL), washed, and resuspended in RPMI-1640 media containing 10% FBS, 2 mmol/L L-glutamine, 100 U/mL Pen, and 100 U/mL Strep. Cells were treated with either medium or with 7C11 anti-Fas MoAb (1:800)23 for the indicated intervals prior to analysis for apoptosis, Northern blotting for c-jun mRNA, as well as in vitro SAPK, p38 MAPK, ERK1, and ERK2 assays. In some experiments, MM cells were treated with IL-6 (100 ng/mL) for 3 to 4 hours before culture with anti-Fas MoAb.
Determination of Apoptosis
Two different methods were used to confirm anti-Fas-induced apoptosis in MM cells.
DNA fragmentation assays.Genomic DNA was isolated from RPMI-8226, IM9, and PCL cells as previously described.40 Briefly, 1 to 2 × 106 cells were washed two times with phosphate buffered saline (PBS) and lysed in buffer (100 mmol/L NaCl, 10 mmol/L Tris-HCL pH 8.0, 25 mmol/L EDTA, 0.5% sodium dodecyl sulfate [SDS], and 0.1 mg/mL proteinase K) at 37°C for 18 hours. DNA was extracted with an equal volume of phenol/chloroform (1:1) and precipitated at −70°C for 2 hours with 0.3 mol/L NaAcetate and 2.5 volume of absolute ethanol. DNA pellets were resuspended in 20 L of 10 mmol/L Tris (pH 7.8) 1 mmol/L EDTA buffer and incubated for 1 hour at 37°C with 1 μg/mL RNase (Boehringer Mannheim Corp, Indianapolis, IN).
DNA-end labeling was performed as previously described.41 Briefly, 0.5 to 1.0 μg of DNA was treated with 5 U of Klenow polymerase with 0.5 μCi of [γ32P]-dCTP in reaction mixture (Tris-HCL pH 7.5, 5 mmol/L MgCl2 ) for 10 minutes at room temperature and terminated by addition of 10 mmol/L EDTA. Unincorporated nucleotides were removed by three consecutive precipitation cycles with ammonium acetate/isopropanol, and labeled DNA was resuspended in 10 mmol/L Tris-HCL (pH 7.5) 1 mmol/L EDTA. Labeled DNA probes were electrophoresed for 2 to 3 hours at 90 V on 1.8% agarose gels, which were dried and exposed for autoradiography.
Flow cytometric analyses.1 ×106 cells were washed twice with cold PBS and fixed by the addition of 40% cold ethanol at 4°C for 30 minutes. Cells were then washed with PBS and treated with RNase (50 μg/mL) for 45 minutes at 37°C. After treatment with RNase, cells were washed with PBS and resuspended in PBS containing 15 μg/mL propidium iodide. Flow cytometric analyses was done using FACScan (Becton Dickinson, San Diego, CA).
RNA Isolation and Northern Blot Analysis
Total cellular RNA was purified using the guanidine isothiocyanate cesium chloride technique as previously described.42 Briefly, total cellular RNA (20 μg/lane) was subjected to electrophoresis through a 1% agarose/2.2 mol/L formaldehyde gel, transferred to nitrocellulose filters, and hybridized to one of the following 32P-labeled DNA probes: (1) the 1.8 kb BamHI/EcoRI insert of a human c-jun gene purified from a pBluescript SK(+) plasmid43; and (2) the 2.0 kb Pst I insert of a chicken β-actin gene purified from the pA1 plasmid.44 Hybridizations were performed at 42°C for 24 hours in 50% (vol/vol) formamide, 2 × SSC (SSC: 0.15 mol/L sodium chloride, 0.015 mol/L sodium citrate), 1 × Denhardt's solution, 0.1% (wt/vol) SDS, and 200 μg/mL salmon sperm DNA. The filters were washed twice, first in 2 × SSC-0.1% SDS at room temperature and next in 0.1 × SSC-0.1% SDS at 60°C for 30 minutes, and then exposed to Kodak X-Omat XAR film (Eastman Kodak, Rochester, NY) using an intensifying screen. The autoradiograms were scanned using an LKB produkter (Bromma, Sweden) Ultrascan XL laser densitometer and analyzed with the Gelscan software package. Signal intensity was determined in a linear range and normalized to that for actin.
Fusion Protein Binding Assays
Cells (2 to 3 × 107) were washed with PBS and lysed in 1 mL of lysis buffer (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 15 mmol/L NP-40, 1 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L DTT, and 10 mg/mL of leupeptin and aprotinin). After incubation on ice for 30 minutes, insoluble material was removed by centrifugation at 14,000 rpm for 10 minutes at 4°C. Lysates were incubated with 5 mg immobilized GST or GST-Jun (2-100)fusion protein for 2 hours at 4°C. GST-Jun fusion protein was prepared as previously described.45 Protein complexes were washed with lysis buffer and incubated in kinase buffer (20 mmol/L HEPES, pH 7.0, 10 mmol/L MgCl2 , and 10 mmol/L MnCl2 ) containing [γ-32P]ATP for 15 minutes at 30°C. Reactions were terminated by boiling in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and phosphorylated proteins analyzed by SDS-PAGE, Coomassie blue staining, and autoradiography.
Immune Complex Kinase Assays
Assays were performed as previously described.46 Lysates were precleared by incubating with 5 mg/mL rabbit-antimouse IgG for 1 hour at 4°C, followed by a 30 minutes incubation with protein A-Sepharose. Supernatants were incubated with either preimmune rabbit serum or specific antibodies for SAPK or ERK1/ERK2 (Santa Cruz Biotechnology, San Diego, CA) or p38 MAPK (provided by Dr Roger Davis, University of Massachusetts Medical School, Worcester) for 2 hours at 4°C before the addition of protein A-Sepharose for 1 hour. Immune complexes were washed three times with lysis buffer, once with kinase buffer, and resuspended in kinase buffer containing 1 mCi/mL [γ-32P]ATP (3,000 ci/mmol; New England Nuclear, Boston, MA) and GST-Jun(2-100) or GST-ATF2 (provided by Dr Roger Davis) or myelin basic protein (MBP) substrate. The reaction was incubated for 15 minutes at 30°C and terminated by the addition of SDS sample buffer. The proteins were analyzed by SDS-PAGE, Coomassie blue staining, and autoradiography.
Phenotypic Analysis of MM Cells
Cells were examined for the expression of Fas Ag before and after culture for 3 to 4 hours with IL-6 (100 ng/mL) using 7C11 anti-Fas MoAb and indirect immunofluorescence and flow cytometric analysis, as in previous studies.6 Antibody-coated cells were enumerated by flow cytometric analysis using an EPICS V cell sorter (Coulter Electronics).
RESULTS
Anti-Fas MoAb Induces Apoptosis in MM Cells
To test for Fas-triggered apoptosis, RPMI-8226 MM cells were cultured in media alone or with anti-Fas MoAb for 3, 6, and 9 hours. DNA cleavage was analysed by agarose gel electrophoresis of the 32P-labeled genomic DNA. Low to undetectable DNA clevage was observed in the cells cultured in media alone (Fig 1A). In contrast, exposure to anti-Fas MoAb resulted in significant clevage of DNA into oligonucleosomal fragments of approximately 200 bp. This pattern was most prominent after 6 and 9 hour culture with anti-Fas MoAb; longer periods of exposure to anti-Fas MoAb (24 hours and 48 hours) resulted in diffuse DNA degradation. Treatment of IM-9 MM cells and PCL patient cells with anti-Fas MoAb for 6 hours and 9 hours also induced apoptosis, evidenced by DNA-ladder formation (data not shown). In contrast, incubation of RPMI-8226, IM-9, and PCL cells with an isotype matched control MoAb did not cause DNA-laddering.
(A) 7C11 anti-Fas MoAb induces DNA fragmentation in RPMI 8226 MM cells. Cells were cultured in media alone and for the indicated times with 5 μg/mL of 7C11 anti-Fas MoAb. Genomic DNA was isolated, end-labeled with 0.5 mCi of [γ32-P]-dCTP, and analyzed by 1.8% agarose gel electrophoresis. MW, molecular weight markers (123-bp ladders and HindIII digest of l-DNA). (B) Quantification of anti-Fas-MoAb induced apoptosis in RPMI-8226 MM cells was performed by using propidium iodide staining technique. Cells were treated with anti-Fas MoAb for the indicated time intervals and stained with propidium iodide; percentage of apoptotic cells was analyzed by FACS. “C” represents control cells.
(A) 7C11 anti-Fas MoAb induces DNA fragmentation in RPMI 8226 MM cells. Cells were cultured in media alone and for the indicated times with 5 μg/mL of 7C11 anti-Fas MoAb. Genomic DNA was isolated, end-labeled with 0.5 mCi of [γ32-P]-dCTP, and analyzed by 1.8% agarose gel electrophoresis. MW, molecular weight markers (123-bp ladders and HindIII digest of l-DNA). (B) Quantification of anti-Fas-MoAb induced apoptosis in RPMI-8226 MM cells was performed by using propidium iodide staining technique. Cells were treated with anti-Fas MoAb for the indicated time intervals and stained with propidium iodide; percentage of apoptotic cells was analyzed by FACS. “C” represents control cells.
Apoptosis was further analyzed by FACS analysis. Cells were treated with anti-Fas MoAb for the indicated time periods and then stained with propidium iodide; percentage of apoptotic cells was determined by flow cytometric analyses. Treatment of RPMI-8226 MM cells with anti-Fas MoAb for 48 hours induced a significant increase in percentage of apoptotic cells (73% ± 5%, three independent experiments) (Fig 1B).
Anti-Fas MoAb Induces c-jun mRNA in MM Cells
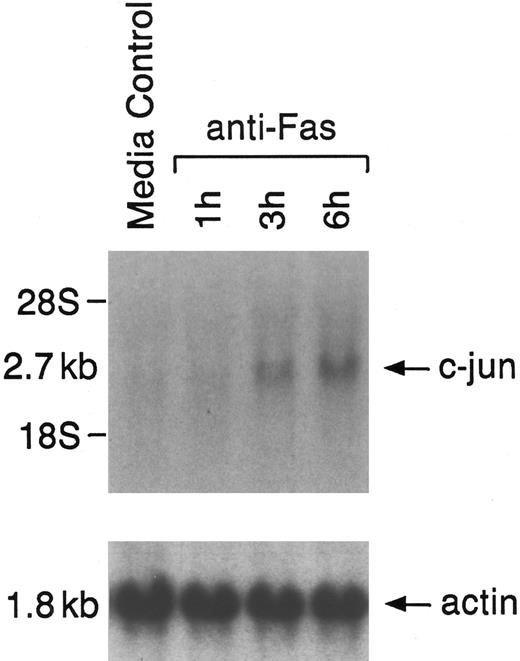
Because our previous studies showed that c-jun, an early response gene, is induced during apoptosis triggered by ara-C,30 we in the present study measured expression of c-jun gene during Fas-induced apoptosis in RPMI 8226 MM cells. Low to undetectable levels of c-jun transcripts were observed in cells cultured in media alone (Fig 2). In contrast, treatment of MM cells with anti-Fas MoAb induced c-jun transcript as early as 1 hour; densitometry showed that c-jun mRNA levels peaked at 6 hours (8.2 ± 0.5-fold increase) and declined thereafter (Fig 2 and data not shown). There were no associated changes in β-actin mRNA.
Effect of anti-Fas MoAb on induction of early response gene c-jun in RPMI 8226 MM cells. Cells were either cultured in media alone or treated with anti-Fas MoAb for the indicated times. Total cellular RNA (20 μg/lane) was hybridized to 32P-labeled c-jun and actin probes.
Effect of anti-Fas MoAb on induction of early response gene c-jun in RPMI 8226 MM cells. Cells were either cultured in media alone or treated with anti-Fas MoAb for the indicated times. Total cellular RNA (20 μg/lane) was hybridized to 32P-labeled c-jun and actin probes.
Effect of Anti-Fas MoAb Treatment on the Activation of SAPK, p38 MAPK, and ERK-1
We next assayed for Fas-induced activation SAPK, p38 MAPK, and ERK-1 since previous studies have shown a role of these kinases in regulation of growth and apoptosis.38 39 To assay for induction of SAPK activity in MM cells, RPMI-8226 MM cells were exposed to anti-Fas MoAb and harvested at 15, 30, and 60 minutes; lysates of control and anti-Fas MoAb-treated cells were subjected to affinity chromatography with GST-Jun (2-100) fusion protein and assayed for c-Jun phosphorylation. As shown in Fig 3A, adsorbates from anti-Fas MoAb-treated MM cells demonstrated increased Jun kinase activity compared to that in control cells. c-Jun kinase activity was detectable at 15 minutes and maximal at 60 minutes. To determine whether the Fas-induced Jun kinase activity is due to parallel increases in SAPK activity, we assayed anti-SAPK immunocomplexes for phosphorylation of GST-Jun. Anti-Fas MoAb induced activation of SAPK at 30 minutes, which peaked at 60 minutes (Fig 3B). Finally, we assayed for induction via Fas of p38 MAPK, ERK1 and ERK2 activities. Anti-Fas MoAb induced p38 MAPK activity at 15 minutes, which peaked at 60 minutes, evidenced by phosphorylation of GST-ATF2 by p38 MAPK immunocomplexes of Fas-treated cells; no similar changes were evident in control cells (Fig 3C). In contrast, Fas did not induce any significant change in ERK1 or ERK2 activity, assayed by phosphorylation of MBP substrate by ERK1 or ERK2 immunocomplexes of Fas-treated cells (Fig 3D and data not shown). Coomassie blue staining confirmed equal protein loading within each lane (data not shown).
Anti-Fas MoAb induces activation of SAPK and p38 MAP kinase. (A) RPMI 8226 MM cells were treated with 5 μg/mL of anti-Fas MoAb for 15, 30, and 60 minutes. Cell lysates prepared from control cells (media alone) and anti-Fas MoAb-treated cells were incubated with glutathione-sepharose beads containing 5 μg of GST-Jun (2-100) for 2 hours. The resulting protein complexes were washed and then incubated in kinase buffer containing GST-Jun fusion protein and [γ-32P]ATP for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE, stained with Coomassie blue, dried, and analyzed by autoradiography. Lysates from control and anti-Fas MoAb-treated RPMI-8226 cells were also immunoprecipitated with anti-SAPK Ab (B), anti-p38 MAP kinase Ab (C), or anti-ERK-1 Ab (D). Immune complex kinase assays were performed by addition of GST-Jun (B), GST-ATF-2 (C), or MBP (D) and [γ32P]ATP and incubation for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE and analyzed by Coomassie blue staining and autoradiography. Anti-SAPK immunocomplexes prepared from control and anti-Fas treated IM-9 (E) and PCL (F ) cells were also assayed for phosphorylation of GST-Jun.
Anti-Fas MoAb induces activation of SAPK and p38 MAP kinase. (A) RPMI 8226 MM cells were treated with 5 μg/mL of anti-Fas MoAb for 15, 30, and 60 minutes. Cell lysates prepared from control cells (media alone) and anti-Fas MoAb-treated cells were incubated with glutathione-sepharose beads containing 5 μg of GST-Jun (2-100) for 2 hours. The resulting protein complexes were washed and then incubated in kinase buffer containing GST-Jun fusion protein and [γ-32P]ATP for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE, stained with Coomassie blue, dried, and analyzed by autoradiography. Lysates from control and anti-Fas MoAb-treated RPMI-8226 cells were also immunoprecipitated with anti-SAPK Ab (B), anti-p38 MAP kinase Ab (C), or anti-ERK-1 Ab (D). Immune complex kinase assays were performed by addition of GST-Jun (B), GST-ATF-2 (C), or MBP (D) and [γ32P]ATP and incubation for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE and analyzed by Coomassie blue staining and autoradiography. Anti-SAPK immunocomplexes prepared from control and anti-Fas treated IM-9 (E) and PCL (F ) cells were also assayed for phosphorylation of GST-Jun.
Additional experiments were performed using IM-9 and patient PCL cells. Cell lysates from unstimulated and anti-Fas treated cells were subjected to immunoprecipitation with anti-SAPK Ab, and anti-SAP immunocomplexes were assayed for phosphorylation of GST-Jun. Triggering via Fas MoAb induced activation of SAPK in both IM-9 (Fig 3E) and PCL (Fig 3F ) cells.
IL-6 Inhibits Fas-Induced DNA Fragmentation in MM Cells
IL-6 triggers a twofold increase in stimulation index of RPMI-8226 MM cells.6 Recent studies have also shown that IL-6 prevents apoptosis in MM cells and B cell hybridomas induced by dexamethasone, factor deprivation and serum starvation11,12; therefore, we determined whether IL-6 inhibits RPMI-8226 MM cell apoptosis triggered via Fas. RPMI-8226 MM cells were cultured with IL-6 (100 ng/mL) for 3 to 4 hours before addition of anti-Fas MoAb for 6 hours; a substantial decrease in the nucleosomal DNA-laddering was evident in cells treated with IL-6 before culture with anti-Fas MoAb, compared to cells cultured in media or with anti-Fas MoAb (Fig 4C). This inhibition of apoptosis was not associated with cell surface changes in the percentage or intensity of Fas Ag expression on RPMI-8226 cells cultured with IL-6 (data not shown).
Effect of IL-6 pretreatment on anti-Fas MoAb-induced SAPK and p38 MAPK activation, as well as DNA fragmentation, in RPMI 8226 MM cells. Cells were cultured in media alone or pretreated with IL-6 as indicated before anti-Fas MoAb treatment. Cell lysates were immunoprecipitated with anti-SAPK Ab (A) or anti-p38 MAPK Ab (B). The resulting protein complexes were washed and then incubated in kinase buffer containing GST-Jun and [γ-32P]ATP for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE, stained with Coomassie blue, dried, and analyzed by autoradiography. (C) Cells were cultured in either media alone or treated with 100 ng/mL of IL-6 for 3 to 4 hours before stimulation with anti-Fas MoAb for 6 hours. Genomic DNA was isolated, end-labeled with [γ32P]ATP, and analyzed by 1.8% agarose gel electrophoresis. MW, molecular weight markers (123-bp ladders and HindIII digest of l-DNA).
Effect of IL-6 pretreatment on anti-Fas MoAb-induced SAPK and p38 MAPK activation, as well as DNA fragmentation, in RPMI 8226 MM cells. Cells were cultured in media alone or pretreated with IL-6 as indicated before anti-Fas MoAb treatment. Cell lysates were immunoprecipitated with anti-SAPK Ab (A) or anti-p38 MAPK Ab (B). The resulting protein complexes were washed and then incubated in kinase buffer containing GST-Jun and [γ-32P]ATP for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE, stained with Coomassie blue, dried, and analyzed by autoradiography. (C) Cells were cultured in either media alone or treated with 100 ng/mL of IL-6 for 3 to 4 hours before stimulation with anti-Fas MoAb for 6 hours. Genomic DNA was isolated, end-labeled with [γ32P]ATP, and analyzed by 1.8% agarose gel electrophoresis. MW, molecular weight markers (123-bp ladders and HindIII digest of l-DNA).
IL-6 Inhibits Induction of SAPK, But Not of p38 MAPK, Triggered via Fas in MM Cells
To test whether the anti-apoptotic effect of IL-6 in Fas-induced MM cell death involves regulation of SAPK and p38 MAPK, we determined whether culture of MM cells with IL-6 altered subsequent Fas-induced phosphorylation of GST-Jun (SAPK activity) or GST-ATF2 (p38 MAPK activity). MM cells were cultured in media or with IL-6 for 3 to 4 hours before addition of anti-Fas MoAb, and cell lysates were subjected to immunoprecipitation with anti-p54 SAPK Ab followed by assay for GST-Jun phosphorylation, or with anti-p38 MAPK Ab followed by GST-ATF2 phosphorylation. As shown in Fig 4A, treatment of MM cells with IL-6 alone did not induce SAPK activation. However, SAPK activation was significantly reduced in MM cells treated with IL-6 before culture with anti-Fas MoAb, compared to controls. No similar effects of IL-6 on Fas-induced p38 MAPK activation in MM cells was observed (Fig 4B). Coomassie blue staining confirmed equal loading of protein in each individual lane (Fig 4A, middle band and 4B, lower band).
DISCUSSION
In the present study, we characterized the signaling mechanism involved in Fas-induced apoptosis of RPMI-8226 MM cells. Anti-Fas MoAb induced DNA fragmentation, which was associated with increased c-jun transcripts, as well as increased SAPK and p38 MAPK activity. Importantly, IL-6 significantly reduced both DNA fragmentation and SAPK activity triggered via Fas. Therefore, our studies suggest that anti-Fas MoAb-induced activation of SAPK is associated with apoptosis, and that IL-6 can abrogate both SAPK activation and apoptosis.
Multiple studies have shown that triggering via Fas(APO-1) Ag induces apoptosis in various cell types.20-24 Fas Ag is a 45-kD type I membrane protein of the TNF/nerve growth factor receptor superfamily, which also includes CD40, CD30, CD27, and OX40.21,22 Fas ligand is a 40 kD type II membrane protein of the TNF family, which includes TNF-α, TNF-β, CD40 ligand, and CD30 ligand.47-49 Shima et al26 have previously reported that five human MM cell lines and the majority of freshly isolated patient MM cells express Fas Ag, and that anti-Fas MoAb treatment triggered loss of cell volume, membrane blebbing, fragmentation of nuclei, and condensed chromatin in some MM cell lines and MM patient samples. Hata et al29 have similarly found Fas Ag to be present on the majority of MM cell lines and patient cells; anti-Fas MoAb triggered apoptosis, confirmed by morphologic analysis alone and/or DNA electrophoresis in some cases. Finally, Westendorf et al25 have demonstrated apoptosis, confirmed both by DNA fragmentation and morphological changes, in some human MM cell lines and patient samples.25 The present studies showed anti-Fas MoAb induced fragmentation of MM cell DNA into multiples of nucleosome sized fragments, changes in chromatin structure characteristic of apoptosis or programmed cell death.50
The signaling mechanism initiated by Fas is not yet delineated. Mutational studies of Fas have shown that an intracellular domain, homologous to a region of the TNF receptor, is essential for Fas-mediated cell death.51 However, the intracellular region of the Fas molecule has no consensus sequence for kinases or phosphatases. A possible mechanism of Fas signaling is via acid sphingomyelinase, analogous to TNF52: TNF induces acid sphingomyelinase activity, resulting in increased production of ceramide which induces apoptosis.53 Alternatively, Fas-mediated apoptosis may involve an IL-1β converting enzyme-like protease.54,55 Recent studies which show that UV light, ionizing radiation, and ceramide activate SAPK activity33,35-37,39 and show a role for SAPK and p38 MAPK in apoptosis38,39 suggest their posible role in Fas-related apoptosis. Our present studies in MM cells also suggest involvement of SAPK and p38 MAPK in apoptosis. They demonstrate that anti-Fas MoAb induced both DNA fragmentation and increased c-jun mRNA levels in MM cells, as well as c-Jun phosphorylation (confirming SAPK activation) and GST-ATF2 phosphorylation (confirming p38 MAPK activation). Moreover, they further support the view that initiation and continuation of apotosis involves activation of transcription factors56,57: phosphorylation of c-Jun upregulates c-jun transcription through AP-1 binding,58 59 and the induction of c-jun expression observed in our study may therefore be mediated by the SAPK signaling pathway.
In previous reports, resistance of MM cells to Fas-related apoptosis has been attributed to BCL-2 expression. For example, the single MM cell line found by Hata et al to be resistant to anti-Fas MoAb treatment despite its cell surface Fas Ag expression highly expressed BCL-2.27 However, others have found BCL-2 expression to be similar in both Fas-sensitive and Fas-resistant MM cell lines,26 suggesting that alternative mechanisms may confer protection against Fas-related apoptosis. In our preliminary studies, anti-Fas MoAb treatment downregulated expression of BCL-2 in RPMI-8226 MM cells, further supporting a protective mechanism other than BCL-2. Importantly, Westendorf et al25 found resistance of MM cells to anti-Fas MoAb-triggered apoptosis to be related either to the lack of cell surface Fas Ag expression; or to a soluble factor present in serum, since MM BMMCs were resistant and purified MM cells were sensitive to anti-Fas MoAb treatment.25
Prior studies have shown that IL-6 is one such soluble factor which can inhibit apoptosis of MM cells induced by Fas or other stimuli. Specifically, studies of MM cell lines and patient MM cells have shown that IL-6 inhibits apoptosis triggered by serum starvation or dexamethasone, but not that stimulated by doxorubicin or etoposide.11,12 Because IL-6 decreased BCL-2 expression in these MM cell lines, its anti-apoptotic effects cannot be directly related to BCL-2. Moreover, Schwarze and Hawley28 have shown that IL-6 mediated suppression of apoptosis in IL-6 dependent B9 cells does not involve induction of endogenous BCL-2 expression, but rather is associated with upregulation of cellular BCL-x mRNA and BCL-x protein. In their study, very high levels of BCL-2 in B9 cells, achievable only using retroviral or bovine papilloma virus-based vectors, suppressed apoptotic death induced by IL-6 deprivation. Therefore, to date, the anti-apoptotic effect of IL-6 on MM cells is not attributable to its effects on BCL-2. The importance of BCL-2 for in vivo survival of MM cells has also been questioned, since recent studies have found higher percentages of BCL-2 positive cells in patients with a long survival, compared to patients with refractory MM and short survival.60
In the present study, we also show that IL-6 is one soluble factor inhibiting apoptosis of MM cells triggered by Fas. In contrast to studies by Hata et al27 in which IL-6 (1 ng/mL) inhibited apoptosis in only a single MM patient sample and in no MM cell lines, we showed inhibition of MM cell apoptosis using 100 ng/mL IL-6. Inhibition of apoptosis at higher IL-6 concentrations in vitro observed in our study suggests that the increased in vivo IL-6 serum levels in patients with advanced stages of disease61-63 may not only mediate tumor cell growth, but also enhance tumor cell survival. Our finding that IL-6 pretreatment alters anti-Fas-MoAb induced SAPK activity and apoptosis without altering p38MAPK activity suggests that IL-6 may act on upstream events involved in activating SAPK, and not p38MAPK. Previous studies in MM and other cells have shown that IL-6 can initiate growth via the Ras dependent MAPK cascade: sequential activation of Shc, Grb2, Sos, Ras, Raf, MEK, and MAPK.8,64-67 In addition, Billadeau et al67 have also reported that expression of mutant ras in the IL-6 dependent ANBL-6 MM cell line facilitates growth in the absence of IL-6 and suppresses apoptosis. Finally, recent studies have shown that activation of ERK signaling pathways can inhibit apoptosis, and conversely, that inactivation of ERK signaling along with activation of SAPK or p38MAPK may be critical for apoptosis.38 These studies, coupled with the present report, suggest that IL-6 may exert an anti-apoptotic effect in MM cells by modulating the Ras-Raf-MAPK signaling cascades. Further characterization of the mechanism whereby IL-6 inhibits apoptosis in MM cells, including abrogation of SAPK activation, may not only provide clues to basic growth control in MM, but also offer new treatment strategies.
Supported by National Institutes of Health Grant No. CA 50947 and the Kraft Family Research Fund.
Address reprint requests to Kenneth C. Anderson, MD, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.

![Fig. 1. (A) 7C11 anti-Fas MoAb induces DNA fragmentation in RPMI 8226 MM cells. Cells were cultured in media alone and for the indicated times with 5 μg/mL of 7C11 anti-Fas MoAb. Genomic DNA was isolated, end-labeled with 0.5 mCi of [γ32-P]-dCTP, and analyzed by 1.8% agarose gel electrophoresis. MW, molecular weight markers (123-bp ladders and HindIII digest of l-DNA). (B) Quantification of anti-Fas-MoAb induced apoptosis in RPMI-8226 MM cells was performed by using propidium iodide staining technique. Cells were treated with anti-Fas MoAb for the indicated time intervals and stained with propidium iodide; percentage of apoptotic cells was analyzed by FACS. “C” represents control cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.227/2/m_bl_0027f1.jpeg?Expires=1769440686&Signature=mU3HLRjnqgw56Vk-PtcL43YIxpfh8VzIDfz2r7qVPiTyMlvshjaJr0t2WDAsrDF3Qxkcjf10JlV76dIQ1tVdMy4rjLfWeHuzbENOWqIbe6fwBJ8TlYF1o8ySOuUucDQNqm49loFNW3q2JNalg8~Xm0o2ClnsSPEt7SmelzvsMNlMbsOHyBKSJtrv5BlZeuvH8A1NCEk6zv3--Vc4VDQ6d30Y4qkL-Bo3xIhtMnX5GZdCpCfOzv8-lmXzNy~MLQ1DtaX45-vB-mT4rxaqDEIRO-gm1OvniK9afdYR74qkvWoPOXtLI3mHU-NEVZzuidIb8MbFxQC~GjgB57lDLbhNxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Anti-Fas MoAb induces activation of SAPK and p38 MAP kinase. (A) RPMI 8226 MM cells were treated with 5 μg/mL of anti-Fas MoAb for 15, 30, and 60 minutes. Cell lysates prepared from control cells (media alone) and anti-Fas MoAb-treated cells were incubated with glutathione-sepharose beads containing 5 μg of GST-Jun (2-100) for 2 hours. The resulting protein complexes were washed and then incubated in kinase buffer containing GST-Jun fusion protein and [γ-32P]ATP for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE, stained with Coomassie blue, dried, and analyzed by autoradiography. Lysates from control and anti-Fas MoAb-treated RPMI-8226 cells were also immunoprecipitated with anti-SAPK Ab (B), anti-p38 MAP kinase Ab (C), or anti-ERK-1 Ab (D). Immune complex kinase assays were performed by addition of GST-Jun (B), GST-ATF-2 (C), or MBP (D) and [γ32P]ATP and incubation for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE and analyzed by Coomassie blue staining and autoradiography. Anti-SAPK immunocomplexes prepared from control and anti-Fas treated IM-9 (E) and PCL (F ) cells were also assayed for phosphorylation of GST-Jun.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.227/2/m_bl_0027f3.jpeg?Expires=1769440686&Signature=bSCW8hCO4~9mc~XNr1sR5XmcUcLVIX~QFsac8yZwJF49lLHk8KK2rBepK2iyww5qgVRIUcMhlBd6KRomYPFQnPslGMVocZoY5iv1rxUxRS6iqfxChAdAL4qqxCgt6UQCDIV-MYzoLGDda2gUFwpEF0uVQoLyErjbfgbZ5PKHwxg9CzzxivU75mUCiwcirkSyjpP1~ZQJFOpwh~ECGEqzJK769Z5B6HAL~mEFV8PnmVeXOlk5yXis3yCvP2GtuPMljSBrBzyUwtIPNxOP0OYIxsfzFgREfl~8H1oWEcz~uuJJiuShtj1wppcnBFmAgkTlPOK4I9L4qTdXU4IhlkaMMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of IL-6 pretreatment on anti-Fas MoAb-induced SAPK and p38 MAPK activation, as well as DNA fragmentation, in RPMI 8226 MM cells. Cells were cultured in media alone or pretreated with IL-6 as indicated before anti-Fas MoAb treatment. Cell lysates were immunoprecipitated with anti-SAPK Ab (A) or anti-p38 MAPK Ab (B). The resulting protein complexes were washed and then incubated in kinase buffer containing GST-Jun and [γ-32P]ATP for 15 minutes at 30°C. The phosphorylated proteins were resolved by 10% SDS-PAGE, stained with Coomassie blue, dried, and analyzed by autoradiography. (C) Cells were cultured in either media alone or treated with 100 ng/mL of IL-6 for 3 to 4 hours before stimulation with anti-Fas MoAb for 6 hours. Genomic DNA was isolated, end-labeled with [γ32P]ATP, and analyzed by 1.8% agarose gel electrophoresis. MW, molecular weight markers (123-bp ladders and HindIII digest of l-DNA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/1/10.1182_blood.v89.1.227/2/m_bl_0027f4.jpeg?Expires=1769440686&Signature=Dp6vf3vEqefxwDxXKcs6SlXTYpc9PeF3NJ-4oxoUjG0gXRnh3KrZ~tTyf3KyKUWR7zDLyYXEKiCxx1mt063sTOPxf8dWhLSVfxxze2RUBY5q24gqiaJSjkstR2u2IATf2jVqf3hG27Ysm52z9OH8vScI-3pV6bjJXoKm-7Na6UGibLyYClg4coKeej2wUCaxDSYU1Av3LDlw9WYzVdr9wGkZxwdvufw0lBBInPiSudbtSncEfYaoav62aCDos-yQ1xXlW2uNKzxiM6DjjJ3Ab1yn8GKiYuSr40a87nZLfuDsx5zpYjhG7Yzq0n~oPucbAMvneygKgez6tNMmRf7BHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal