Key Points
Transcriptional dependency of RIPK1-driven necroptosis is a key regulatory mechanism in ALL, modulated by SP1, p300, and HDAC2.
Combination of SMAC mimetics and HDAC inhibitors can be a clinically relevant option for resensitizing resistant ALL toward cell death.
Visual Abstract
Insufficient eradication of cancer cells and survival of drug tolerant clones are major relapse driving forces. Underlying molecular mechanisms comprise activated prosurvival and antiapoptotic signaling, leading to insufficient apoptosis and drug resistance. The identification of programmed cell death pathways alternative to apoptosis opens up possibilities to antagonize apoptosis escape routes. We have earlier shown that acute lymphoblastic leukemia (ALL) harbors a distinct propensity to undergo cell death by receptor-interacting protein kinase 1 (RIPK1)−dependent necroptosis, activated by small-molecule second mitochondria-derived activators of caspase (SMAC) mimetics. Despite demonstrated safety and tolerability of SMAC mimetics in clinical trials, their efficacy as single agent seems still limited, highlighting the need for combinatorial treatments. Here, we investigate so far unexplored regulatory mechanisms of necroptosis and identify targets for interference to augment the necroptotic antileukemia response. Ex vivo drug response profiling in a model of the bone marrow microenvironment reveals powerful synergy of necroptosis induction with histone deacetylase (HDAC) inhibition. Subsequent transcriptome analysis and functional in vivo CRISPR screening identify gene regulatory circuitries through the master transcription regulators specificity protein 1 (SP1), p300, and HDAC2 to drive necroptosis. Although deletion of SP1 or p300 confers resistance to necroptosis, loss of HDAC2 sensitizes cells to RIPK1-dependent cell death by SMAC mimetics. Consequently, our data inform strong in vivo antileukemic activity of combinatorial necroptosis induction and HDAC inhibition in patient-derived human leukemia models. Thus, transcriptional dependency of necroptosis activation is a key regulatory mechanism that identifies novel targets for interference, pointing out a strategy to exploit alternative nonapoptotic cell death pathways to eradicate resistant disease.
Introduction
Despite considerable advances in pediatric acute lymphoblastic leukemia (ALL) treatment,1 relapses still represent a clinical challenge with poor prognosis and dismal outcomes.2 One potential source of treatment failure is apoptosis evasion, owing to insufficient mitochondrial priming.3 Apart from approaches to decrease the threshold for mitochondrial apoptosis,4 induction of nonapoptotic cell death has emerged as attractive strategy,5 to elicit cell death even under apoptosis-insensitive conditions.6 Receptor-interacting protein kinase 1 (RIPK1), a central regulator of cell survival, inflammation, and death,7 mediates the nonapoptotic cell death mechanism necroptosis, activating RIPK3 and mixed lineage kinase domain-like pseudokinase (MLKL). Further to the regulation of distinct RIPK1-governed protein-protein interactions and phosphorylation events,8 the regulatory circuitries upon necroptosis induction remain to be elucidated. Small-molecule second mitochondria-derived activators of caspase (SMAC) mimetics (SM) potently induce RIPK1-dependent necroptosis in primary human ALL downstream depletion of inhibitor of apoptosis proteins (IAPs),9 revealing a distinct vulnerability that relies on tumor necrosis factor (TNF) receptor 1 and 2 signaling, yet is independent of TNF-α.10 Key features such as posttranslational modification of RIPK1, activation status of caspase-8, RIPK3 homodimerization and MLKL phosphorylation status, or ESCRT (endosomal sorting complex required for transport)–mediated membrane repair contribute to decisions to turn cells into a necroptotic state.11,12 However, these mechanisms alone cannot explain the potential of cells to undergo necroptosis, and further regulatory events are required for cell death execution downstream of RIPK1. Several in-human studies have demonstrated the tolerability of the bivalent SM birinapant (reviewed in a previous study13), yet displaying limited single-agent activity. This illustrates the critical need for combinatorial treatment strategies, as exemplified by the synergistic activity of SM with anti-CD3 immunotherapy in T-cell ALL.14 Here, we investigate the regulation of RIPK1-dependent cell death in pediatric ALL by combining functional drug response profiling (DRP), transcriptomics, and CRISPR-based genome editing. Integrative analyses uncover the importance of transcriptional activity driven by the master regulators specificity protein 1 (SP1), p300, and histone deacetylase 2 (HDAC2) in necroptosis activation and nominate a combination of SM with HDAC inhibition as a potential antileukemic strategy to augment RIPK1-dependent necroptosis and counteract apoptosis evasion.
Methods
Human samples
Primary human ALL samples originated from cryopreserved bone marrow (BM) aspirates of patients from the AIEOP-BFM 2000, the AIEOP-BFM 2009, and the ALL-REZ 2002 studies. Informed consent was given in accordance with the principles of the Declaration of Helsinki. Approval was granted by the ethics commission of the Kanton Zürich (approval number 2014-0383). Patient characteristics are presented in supplemental Table 3, available on the Blood website.
PDXs and in vivo experiments
In vivo experiments were approved by the veterinary office of the Kanton of Zürich. For generating patient-derived xenografts (PDXs), primary human ALL cells were transplanted into 5- to 12-week-old immunodeficient NOD/SCID/IL2rγnull (NSG) mice via tail vein injection. To assess drug activity, engrafted mice were treated with vehicle, birinapant (15 mg/kg, or 5 mg/kg), mocetinostat (Mo; 25 mg/kg), or the combination of birinapant (15 mg/kg) and Mo (25 mg/kg), via intraperitoneal injections, 5 times per week for 3 weeks. Leukemia progression was monitored by staining peripheral blood with ɑ-hCD19-PE (catalog no. 302208, BioLegend), ɑ-hCD45-Alexa Fluor 647 (catalog no. 304018, BioLegend), and ɑ-mCD45-eFluor 450 (catalog no. 48-0451-82, Invitrogen), analyzed by flow cytometry (LSRFortessa, BD Biosciences) and FlowJo software (10.0 8rl). Cells transduced with RFP657-containing plasmids were stained with ɑ-hCD19-PE and ɑ-mCD45-eFluor 450. Engrafted ALL cells were collected from the spleen and BM and analyzed using the same markers. Animals were randomized into treatment arms. No animals were excluded from analyses.
DRP
Human telomerase reverse transcriptase mesenchymal stem cells were plated at 50 000 cells per mL in serum-free AIM V medium in 384-well plates. After 24 hours, ALL cells were added on top of the mesenchymal stem cells at 400 000 cells per mL. Cells were treated after 4 to 6 hours as indicated, using an HP D300 Digital Dispenser (Tecan). For matrix-based screens, drug concentrations were used to reach a maximum decrease in ALL viability of ∼50%. A-485 and inobrodib (INB) were used at different concentration ranges, based on their different potencies.15,16 LD4172, degrader of CREB-binding protein 1 (dCBP1), A-485, and INB were added to the coculture system right after the plating of ALL PDXs, to allow a 6-hour preincubation. After 72 hours of treatment, live cells were stained with CyQuant (catalog no. C35012, Invitrogen), imaged with Operetta CLS High-Content Analysis System and quantified using Biology Image Analysis Software (Single Cell Technologies). Viable ALL numbers were quantified for viability analyses, as described in supplemental Methods.
Transcriptomics
PID0117 PDXs were plated at a concentration of 1 million cells per mL in serum-free AIM V and treated with 50 nM birinapant (S7015, Selleckchem) for 2.5 or 5 hours or left untreated. RNA was extracted using RNeasy Plus Mini Kit (catalog no. 74134, Qiagen) following the manufacturer’s instructions. Quality control, library preparation, paired-end sequencing at 25 million clusters per sample, and bioinformatic analysis of RNA samples were executed using GalSeq srl. Detailed information about downstream analysis is described in the supplemental Methods.
sgRNA CRISPR screen
Five million ALL cells were transduced with the single guide RNA (sgRNA) library (multiplicity of infection of 0.3 and coverage of ∼500 transduced cells per sgRNA) and injected into 1 mouse. Every mouse represented a singular condition and replicate. Treatment was executed as described under “PDXs and in vivo experiments” section. The experiment was conducted in triplicate. At the end of treatment window, genomic DNA from ALL cells collected from mice’s spleens was extracted using a column-based method (GenElute HP Plasmid Maxiprep Kit, catalog no. NA0310-KIT; Sigma-Aldrich). Further processing and analysis were performed as described in supplemental Methods by the Functional Genomic Center Zürich.
Statistical analyses
Statistical significance of differences was calculated using a 2-tailed Wilcoxon matched-pairs signed rank test for paired samples, to account for inter-PDX variations, when comparing multiple PDXs. The 2-way analysis of variance was used for multiple comparisons within the same PDX. The Mann-Whitney U test was used to calculate significant differences between independent PDX groups. The Pearson correlation coefficient (r) was calculated to detect linear correlations between drug sensitivities and synergy scores. The log-rank (Mantel-Cox) test was used to detect significant differences in the survival distributions between treatment groups. All statistical analyses were performed using Prism 10.0.3 (GraphPad software; not significant, P > .05; ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001).
Results
Pharmacological interference with apoptosis pathways and acetylation processes augments SM-induced cell death in ALL
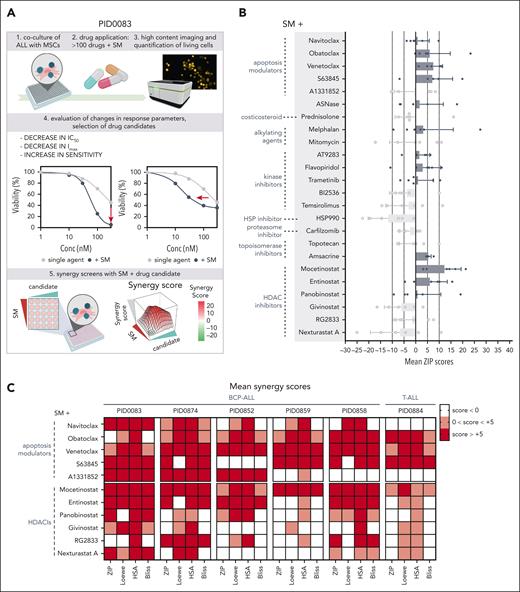
To identify the regulatory mechanisms that drive RIPK1-dependent cell death, we designed a combinatorial drug screen to improve SM action and thus RIPK1-dependent necroptosis in ALL. A library of 106 compounds (supplemental Table 1) was used in combination with fixed doses of SM (IC20 and IC50) on a primary B-cell precursor (BCP) patient-derived xenograft (PDX) ALL, using our established coculture DRP pipeline.17 Evaluation of response parameters identified 24 candidates with improved antileukemic effect in combination with SM (supplemental Figure 1). We subsequently used these compounds to perform a matrix-based synergy screen with SM in 5 different BCP-ALL and 1 T-cell ALL (T-ALL) PDX (Figure 1A-B). Several apoptosis modulators and HDAC inhibitors (HDACis) were among the classes of compounds with remarkable positive mean synergy scores (zero interaction potency [ZIP] scores) compared with SM, with Mo holding the highest average in mean ZIP scores across the samples (supplemental Table 2). Thus, activation of apoptosis through targeted B-cell lymphoma 2 (BCL2) antagonism potentiates the necroptotic SM response as shown earlier for glioblastoma18 and inhibition of transcriptional activity by HDACs is similarly effective in enhancing SM-driven cell death (Figure 1C). HDACis are of growing interest in the cancer treatment field because they restore normal acetylation patterns that are often disrupted during tumorigenesis.19 This results in the modulation of chromatin accessibility and transcription factor (TF) activities, both essential for transcriptional activity20,21 and for SM-induced cell death.
Apoptosis modulators and HDACis potentiate SM response, showing synergistic potential in ALL PDXs. (A) Schematic representation of experimental set up. (B) DRP from 6 different PDXs, screened for synergy between the SM birinapant and 24 anticancer compounds. Each dot represents a sample. Mean, and standard deviation are also shown. Samples included BCP-ALL (PID0083, PID0874, PID0852, PID0859, and PID0858) and T-ALL (PID0884). Within each PDX, n = 1. (C) Heat map depicting mean synergy scores (ZIP, Loewe, HSA, and Bliss) for combinations of SM + apoptosis modulators and SM + HDACis, indicated for each PDX. White represents synergy scores <0; pink represents synergy scores between 0 and +5; red represents synergy scores above +5. HSA, highest single agent; IC50, 50% inhibitory concentration; MSC, mesenchymal stem cell.
Apoptosis modulators and HDACis potentiate SM response, showing synergistic potential in ALL PDXs. (A) Schematic representation of experimental set up. (B) DRP from 6 different PDXs, screened for synergy between the SM birinapant and 24 anticancer compounds. Each dot represents a sample. Mean, and standard deviation are also shown. Samples included BCP-ALL (PID0083, PID0874, PID0852, PID0859, and PID0858) and T-ALL (PID0884). Within each PDX, n = 1. (C) Heat map depicting mean synergy scores (ZIP, Loewe, HSA, and Bliss) for combinations of SM + apoptosis modulators and SM + HDACis, indicated for each PDX. White represents synergy scores <0; pink represents synergy scores between 0 and +5; red represents synergy scores above +5. HSA, highest single agent; IC50, 50% inhibitory concentration; MSC, mesenchymal stem cell.
HDAC inhibition potentiates SM antileukemic activity in BCP-ALL PDXs in a RIPK1-dependent fashion
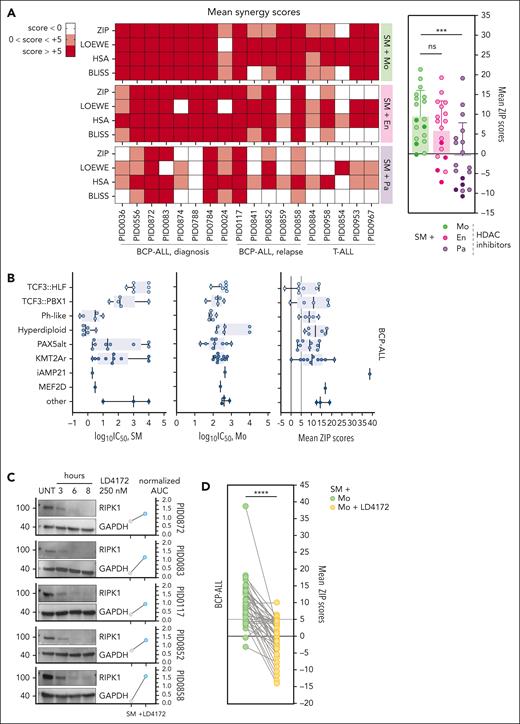
We further validated the synergistic potential among our top 3 HDACi candidates (Mo, entinostat, and panobinostat) and SM in a larger cohort of pediatric ALL PDXs (n = 18), including diagnosis and relapse cases of both BCP-ALL and T-ALL (supplemental Figure 2; supplemental Table 3). Although panobinostat broadly inhibits the entire HDAC family, entinostat selectively targets HDAC1 and HDAC3,22 and Mo preferentially targets HDAC1, HDAC2, HDAC3, and HDAC11.23 Our screen highlighted a positive synergy pattern more pronounced in BCP-ALL than T-ALL, with Mo being overall more synergistic with SM than the other HDACis (Figure 2A). To further confirm the synergistic potential of this combination in BCP-ALL, we expanded our PDX cohort (final n = 47) to comprise different leukemia subtypes (supplemental Figure 2; supplemental Table 3). Notably, 45 of 47 BCP-ALL PDXs showed consistently positive synergy scores for at least 2 of the 4 scoring methods evaluated (ZIP, Loewe, highest single agent, and Bliss) indicating a robust synergistic effect23 (supplemental Figure 3A; supplemental Table 4). Sensitivities to single-agent SM vary among subtypes, given that TCF3::HLF+ and TCF3::PBX1+ BCP-ALL showed lower responses to treatment than the more SM-sensitive hyperdiploid or philadelphia-like leukemias. Conversely, sensitivity to Mo did not follow a subtype-specific trend. Interestingly, the synergistic potential of the SM and Mo combination was not determined by the cytogenetic subtype (Figure 2B), the disease stage (diagnosis/relapse), or single-agent sensitivities (supplemental Figure 3B-D). Considering the central role of RIPK1 in SM-driven cell death,9 we hypothesized that RIPK1 depletion attenuates the synergistic potential of HDACi with SM. Therefore, we used the RIPK1 proteolysis-targeting chimera (PROTAC) LD4172,24 which effectively led to RIPK1 depletion within 6 hours, while also protecting the PDXs from SM-induced cell death (Figure 2C). Depletion of RIPK1 decreased the synergy scores between SM and the HDACi Mo, highlighting RIPK1’s importance for the synergistic activity of this combination (Figure 2D). Downstream of RIPK1, the pseudokinase MLKL is a key necroptosis executor.25 PROTAC-mediated MLKL depletion had a moderate impact on the synergistic activity of SM and HDACi. However, MLKL depletion in PDXs deficient for the executioner caspases 3 and 7 led to a complete attenuation of the synergistic score between SM and HDACi, further supporting necroptosis as a key downstream cell death execution mechanism (supplemental Figure 3A). Given that inhibition of HDACs results in rapid acetylation of several lysine residues of histone 3 (H3),26 we investigated the acetylation levels of H3 at lysine 27 (H3K27ac) and lysine 36 (H3K36ac) upon combinatorial treatment. Although the acetylation patterns upon treatment with SM, Mo, or the combination were variable among different PDXs, H3K36ac was significantly higher upon combinatorial treatment than upon single-agent treatment. The increase in H3K27ac was less pronounced (supplemental Figure 3B). Thus, interference with transcriptional regulation by HDAC inhibition is a potential mechanism to amplify SM antitumor activity. Similar drug combinations have been described to be effective in myeloid leukemia27 and ovarian cancer28 cells where they promote TNF-α expression and its autocrine signaling. However, our previous studies failed to correlate TNF-α levels with SM sensitivity, whereas the ligand could only partially modulate SM response in ALL PDXs.9,10 We could not detect a significant increase in the secretion of TNF-α upon SM-HDACi combination, with overall very low TNF-α levels (<5 pg/mL) (supplemental Figure 3C), whereas the synergistic potential of the cotreatment was maintained in TNF-α-depleted PID0117 cells10 (supplemental Figure 3D). Therefore, our data rule out the contribution of TNF-α to the synergistic effect of SM and Mo.
SM synergizes with HDACi across ALL PDXs in a RIPK1-dependent fashion. (A) Heat map depicting mean synergy scores (ZIP, Loewe, HSA, and Bliss) obtained from matrix synergy screens performed in 18 BCP-ALL and T-ALL, including relapse and diagnosis samples. White represents synergy scores <0; pink represents synergy scores between 0 and +5; red represents synergy scores above +5 (left). The Wilcoxon matched-pairs signed rank test revealing significant differences in mean ZIP scores between SM + Mo combination and the other combinations. Lighter dots, BCP-ALL; darker dots, T-ALL (right). (B) Individual sensitivities (log10IC50) toward SM or Mo and mean ZIP scores value for the SM + Mo combination, calculated for 47 BCP-ALL PDXs, grouped by subtype. (C) Immunoblot showing RIPK1 depletion after 6-hour treatment with LD4172 in 5 PDXs. GAPDH is used as a loading control. Dot plot depicting normalized logarithmic SM AUC (normalized AUC) upon SM treatment alone vs pretreatment with LD4172. (D) The Wilcoxon matched-pairs signed rank test indicating the significance of the variation in ZIP scores between SM + Mo combination with and without LD4172-mediated RIPK1 degradation, in 42 BCP-ALL PDXs. ns, P > .05; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. Replicates within each PDX are indicated on respective underlying data table (supplemental Data 1). SM, birinapant. AUC, area under the curve; En, entinostat; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant; Pa, panobinostat; UNT, untreated.
SM synergizes with HDACi across ALL PDXs in a RIPK1-dependent fashion. (A) Heat map depicting mean synergy scores (ZIP, Loewe, HSA, and Bliss) obtained from matrix synergy screens performed in 18 BCP-ALL and T-ALL, including relapse and diagnosis samples. White represents synergy scores <0; pink represents synergy scores between 0 and +5; red represents synergy scores above +5 (left). The Wilcoxon matched-pairs signed rank test revealing significant differences in mean ZIP scores between SM + Mo combination and the other combinations. Lighter dots, BCP-ALL; darker dots, T-ALL (right). (B) Individual sensitivities (log10IC50) toward SM or Mo and mean ZIP scores value for the SM + Mo combination, calculated for 47 BCP-ALL PDXs, grouped by subtype. (C) Immunoblot showing RIPK1 depletion after 6-hour treatment with LD4172 in 5 PDXs. GAPDH is used as a loading control. Dot plot depicting normalized logarithmic SM AUC (normalized AUC) upon SM treatment alone vs pretreatment with LD4172. (D) The Wilcoxon matched-pairs signed rank test indicating the significance of the variation in ZIP scores between SM + Mo combination with and without LD4172-mediated RIPK1 degradation, in 42 BCP-ALL PDXs. ns, P > .05; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. Replicates within each PDX are indicated on respective underlying data table (supplemental Data 1). SM, birinapant. AUC, area under the curve; En, entinostat; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant; Pa, panobinostat; UNT, untreated.
Transcriptional activation operates an early necroptotic cell death response with NF-κB and HDAC signatures
Acetylation processes at the histone level are known to positively regulate transcriptional activity.29 In addition, sustained protein translation marks an important feature of necroptosis, which supports the proinflammatory nature of this pathway by guaranteeing cytokine and chemokine production.30 Therefore, we sought to identify changes in the transcriptional landscape during early responses to SM in PID0117, a BCP-ALL PDX showing a necroptotic cell death phenotype upon SM treatment.9 To address the importance of RIPK1, we used a previously established isogenic RIPK1-deficient BCP-ALL PDX, generated by CRISPR-mediated editing,9 which shows resistance to SM (Figure 3A). RNA sequencing analysis was performed in RIPK1 proficient (RIPK1+/+) and RIPK1-deficient cells (RIPK1−/−) upon short SM treatment (2.5 and 5 hours; Figure 3B). To obtain a RIPK1-specific gene signature, we compared SM treated (5 hours) with untreated conditions, respectively, within RIPK1+/+ and RIPK1−/− cells, followed by a comparison between the 2 isogenic PDXs. Kyoto Encyclopedia of Genes and Genomes pathways analysis of upregulated genes revealed TNF and NF-κB signaling as the most significantly enriched pathways upon 5 hours, but not 2.5 hours, of SM treatment (Figure 3C; supplemental Figure 5A) as part of a RIPK1-specific signature (Figure 3D). Of note, the missing correlation between TNF-α levels and SM response already reported in our PDXs10 does not exclude involvement of TNF signaling pathway genes upon necroptosis induction, as evident from the transcriptomic data, supporting a TNF receptor–dependent but ligand-independent role for RIPK1-mediated death.10 In addition, SM treatment increased the expression of genes involved in DNA-binding transcription activity (GO:MF), whereas genes with DNA modification functions (GO:MF) were downregulated (Figure 3E). This suggests a potential role for RIPK1 during transcriptional activation, which dictates necroptosis initiation. When focusing on RIPK1-regulated genes, we found, among others, TNFAIP3 as significantly upregulated upon SM treatment in RIPK1+/+ cells (Figure 3F). TNFAIP3, together with its binding partner TNIP1, acts as a negative modulator of NF-κB, described to counteract TNF-mediated cell death by promoting RIPK1 ubiquitylation.31,32 Analysis of TRRUST (transcriptional regulatory relationships unraveled by sentence-based text mining) identified the TFs that may modulate the expression of the differentially regulated genes activated upon SM treatment. Among them, we found NF-κB subunits (RELA, NFKB1, and REL), HDACs (HDAC1 and HDAC2), and SP1 as master regulators of gene expression upon SM treatment (Figure 3G-H). These data support the key role of NF-κB–driven gene expression33,34 and extend into broader relevance of transcriptional modulation for RIPK1-driven cell death. Remarkably, the master regulator SP1 seemed to modulate both upregulated and downregulated genes upon treatment (Figure 3G; supplemental Figure 5B), emphasizing its importance in necroptosis modulation. SP1 activity is negatively regulated by HDAC1 via direct binding of the protein to the E2F1-binding site of SP1.35 Moreover, the SP1-HDAC1/2 interaction has been described to form a corepressor complex, which maintains the hypoacetylated status of H3 and H4. This can be reversed by treatment with HDACis, which allows SP1 association with the HAT p300, leading to transcriptional activation.36,37 These findings further highlight the connection between SM-induced cell death, regulation of acetylation processes, and TF activity. Thus, gene modulation is key to necroptosis induction in ALL, which could be further enhanced by pharmacological inhibition of HDACs-operated deacetylation processes.
Transcriptome analysis indicates early events during necroptosis, suggesting transcriptional dependency. (A) Validation of RIPK1−/− PDX: immunoblot showing efficient knockout of RIPK1, whereas GAPDH is used as a loading control (left), and viability curves indicate response to the SM birinapant in RIPK1+/+ vs RIPK1−/− PDX (right). (B) Schematic representation of experimental setup. (C) Functional enrichment analysis (displayed: Kyoto Encyclopedia of Genes and Genomes [KEGG] pathways) upon 5 hours of SM treatment in PID0117 RIPK1−/− cells (log2FC > 1). (D) Functional enrichment analysis (displayed: KEGG pathways) for the RIPK1-specific signature (log2FC > 0.58). To obtain this signature, log2FC values resulting from SM treatment vs untreated conditions were additionally compared between the 2 isogenic PDXs. Overrepresentation of genes belonging to specific pathways (fold enrichment) is shown on the x-axis; dot sizes represent the number of genes belonging to the specific pathway; FDR is indicated using different colors (green, lower FDR; the enrichment is less likely to be caused by chance). (E) Volcano plot showing differential gene expression (upregulation/downregulation) of genes in RIPK1+/+ cells upon 5 hours of SM treatment. Highlighted are genes upregulated with DNA-binding transcription activity (GO:MF, red) and downregulated with DNA modification activity (GO:MF, blue) in RIPK1+/+ cells upon SM treatment. Horizontal dotted line, −log10 (.05); vertical dotted lines, log2FC (±1). (F) Representation of differentially regulated genes in RIPK1+/+ cells (x-axis) in relation to RIPK1 signature (y-axis) after 5 hours of SM treatment. Colored, labeled dots: genes differentially regulated by |log2FC| >0.58 and P value (adjusted) < .05. (G) TRRUST analysis of differentially regulated genes for RIPK1+/+ cells (log2FC > 1), highlighting the regulators of genes of interest upon 5 hours SM treatment. (H) TRRUST analysis of differentially regulated genes for RIPK1 signature (log2FC > 0.58). RNA sequencing was conducted in triplicates. CTRL, control; FC, fold cange; FDR, false discovery rate; UNTR, untreated.
Transcriptome analysis indicates early events during necroptosis, suggesting transcriptional dependency. (A) Validation of RIPK1−/− PDX: immunoblot showing efficient knockout of RIPK1, whereas GAPDH is used as a loading control (left), and viability curves indicate response to the SM birinapant in RIPK1+/+ vs RIPK1−/− PDX (right). (B) Schematic representation of experimental setup. (C) Functional enrichment analysis (displayed: Kyoto Encyclopedia of Genes and Genomes [KEGG] pathways) upon 5 hours of SM treatment in PID0117 RIPK1−/− cells (log2FC > 1). (D) Functional enrichment analysis (displayed: KEGG pathways) for the RIPK1-specific signature (log2FC > 0.58). To obtain this signature, log2FC values resulting from SM treatment vs untreated conditions were additionally compared between the 2 isogenic PDXs. Overrepresentation of genes belonging to specific pathways (fold enrichment) is shown on the x-axis; dot sizes represent the number of genes belonging to the specific pathway; FDR is indicated using different colors (green, lower FDR; the enrichment is less likely to be caused by chance). (E) Volcano plot showing differential gene expression (upregulation/downregulation) of genes in RIPK1+/+ cells upon 5 hours of SM treatment. Highlighted are genes upregulated with DNA-binding transcription activity (GO:MF, red) and downregulated with DNA modification activity (GO:MF, blue) in RIPK1+/+ cells upon SM treatment. Horizontal dotted line, −log10 (.05); vertical dotted lines, log2FC (±1). (F) Representation of differentially regulated genes in RIPK1+/+ cells (x-axis) in relation to RIPK1 signature (y-axis) after 5 hours of SM treatment. Colored, labeled dots: genes differentially regulated by |log2FC| >0.58 and P value (adjusted) < .05. (G) TRRUST analysis of differentially regulated genes for RIPK1+/+ cells (log2FC > 1), highlighting the regulators of genes of interest upon 5 hours SM treatment. (H) TRRUST analysis of differentially regulated genes for RIPK1 signature (log2FC > 0.58). RNA sequencing was conducted in triplicates. CTRL, control; FC, fold cange; FDR, false discovery rate; UNTR, untreated.
Functional in vivo CRISPR screen reveals an essential role of transcriptional regulation for necroptosis
RIPK1 activation, regulation, and downstream signaling during necroptosis have been broadly studied.38 However, knowledge of additional genetic drivers that functionally regulate RIPK1-dependent cell death outcome is still scarce. With a targeted in vivo sgRNA CRISPR-based genome editing screen, we aimed to uncover thus far unrecognized genes that contribute to the SM response in ALL and to functionally determine the transcriptional regulation as a key mechanism for RIPK1-dependent cell death. We designed a targeted sgRNA library comprising the SM-induced genes detected by the transcriptome analysis for functional validation. In addition, genes encoding for HDACs/HATs and TFs of relevance were included (supplemental Material; supplemetal Data Set 2). The stably Cas9-expressing BCP-ALL PID0117 PDX9 was transduced with the sgRNA library and transplanted into NSG mice, which were subsequently treated with either vehicle or birinapant (Figure 4A). Although vehicle-treated cells expanded rapidly, SM treatment induced a delay in progression (supplemental Figure 6A). Upon targeted sequencing of the guides in harvested PDX cells, we identified a significant enrichment of guides including sgRNAs against SP1 and EP300. sgRNAs against RIPK1 served as positive controls and resulted to be enriched under both treatment conditions. In addition, we observed a consistent dropout of guides against HDAC2, TNIP1, and TNFAIP3 (Figure 4B-C). EP300 encodes for the acetyltransferase p300, which not only acetylates histones and TFs but also binds TFs, resulting in transcriptional activation of target genes in a feed-forward fashion, which is counteracted by HDACs.39,40 Of note, the TF SP1 can be recruited by p300 through direct interaction with the N-terminal domain of SP1.41 The observed enrichment of cell populations with knockout of either SP1 or p300 upon SM treatment (Figure 4B) confirms their critical role in mediating sensitivity of ALL toward SM, in line with transcriptional activation being key for SM response (Figure 3E). Furthermore, depletion of HDAC2 (Figure 4B), an epigenetic transcriptional repressor, holds potential in sensitizing to SM treatment, which is supported by observed potentiated antitumor activity of SM by pharmacological HDAC inhibition (Figure 2A; supplemental Figure 3A). Interestingly, HDAC2 is specifically targeted by Mo,42 which we showed to have the highest synergistic potential when combined with SM (Figure 2A), but not by entinostat, another HDACi targeting mainly HDAC1 and HDAC3.22 Therefore, this functional screen highlights the pivotal role of HDAC2 and its inhibition by Mo in the observed synergy with SM.
sgRNA CRISPR screen highlights key regulators of SM response, including SP1 and acetylation-modulating enzymes. (A) Schematic representation of experimental setup. (B) Volcano plot displaying significantly enriched or depleted sgRNAs (cutoffs: |robust rank aggregation [score]| > 0.495; FDR < 0.05; left). sgRNA rank plot depicting enriched (red) or dropout (blue) sgRNAs for the genes shown with the volcano plot plus intergenic controls (right). Each line represents 1 sgRNA; total sgRNAs per gene are 12 (4 sgRNAs from the Brunello library, 3 replicates). (C) Transcription frequency in TPM of sgRNAs for genes of interest. RIPK1 is included as a positive control. One dot represents 1 replicate. TPM, transcript per million.
sgRNA CRISPR screen highlights key regulators of SM response, including SP1 and acetylation-modulating enzymes. (A) Schematic representation of experimental setup. (B) Volcano plot displaying significantly enriched or depleted sgRNAs (cutoffs: |robust rank aggregation [score]| > 0.495; FDR < 0.05; left). sgRNA rank plot depicting enriched (red) or dropout (blue) sgRNAs for the genes shown with the volcano plot plus intergenic controls (right). Each line represents 1 sgRNA; total sgRNAs per gene are 12 (4 sgRNAs from the Brunello library, 3 replicates). (C) Transcription frequency in TPM of sgRNAs for genes of interest. RIPK1 is included as a positive control. One dot represents 1 replicate. TPM, transcript per million.
The key transcriptional regulators p300, SP1, and HDAC2 modulate RIPK1-dependent necroptosis
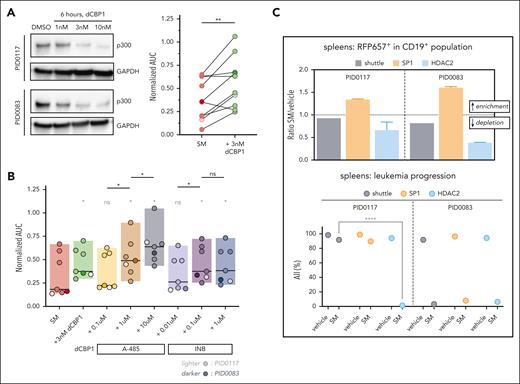
To confirm a sensitizing role for p300 in SM-induced necroptosis, we compared SM sensitivities in different BCP-ALL PDXs upon degradation of p300 by the PROTAC dCBP1. Preincubation with 3 nM dCBP1 for 6 hours was sufficient for p300 degradation (Figure 5A, left). We detected a significant decrease in sensitivity to RIPK1-dependent cell death upon dCBP1 treatment (Figure 5A, right), confirming the sensitizing role of p300 suggested by the CRISPR screen. T-ALL and SM-insensitive B-ALL PDXs did not show a significant difference in sensitivity upon cotreatment (supplemental Figure 6B). To investigate which specific property of p300 rescues the response to SM in ALL, we combined SM with A-485, a p300 catalytic inhibitor,15 and INB (CCS1477), a p300 bromodomain inhibitor.43 Cotreatment with A-485 caused a significant dose-dependent decrease in SM sensitivity, whereas for INB it plateaued at the second concentration used (Figure 5B). Thus, our data suggest that the catalytic activity of p300 might be predominant during SM response in ALL, whereas its ability to bind to acetylated proteins via the bromodomain could only have a limited effect. To confirm the functional relevance of SP1 and HDAC2 in RIPK1-dependent cell death, we performed an in vivo competition assay in which 2 stably Cas9-expressing BCP-ALL PDXs were used to first deplete SP1 or HDAC2 in ∼50% of the population (supplemental Figure 6C) with subsequent selective pressure by SM in vivo. Indeed, in the spleens and BM from both PDXs, the SP1-deleted populations were highly enriched in SM-treated mice compared with vehicle-treated mice. In contrast, we detected depletion of HDAC2-deficient populations in SM-treated vs vehicle-treated mice. Finally, we noticed a significantly lower human leukemic load upon HDAC2 depletion by SM treatment than the shuttle construct-containing counterpart, suggesting that even partial depletion of HDAC2 can sensitize leukemic cells to SM treatment (Figure 5C; supplemental Figure 6D). Taken together, these findings further support the functional role of SP1, p300, and HDAC2 in the regulation of necroptosis by SM, suggesting that transcriptional regulation is a potent driver of necroptosis.
Depletion of SP1, p300, or HDAC2 alters sensitivity toward SM in different PDXs. (A) Immunoblot showing depletion of p300 upon treatment for 6 hours with various concentrations of dCBP1 or DMSO in 2 PDXs. GAPDH is used as a loading control (left). Dot plot showing normalized AUC upon SM treatment in 10 SM-sensitive BCP-ALL with or without pretreatment with dCBP1 for 6 hours (right). The Wilcoxon matched-pairs signed rank test revealing significant differences in normalized AUC. PDXs included PID0036, PID0556, PID0872, PID0083, PID0788, PID0874, PID0117, PID0852, PID0859, and PID0858. (B) Box plot showing SM normalized AUC from 7 BCP-ALL PDXs treated with indicated p300 inhibitors (A-485, 0.1-10 μM; INB, 0.01-1 μM; concentration ranges were selected based on different potencies of the compounds). The Wilcoxon matched-pairs signed rank test detecting differences in the normalized AUC upon SM treatment after pretreatment with different p300 inhibitors compared with SM alone (gray∗) or compared with increasing concentrations of the same inhibitors (black∗). Median is displayed for each condition. PDXs included PID0556, PID0083, PID0874, PID0117, PID0852, PID0859, and PID0858. (C) Bar plot depicting the ratio of RPF657+ population (construct-containing population) calculated on human CD19+ (hCD19+) population in spleens of mice treated with SM over spleens of mice treated with vehicle. HDAC2, population containing LC.RFP657.HDAC2 construct; shuttle, population containing LC.RFP657.shuttle construct (used as control); SP1, population containing LC.RFP657.SP1 construct. No difference, y = 1; enrichment, y > 1; depletion, y < 1. Dot plot depicts hCD19+ cells percent over the total lymphocyte population (hCD19+ + mCD45+) in mice’s spleens at the end of treatment. Multiple comparison 2-way analysis of variance (ANOVA) analysis showing significant difference in ALL population in SM-treated HDAC2-depleted PID0117 spleen compared with the shuttle counterpart. ns, P > .05; ∗P ≤ .05; ∗∗P ≤ .01 ∗∗∗∗P ≤ .0001.
Depletion of SP1, p300, or HDAC2 alters sensitivity toward SM in different PDXs. (A) Immunoblot showing depletion of p300 upon treatment for 6 hours with various concentrations of dCBP1 or DMSO in 2 PDXs. GAPDH is used as a loading control (left). Dot plot showing normalized AUC upon SM treatment in 10 SM-sensitive BCP-ALL with or without pretreatment with dCBP1 for 6 hours (right). The Wilcoxon matched-pairs signed rank test revealing significant differences in normalized AUC. PDXs included PID0036, PID0556, PID0872, PID0083, PID0788, PID0874, PID0117, PID0852, PID0859, and PID0858. (B) Box plot showing SM normalized AUC from 7 BCP-ALL PDXs treated with indicated p300 inhibitors (A-485, 0.1-10 μM; INB, 0.01-1 μM; concentration ranges were selected based on different potencies of the compounds). The Wilcoxon matched-pairs signed rank test detecting differences in the normalized AUC upon SM treatment after pretreatment with different p300 inhibitors compared with SM alone (gray∗) or compared with increasing concentrations of the same inhibitors (black∗). Median is displayed for each condition. PDXs included PID0556, PID0083, PID0874, PID0117, PID0852, PID0859, and PID0858. (C) Bar plot depicting the ratio of RPF657+ population (construct-containing population) calculated on human CD19+ (hCD19+) population in spleens of mice treated with SM over spleens of mice treated with vehicle. HDAC2, population containing LC.RFP657.HDAC2 construct; shuttle, population containing LC.RFP657.shuttle construct (used as control); SP1, population containing LC.RFP657.SP1 construct. No difference, y = 1; enrichment, y > 1; depletion, y < 1. Dot plot depicts hCD19+ cells percent over the total lymphocyte population (hCD19+ + mCD45+) in mice’s spleens at the end of treatment. Multiple comparison 2-way analysis of variance (ANOVA) analysis showing significant difference in ALL population in SM-treated HDAC2-depleted PID0117 spleen compared with the shuttle counterpart. ns, P > .05; ∗P ≤ .05; ∗∗P ≤ .01 ∗∗∗∗P ≤ .0001.
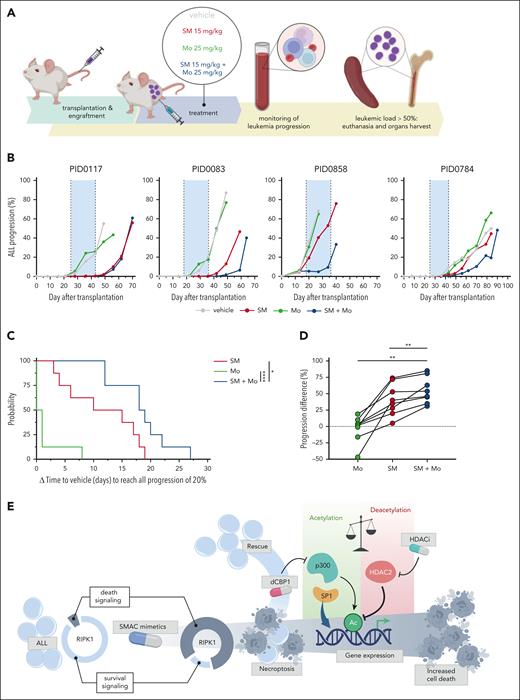
HDAC inhibition potentiates antileukemia effects of SM in vivo
The superior antitumor activity of the SM and Mo combination was assessed in vivo using 8 different BCP-ALL PDXs. ALL cells from each PDX were transplanted in 4 NSG mice, resulting in a 1-mouse-per-cohort study design.44 ALL progression was monitored throughout the duration of the experiment, until the end point (ALL in peripheral blood of ≥50%) was met (Figure 6A). Within each PDX, the SM + Mo combination delayed ALL progression compared with the single agents, with ALL values remaining below 1% (in 6/8 PDXs) during the treatment window. The combination delayed progression even in cases where Mo treatment alone was not effective (Figure 6B-C; supplemental Figure 7A). Overall, the ALL-progression difference between control (vehicle) and treated mice, calculated at the control end point, was significantly greater for SM + Mo than the single agents (Figure 6D). There was no evidence of toxicity or body weight loss over the course of the treatment (supplemental Figure 7B). ALL-containing spleens from the mice were collected at individual end points, and ALL cells were screened ex vivo against SM and Mo to confirm that no resistant clones were selected during in vivo treatment (supplemental Figure 7C). These data confirm the combinatorial antileukemic action of SM and Mo in xenograft mouse models.
SM and HDACi cooperate to delay leukemia progression in vivo. (A) Schematic representation of experimental setup. (B) Representative examples of leukemia progression dynamics during treatment of 4 PDXs. Shaded in blue is the treatment window. (C) Time elapse (in days) to reach ALL progression of 20% in peripheral blood, calculated between treated mouse and the respective control (vehicle) for each PDX (vehicle – treatment). A log-rank (Mantel-Cox) test revealing significant differences in progression for mice treated with the single agents vs combinatorial treatment. (D) ALL-progression difference, indicating the difference in leukemia percent (in peripheral blood) calculated between control mouse and treated mouse (vehicle – treatment) at the end point of control mice, relative to each PDX. Same treatment conditions are plotted together. The Wilcoxon matched-pairs signed rank test revealing significant differences in progression for mice treated with the single agents vs combinatorial treatment. Median is depicted. Leukemia percent is calculated based on hCD19+ cells over the total lymphocyte population (hCD19+ + mCD45+). Gray, vehicle; red, birinapant; green, Mo; blue, combination. SM, birinapant 15 mg/kg; Mo, 25 mg/kg; SM + Mo, birinapant 15 mg/kg + Mo 25 mg/kg. Eight PDXs are included. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001. (E) Graphical representation of the proposed mechanism of transcriptional regulation of SM-induced cell death in ALL.
SM and HDACi cooperate to delay leukemia progression in vivo. (A) Schematic representation of experimental setup. (B) Representative examples of leukemia progression dynamics during treatment of 4 PDXs. Shaded in blue is the treatment window. (C) Time elapse (in days) to reach ALL progression of 20% in peripheral blood, calculated between treated mouse and the respective control (vehicle) for each PDX (vehicle – treatment). A log-rank (Mantel-Cox) test revealing significant differences in progression for mice treated with the single agents vs combinatorial treatment. (D) ALL-progression difference, indicating the difference in leukemia percent (in peripheral blood) calculated between control mouse and treated mouse (vehicle – treatment) at the end point of control mice, relative to each PDX. Same treatment conditions are plotted together. The Wilcoxon matched-pairs signed rank test revealing significant differences in progression for mice treated with the single agents vs combinatorial treatment. Median is depicted. Leukemia percent is calculated based on hCD19+ cells over the total lymphocyte population (hCD19+ + mCD45+). Gray, vehicle; red, birinapant; green, Mo; blue, combination. SM, birinapant 15 mg/kg; Mo, 25 mg/kg; SM + Mo, birinapant 15 mg/kg + Mo 25 mg/kg. Eight PDXs are included. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001. (E) Graphical representation of the proposed mechanism of transcriptional regulation of SM-induced cell death in ALL.
Discussion
Insufficient activation of apoptosis represents a hallmark of cancer, with leukemia constituting a remarkable example.3,45 Therefore, alternative strategies are needed to resensitize cancer cells to treatment, circumventing therapy resistance. Deregulation in the expression of IAPs is a strategy adopted by tumor cells, especially in hematologic malignancies, which contributes to disease progression and poor treatment response. Consequently, compounds including SM have been developed for targeting and inhibiting IAPs, along with RIPK1 deubiquitylation and necroptotic cell death engagement.46 Efforts have been made to elucidate the molecular mechanisms behind RIPK1 switch from prosurvival to death signaling, describing distinct protein-protein interactions, alterations in NF-κB signaling,38,47 and metabolic regulation.48 Our data extend knowledge on the regulatory mechanisms of RIPK1-mediated cell death, revealing transcriptional aspects of necroptosis that are amenable to pharmacological interference to deepen or inhibit the response to SM treatment. Here, extending data on solid tumors,49 we show that decreasing the threshold for mitochondrial membrane depolarization with BCL2 antagonists enabled sensitization to necroptosis by SM in our human leukemia model. Nevertheless, insufficient mitochondrial priming in drug-resistant leukemia may hamper a strategy of using BCL2 antagonists,3 and there is still a need for alternative approaches. Our data, combining DRP with transcriptome analysis and functional in vivo CRISPR screening, indicate that RIPK1-driven cell death is notably dependent on transcriptional regulation by the master regulators SP1 and HDAC2, where SP1 activity is integral to ALL response to SM, whereas HDAC2 antagonizes this process. In addition, epigenetic remodeling by the acetyltransferase p300 contributes to the necroptotic response, extending data from other model systems.50 Consequently, pharmacological interference with acetylation/deacetylation programs alters the SM response and nominates the combination of SM and HDAC inhibition as an attractive, so far unexplored, starting point for future development of SM-potentiating strategies for ALL (Figure 6E). Accordingly, combinatorial treatment revealed a significant delay in leukemia progression in xenografted mouse models. Therefore, preclinical combinatorial DRP on patient samples represents a powerful tool to detect promising combinations and may inform individualized treatment options. This concept is being tested currently in an observational registry (ClinicalTrials.gov identifier: NCT06550102), in which this combination is a prime candidate for inclusion, also given the acceptable safety profiles of birinapant51 and Mo.52 The patient cohort tested here may be biased toward rare and high-risk subtypes. Although the combinatorial effect of SM and HDACi may be less efficient in subtypes not included in our cohort, the herein shown activity of this combination representing patient population at critical unmet medical need further supports efforts to translate this concept into clinical application. Confirming our previous findings on TNF-α–independent regulation of SM-induced cell death,10 the synergistic action between SM and HDACi in ALL is not driven by TNF-α secretion. Therefore, further investigation at the mechanistic level is needed to identify specific downstream players whose expression is influenced by the HDAC-dependent manipulation of transcription during SM-induced cell death. This will be beneficial for the development of more targeted HDACi treatments that, along with combinatorial partners like SM, could achieve higher therapeutic potential and lower toxicities in humans.53 Although we highlight a contribution of the NF-κB signaling modulators TNFAIP3 and TNIP1 as negative regulators of necroptosis downstream of RIPK1, consistent with data from different models,31,54,55 RIPK1 drives regulatory events independent of NF-κB, which may rely on protein-protein interactions.56 These data allow for a reflection on strategies to manipulate necroptosis also in the context of other RIPK1-driven mechanisms such as antitumor immunity,57 inflammatory responses,58 or in degenerative diseases.59 Necroptosis may have primarily evolved to cope with inflammatory signaling stress,60 yet the underlying regulatory programs may be exploited to develop strategies to eradicate resistant leukemia cells, particularly under apoptosis-insufficient circumstances.
Acknowledgments
The authors are grateful to all members of the Bourquin and Bornhauser group for support and discussions. They thank the staff at the animal facility, the flow cytometry, and the microscopy core units at University Children’s Hospital Zürich (UZH), and acknowledge members of the Functional Genomic Center Zürich and GalSeq srl for their support.
B.C.B. was supported by funding from the Swiss Cancer Research Foundation (KFS-4384-02-2018), the Swiss Cancer League (KLS-5396-08-2021), the Swiss National Science Foundation (215340), and the Stefanini-Foundation Switzerland. J.-P.B. and B.C.B were supported by funding from the Stiftung Kinderkrebsforschung Schweiz, the Swiss Biobank Society of Pediatric Oncology and Hematology, and the clinical research priority program “Precision Hematology/Oncology” of UZH. The research was supported, in part, by the National Institutes of Health (Baylor College of Medicine, Houston [R01-CA268518; J.W.]), the Cancer Prevention and Research institute of Texas (RP220480 [J.W.]), and the Michael E. DeBakey Professorship in Pharmacology (J.W.).
Authorship
Contribution: A.S. and B.C.B. conceptualized the study; A.S. curated the data; A.S. and B.G. performed the formal analysis; J.-P.B. and B.C.B. acquired funding; A.S., A. Dehler, B.G., F.S., M.R., J.K., and A. Drakul performed the investigation; A.S., A. Dehler, B.G., F.S., X.Y., and S.K. performed the methodology; A.S. and B.C.B. performed the project administration; J.-P.B. and B.C.B. were responsible for the resources; B.G. was responsible for the software; J.-P.B. and B.C.B. performed the supervision; A.S. and B.C.B. performed the validation; A.S., B.G., and F.S. performed the visualization; A.S. and B.C.B. wrote the original draft; and A.S., A. Dehler, B.G., F.S., M.R., J.K., A. Drakul, S.K., J.W., J.P.B., and B.C.B. reviewed and edited the manuscript.
Conflict-of-interest disclosure: J.W. is the cofounder of Chemical Biology Probes LLC and Fortitude Biomedicines, Inc; holds equity interest in Fortitude Biomedicines, Inc; and has stock ownership in, and serves as a consultant for, CoRegen Inc. The remaining authors declare no competing financial interests.
Correspondence: Beat C. Bornhauser, Department of Oncology and Children’s Research Centre, University Children’s Hospital Zürich, Lenggstrasse 30, 8008, Zurich, Switzerland; email: beat.bornhauser@kispi.uzh.ch.
References
Author notes
Transcriptome and sequencing data were deposited in Zenodo and can be accessed using the links provided in the supplemental Methods.
All data are available in the main text or in the supplemental Material. The supplemental Material includes supplemental figures, methods, tables, and 2 auxiliary tables (supplemental Data 1 and supplemental Data 2). Supplemental Data 1 contains underlying data for all the display items, whereas supplemental Data 2 lists details about the single guide RNA library. Material can be available on request from the corresponding author, Beat C. Bornhauser (beat.bornhauser@kispi.uzh.ch).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Transcriptome analysis indicates early events during necroptosis, suggesting transcriptional dependency. (A) Validation of RIPK1−/− PDX: immunoblot showing efficient knockout of RIPK1, whereas GAPDH is used as a loading control (left), and viability curves indicate response to the SM birinapant in RIPK1+/+ vs RIPK1−/− PDX (right). (B) Schematic representation of experimental setup. (C) Functional enrichment analysis (displayed: Kyoto Encyclopedia of Genes and Genomes [KEGG] pathways) upon 5 hours of SM treatment in PID0117 RIPK1−/− cells (log2FC > 1). (D) Functional enrichment analysis (displayed: KEGG pathways) for the RIPK1-specific signature (log2FC > 0.58). To obtain this signature, log2FC values resulting from SM treatment vs untreated conditions were additionally compared between the 2 isogenic PDXs. Overrepresentation of genes belonging to specific pathways (fold enrichment) is shown on the x-axis; dot sizes represent the number of genes belonging to the specific pathway; FDR is indicated using different colors (green, lower FDR; the enrichment is less likely to be caused by chance). (E) Volcano plot showing differential gene expression (upregulation/downregulation) of genes in RIPK1+/+ cells upon 5 hours of SM treatment. Highlighted are genes upregulated with DNA-binding transcription activity (GO:MF, red) and downregulated with DNA modification activity (GO:MF, blue) in RIPK1+/+ cells upon SM treatment. Horizontal dotted line, −log10 (.05); vertical dotted lines, log2FC (±1). (F) Representation of differentially regulated genes in RIPK1+/+ cells (x-axis) in relation to RIPK1 signature (y-axis) after 5 hours of SM treatment. Colored, labeled dots: genes differentially regulated by |log2FC| >0.58 and P value (adjusted) < .05. (G) TRRUST analysis of differentially regulated genes for RIPK1+/+ cells (log2FC > 1), highlighting the regulators of genes of interest upon 5 hours SM treatment. (H) TRRUST analysis of differentially regulated genes for RIPK1 signature (log2FC > 0.58). RNA sequencing was conducted in triplicates. CTRL, control; FC, fold cange; FDR, false discovery rate; UNTR, untreated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/7/10.1182_blood.2025028938/1/m_blood_bld-2025-028938-gr3.jpeg?Expires=1770988432&Signature=g~5IP04549n6DZC9TtY3B8YTEBsc4uZSEYXIA2kkRvLrilrM9vlNmXr5ht1EZmxvpbUv7wE8zZfT-slLBqlf~SDXnNoZACVP9uNuttv20oygc0E~BrrxxfCcgzwKY6w-7Cy~xufFnSwNiqalPu05SvkGRMhsZ2Z0KC4FGRO6W46r41THdgL5VbnfX7399Oyl0JWgwK8Vo105Sk-sK0bpFtK7aWnR65VBzCgkE~GUsCAuvZxHhrT1P9T2a~DX6-Gz~37wkMhCE5MHlbAi3yRqH~EZ6etVDS6YEkATOHpZs~ol6yI84PZFc6WoZkK0Ohq72XFlMpMkzTG5UGhsl3At2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![sgRNA CRISPR screen highlights key regulators of SM response, including SP1 and acetylation-modulating enzymes. (A) Schematic representation of experimental setup. (B) Volcano plot displaying significantly enriched or depleted sgRNAs (cutoffs: |robust rank aggregation [score]| > 0.495; FDR < 0.05; left). sgRNA rank plot depicting enriched (red) or dropout (blue) sgRNAs for the genes shown with the volcano plot plus intergenic controls (right). Each line represents 1 sgRNA; total sgRNAs per gene are 12 (4 sgRNAs from the Brunello library, 3 replicates). (C) Transcription frequency in TPM of sgRNAs for genes of interest. RIPK1 is included as a positive control. One dot represents 1 replicate. TPM, transcript per million.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/7/10.1182_blood.2025028938/1/m_blood_bld-2025-028938-gr4.jpeg?Expires=1770988432&Signature=mOcxgDOhn1NlQ5yIEi8CtAqbgoZ4lKfNABS-n7SSwzcaydW0-nEpGZdH~FpifGiafWWOZmgeJLLcWT5G7J4QqjpGednkk75DFFYImf4c46V5xZ0iuVNT3k8Yh7qK8cQejRQ7f9MNI8P4~N0rISliYYmzuQXVdkjC0vJLzDaShNEfOtYE-F3GUFPlB9hgfo~XVR0kkbWp6ONDXLmDLOW9D8GbnFA5BX-8vzU-jBHad45qWkVNuyqwJbuIA4R3rtlH~aATXKpXJPbCxs3ndjitdzS15FTuUvuMc9v2wj~fN6gIJ2pURDicJLnWq0krJwl2A-8Hi8VigLPHpizidoMrdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal