Key Points
Complement MAC triggers the development of anti-CD36–mediated murine TRALI.
Blockage of MAC formation by monoclonal anti-C7 prevented and ameliorated antibody-mediated murine TRALI.
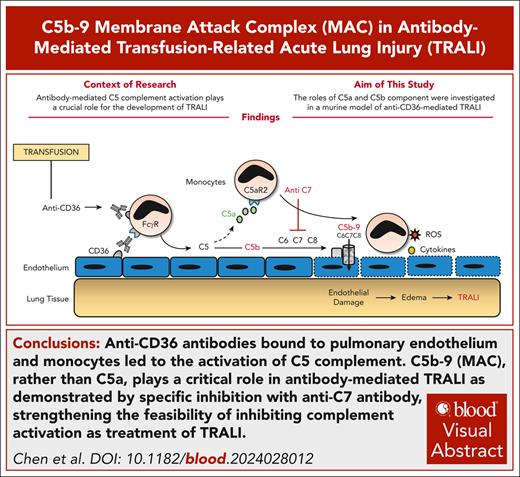
Visual Abstract
Transfusion-related acute lung injury (TRALI) is a leading cause of blood transfusion–triggered mortality. Recently, we demonstrated the critical role of Fc-dependent complement activation in anti-CD36–mediated murine TRALI. In this study, we found that C5−/− mice were protected, and administration of anti-C5 rescued wild-type mice from anti-CD36–mediated TRALI. However, C5aR1−/− mice were not protected against anti-CD36–mediated TRALI, implying a possible role of C5b-9 (membrane attack complex [MAC]). Accordingly, elevated levels of MAC were detected in bronchoalveolar lavage fluid and lung tissue of mice with anti-CD36–induced TRALI. Inhibition of MAC formation by administration of anti-C7–blocking monoclonal antibody alleviated TRALI in mice, suggesting the critical role of the MAC in the pathology of anti-CD36–mediated TRALI. Furthermore, anti-C7 treatment also led to favorable outcome in murine TRALI induced by anti–major histocompatibility complex class 1, indicating the potential broader applicability of MAC inhibitors in the treatment of antibody-mediated TRALI. Therefore, this approach may be promising to further explore the treatment of patients with TRALI.
Introduction
Transfusion-related acute lung injury (TRALI) is a clinical syndrome characterized by hypoxia and noncardiogenic pulmonary edema that occurs during or after blood transfusion.1 TRALI is a leading cause of transfusion-related diseases and death. However, no specific therapy is available for TRALI.2,3 The presence of anti-leukocyte antibodies in blood products is a risk factor. These antibodies react with HLA class 1, class 2, and human neutrophil antigens expressed on recipient cells.4
Studies in the past suggested the role of complement in TRALI, but conflicting results were found.5 Nonetheless, recent systematic in vitro and in vivo studies using monoclonal antibodies (mAbs) against major histocompatibility complex class 1 (MHC 1), in combination with analysis of complement levels in patients with TRALI, revealed a crucial role for Fc-mediated complement activation in TRALI.6,7 Hereafter, it was found that anti-MHC 1 immunoglobulin G (IgG) hexamerization initiated complement-dependent acute lung injury in mice.8
Recently, we reported that CD36 antibodies also triggered the development of murine TRALI via Fc-dependent complement activation.9 Administration of a blocking mAb, anti-C5 (BB5.1), alleviated anti-CD36–mediated TRALI in mice.9 The BB5.1 antibody is able to prevent the production of C5a and C5b, forming a nidus for the formation of the proinflammatory and cytolytic membrane attack complex (MAC).10 C5a might attract macrophages and stimulate the formation of neutrophil extracellular traps as described in anti-MHC 1–mediated TRALI.7 MAC insertion in the cell membrane activates multiple intracellular signaling pathways, leading to cell activation and thereby altering cell functions. In vitro studies showed that allo-antibody–mediated MAC internalization by endothelial cells induced cell death.11 However, the role of the MAC in antibody-mediated TRALI remains unclear. To shed light this, we used a blocking mAb against C7 (mAb 73D1) to prevent MAC generation without affecting C5 cleavage.
Study design
Two-hit murine TRALI was induced by priming with a low dose of lipopolysaccharide (LPS; 0.1 mg/kg, intraperitoneal) 24 hours before administration of mAb against CD36 (GZ1; IgG2a, 0.4 mg/kg, IV) or against MHC 1 (34-1-2S; IgG2a, 4.5 mg/kg, IV) as described previously.9,12 Anti-CD36–mediated TRALI model was conducted in wild-type, C5−/−, and C5aR1−/− C57BL/6J male mice. Anti-MHC 1–mediated TRALI was induced in wild-type BALB/c male mice. The analysis of rectal temperature, lung wet/dry weight ratio (W/D weight ratio), bronchoalveolar lavage fluid (BALF), C5a supplementation, C5aR antagonists, and treatment with anti-C7 mAb are detailed in the supplemental Methods (available on the Blood website). This study was approved by the animal care committee of Sun Yat-Sen University, Guangzhou, China (SYSU-IACUC-2020-000185).
Results and discussion
C5a-CaR2, but not the C5a-C5aR1 axis, affects anti-CD36–mediated TRALI
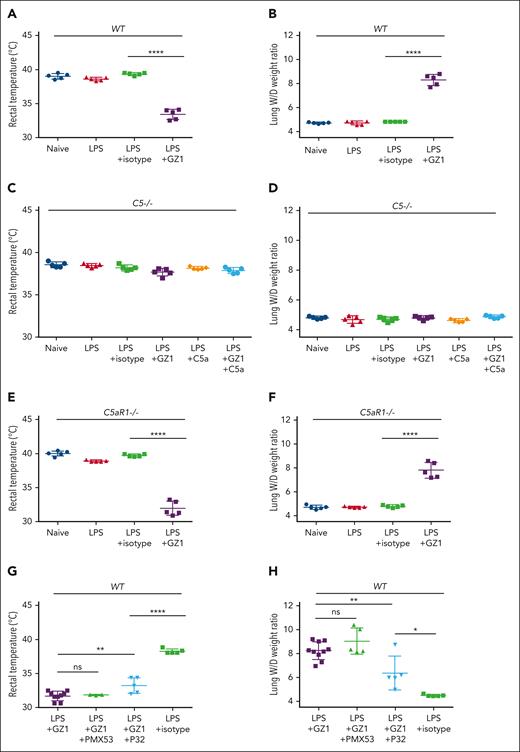
We found that administration of mAb GZ1 against CD36, upon priming with LPS, could induce a decrease in rectal temperature and an increase in TRALI severity as measured by elevated lung W/D weight ratios in wild-type (Figure 1A-B) but not in C5−/− mice (Figure 1C-D). Overall, there was no significant difference between naïve and LPS-treated mice in this study, which supports the nature of the 2-hit murine model. Compared with isotype-antibody treated mice, no significant sign of TRALI was observed in LPS-primed C5−/− mice that received mAb GZ1, even upon C5a supplementation (rectal temperature: GZ1 vs isotype, 37.68°C ± 0.43°C vs 38.20°C ± 0.31°C; P > .05; lung W/D weight ratio: GZ1 vs isotype, 4.80 ± 0.11 vs 4.68 ± 0.15; P > .05; Figure 1C-D). However, mAb GZ1 still caused TRALI in LPS-primed C5aR1−/− mice, as observed by a significant decrease in rectal temperature (GZ1 vs isotype: 31.94°C ± 1.04°C vs 39.70°C ± 0.19°C; P < .0001) and an increase in the lung W/D weight ratio compared with isotype control (GZ1 vs isotype: 7.81 ± 0.65 vs 4.78 ± 0.10; P < .0001; Figure 1E-F). Accordingly, administration of the C5aR1 antagonist PMX53 did not change the rectal temperature (GZ1 + PMX53 vs GZ1: 31.87°C ± 0.06°C vs 31.71°C ± 0.71°C; P > .05) and the lung W/D weight ratio (GZ1 + PMX53 vs GZ1: 9.03 ± 1.10 vs 8.24 ± 0.75; P > .05) of mice treated with mAb GZ1 and PMX53 compared with those treated with mAb GZ1 alone (Figure 1G-H). This suggests that the C5aR1-C5a axis does not play significant role in anti-CD36–induced murine TRALI.
Analysis of anti-CD36–mediated TRALI in wild-type, C5−/−, C5aR1−/−, and wild-type mice receiving C5aR1 or C5aR2 agonist. Wild-type, C5−/−, and C5aR1−/− C57BL/6J male mice were treated with different reagents as indicated. The signs of TRALI including rectal temperatures and lung W/D weight ratios changes in wild-type mice (A-B), C5−/− mice (C-D), C5aR1−/− mice (E-F), and wild-type mice receiving C5aR1 agonist (PMX53) or C5aR2 agonist (P32) (G-H) are presented. In the control experiment (A-B), untreated wild-type mice (naïve), or treated with LPS, with LPS alone, or LPS and afterward isotype control or mAb GZ1 (0.4 mg/kg) were shown (n = 5 in each cohort). Similar experiments were performed with C5−/− mice (C-D). In addition, rectal temperatures and lung W/D weight ratios of C5−/− mice supplemented with C5a (100 ng) and C5a together with mAb GZ1 (0.4 mg/kg) are shown (n = 5 in each cohort). Similar experiments as wild-type mice (see earlier) were performed with C5aR1−/− mice (E-F). (G-H) Rectal temperatures and lung W/D weight ratios of LPS-treated wild-type mice receiving either mAb GZ1 (n = 10), GZ1 together with PMX53 (10 mg/kg; n = 5), or (P32: 3 mg/kg; n = 5) or isotype control (n = 5). Rectal temperatures could be measured in only 9 of 10 LPS + GZ1–treated mice (1 mouse died within 30 minutes) and in 3 of 5 LPS + GZ1 + PMX53–treated mice (2 mice died within 30 minutes). Statistical analysis was performed with 1-way analysis of variance with Bonferroni correction for multiple comparisons. Each dot represents 1 mouse and error bars represent the standard deviations. ∗∗∗∗P < .0001; ∗∗P < .01; ∗P < .05. ns, nonsignificant.
Analysis of anti-CD36–mediated TRALI in wild-type, C5−/−, C5aR1−/−, and wild-type mice receiving C5aR1 or C5aR2 agonist. Wild-type, C5−/−, and C5aR1−/− C57BL/6J male mice were treated with different reagents as indicated. The signs of TRALI including rectal temperatures and lung W/D weight ratios changes in wild-type mice (A-B), C5−/− mice (C-D), C5aR1−/− mice (E-F), and wild-type mice receiving C5aR1 agonist (PMX53) or C5aR2 agonist (P32) (G-H) are presented. In the control experiment (A-B), untreated wild-type mice (naïve), or treated with LPS, with LPS alone, or LPS and afterward isotype control or mAb GZ1 (0.4 mg/kg) were shown (n = 5 in each cohort). Similar experiments were performed with C5−/− mice (C-D). In addition, rectal temperatures and lung W/D weight ratios of C5−/− mice supplemented with C5a (100 ng) and C5a together with mAb GZ1 (0.4 mg/kg) are shown (n = 5 in each cohort). Similar experiments as wild-type mice (see earlier) were performed with C5aR1−/− mice (E-F). (G-H) Rectal temperatures and lung W/D weight ratios of LPS-treated wild-type mice receiving either mAb GZ1 (n = 10), GZ1 together with PMX53 (10 mg/kg; n = 5), or (P32: 3 mg/kg; n = 5) or isotype control (n = 5). Rectal temperatures could be measured in only 9 of 10 LPS + GZ1–treated mice (1 mouse died within 30 minutes) and in 3 of 5 LPS + GZ1 + PMX53–treated mice (2 mice died within 30 minutes). Statistical analysis was performed with 1-way analysis of variance with Bonferroni correction for multiple comparisons. Each dot represents 1 mouse and error bars represent the standard deviations. ∗∗∗∗P < .0001; ∗∗P < .01; ∗P < .05. ns, nonsignificant.
Because C5a can interact with C5aR1 and C5aR2 that exhibit similar binding affinities,13 we tested the contribution of C5aR2 to the occurrence of anti-CD36 induced TRALI in LPS-primed mice using a C5aR2-selective functional ligand, P32.14 Compared with GZ1-only treatment, P32 administration decreased the severity of TRALI as shown by a significantly increased rectal temperature (GZ1 vs GZ1 + P32: 31.71°C ± 0.71°C vs 33.24°C ± 1.15°C; P < .01) and a decreased lung W/D weight ratio (GZ1 + P32 vs GZ1: 8.24 ± 0.75 vs 6.36 ± 1.41; P < .01; Figure 1G-H). However, higher lung W/D weight ratio was still observed in LPS-primed and GZ1-treated P32-treated mice compared with LPS-primed and isotype-treated mice (GZ1 + P32 vs isotype alone: 6.36 ± 1.41 vs 4.49 ± 0.08; P < .05).
Previous research has confirmed the proinflammatory effect of C5aR1, but the function of C5aR2 is not fully understood.13,15 Although C5aR2 can exacerbate sepsis-induced intestinal injury through cytokine production,16 it protects against ischemia-mediated intestinal injury through neutrophil modulation.17 A recent study showed that C5a-C5aR2 interaction could drive cytokine induction in vivo, presumably through monocytes/macrophages.18 Our previous study demonstrated the key role of monocytes as pathogenic cells in anti-CD36–mediated murine TRALI.9 In accordance, we found increased levels of monocyte chemoattractant protein 1 in serum of TRALI mice (supplemental Figure 1). In this setting, however, we demonstrated that C5aR2-selective functional ligand P32 decreased the TRALI severity. The question of whether this interaction could induce reactive oxygen species production leading to neutrophil extracellular trap production and worsening TRALI is intriguing.7 In a previous study, however, both C5aR2–/– and wild-type mice suffered TRALI caused by anti–MHC 1.19 Therefore, the role of C5aR2 in antibody-mediated TRALI needs further investigation.
C5b-9 is involved in the development of anti-CD36–mediated murine TRALI, which can be alleviated through the administration of anti-C7 IgG
We further explored the role of C5b-9 in TRALI and found that higher C5b-9 deposition was detected in lung tissue of LPS-primed mice treated with mAb GZ1 (TRALI mice), compared with LPS-primed mice treated with isotype control, and this was reversed in TRALI mice pretreated with anti-C7 (mAb 73D1) IgG (Figure 2A). In agreement, the concentration of C5b-9 was significantly higher in the BALF of LPS-primed mice treated with GZ1 than in that of LPS-primed mice treated with isotype control (GZ1 vs isotype: 91.32 ± 14.84 vs 13.13 ± 0.30; P < .0001; Figure 2B). This upregulation of C5b-9 in the BALF was abolished when anti-C7 IgG was administered to mAb GZ1–treated mice (Figure 2B).
TRALI induced by anti-CD36 led to upregulation of C5b-9 in the lungs and could be inhibited by administration of anti-C7. (A) Immunohistochemical detection of C5b-9 (MAC) in mice lung tissue from untreated wild-type mice (naïve) compared with LPS pretreated and received afterward mAb GZ1 (0.4 mg/kg) or isotype control, and pretreated with anti-C7 before GZ1 (n = 5 in each group). Lung tissue sections were stained with rabbit anti-C5b-9 (top panel) or normal rabbit IgG (normal IgG control, bottom panel), and images were taken at ×80 original magnification. Representative images from each indicated group are shown. Scale bars, 25 μm. (B) C5b-9 concentration in BALF of the indicated mouse groups were measured by enzyme-linked immunosorbent assay. In the prophylactic approach, anti-C7 (dose: 1 mg/200 μL in PBS; n = 5), or anti-C7 F(ab′)2 (dose: 1 mg/200 μL in PBS; n = 5) was administered before TRALI induction with mAb GZ1 (0.4 mg/kg). In the therapeutic approach, TRALI was first induced with mAb GZ1 (0.4 mg/kg), then mice were treated with anti-C7 (dose: 1 mg/200 μL in PBS; n = 10), or anti-C7 F(ab′)2 (dose: 1 mg/200 μL in PBS; n = 10) after TRALI. PBS was treated as a control. Rectal temperatures (C) and lung W/D weight ratios (D) were measured as described earlier. Statistical analysis was performed with 1-way analysis of variance with Bonferroni correction for multiple comparisons (B-D). Each dot represents 1 mouse and error bars represent the standard deviations. ∗∗∗∗P < .0001; ∗∗P < .01; ∗P < .05. ns, nonsignificant.
TRALI induced by anti-CD36 led to upregulation of C5b-9 in the lungs and could be inhibited by administration of anti-C7. (A) Immunohistochemical detection of C5b-9 (MAC) in mice lung tissue from untreated wild-type mice (naïve) compared with LPS pretreated and received afterward mAb GZ1 (0.4 mg/kg) or isotype control, and pretreated with anti-C7 before GZ1 (n = 5 in each group). Lung tissue sections were stained with rabbit anti-C5b-9 (top panel) or normal rabbit IgG (normal IgG control, bottom panel), and images were taken at ×80 original magnification. Representative images from each indicated group are shown. Scale bars, 25 μm. (B) C5b-9 concentration in BALF of the indicated mouse groups were measured by enzyme-linked immunosorbent assay. In the prophylactic approach, anti-C7 (dose: 1 mg/200 μL in PBS; n = 5), or anti-C7 F(ab′)2 (dose: 1 mg/200 μL in PBS; n = 5) was administered before TRALI induction with mAb GZ1 (0.4 mg/kg). In the therapeutic approach, TRALI was first induced with mAb GZ1 (0.4 mg/kg), then mice were treated with anti-C7 (dose: 1 mg/200 μL in PBS; n = 10), or anti-C7 F(ab′)2 (dose: 1 mg/200 μL in PBS; n = 10) after TRALI. PBS was treated as a control. Rectal temperatures (C) and lung W/D weight ratios (D) were measured as described earlier. Statistical analysis was performed with 1-way analysis of variance with Bonferroni correction for multiple comparisons (B-D). Each dot represents 1 mouse and error bars represent the standard deviations. ∗∗∗∗P < .0001; ∗∗P < .01; ∗P < .05. ns, nonsignificant.
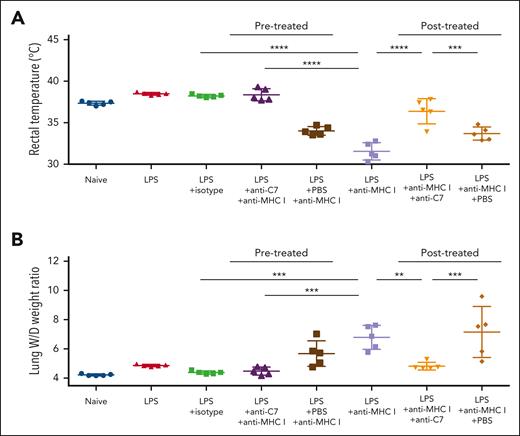
Next, we examined the efficacy of anti-C7 in preventing and ameliorating anti-CD36 antibody–induced TRALI, in LPS-primed mice (Figure 2C). When the anti-C7 IgG or F(ab′)2 fragment was administered before the induction of TRALI with mAb GZ1, TRALI was completely prevented. The rectal temperature of mice treated with GZ1 + anti-C7 IgG (37.30°C ± 0.27°C) or GZ1 + anti-C7 F(ab′)2 (37.66°C ± 0.18°C) was significantly higher than that of mice treated with GZ1 + phosphate-buffered saline (PBS; 34.44°C ± 1.56°C; P < .001). Accordingly, a significantly higher lung W/D weight ratio was found in the GZ1 + PBS group than in the GZ1 + anti-C7 IgG or F(ab′)2 treatment group (8.03 ± 1.42 vs 4.78 ± 0.30 and 4.75 ± 0.17, respectively; P < .0001). Survival rate analysis using Kaplan-Meier curves showed that only 6 of 10 (60%) GZ1 + PBS–treated mice survived beyond 2 hours after TRALI induction with mAb GZ1 compared with 100% survival of the anti-C7 IgG and F(ab′)2 treatment group (supplemental Table 1). Similar results were obtained in LPS-primed mice after TRALI induction with mAb GZ19 when anti-C7 IgG or F(ab′)2 was administered. A significant decrease in the lung W/D weight ratio was observed in mice treated with GZ1 + anti-C7 (IgG or F[ab′]2: 6.36 ± 1.64 or 6.54 ± 1.53, respectively) compared with mice treated with GZ1 + PBS (9.28 ± 0.64; P < .0001). Of the anti-C7 IgG and F(ab′)2 treated mice, 9 of 10 (90%) and 10 of 10 (100%), respectively, survived severe TRALI, whereas the survival rate was only 2 of 10 (20%) in the PBS treatment group (supplemental Table 1).
Inhibition of C5b-9 prevents anti-MHC 1–mediated TRALI
To evaluate the potential broader application of targeting C5b-9 in antibody-mediated TRALI, we examined the inhibitory effect of anti-C7 in the anti-MHC 1–mediated murine TRALI model and observed a favorable outcome regarding rectal temperatures (Figure 3A) and lung W/D weight ratios (Figure 3B). After TRALI induction, administration of anti-C7 restored lung W/D weight ratios (anti-C7 vs PBS: 4.81 ± 0.27 vs 7.15 ± 1.74; P < .001; Figure 3B) and rescued mice from mAb 34-1-2S–induced TRALI in LPS-treated mice (supplemental Table 2).
Anti-C7 inhibited anti-MHC 1–induced murine TRALI. Wild-type BALB/c male mice were treated with mAbs against MHC 1 (anti-MHC 1: mAb 34-1-2S) for TRALI induction as indicated. In the prophylactic approach, anti-C7 (dose: 1 mg/200 μL in PBS) was injected before TRALI induction with mAb 34-1-2S (4.5 mg/kg). In the therapeutic approach, TRALI was first induced with mAb 34-1-2S (4.5 mg/kg), then treated with anti-C7 (dose: 1 mg/200 μL in PBS) after TRALI. PBS was treated as a control. Rectal temperatures (A) and lung W/D weight ratios (B) were measured as described earlier (n = 5 in each cohort). Statistical analysis was performed with 1-way analysis of variance with Bonferroni correction for multiple comparisons. Each dot represents 1 mouse and error bars represent the standard deviations. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01.
Anti-C7 inhibited anti-MHC 1–induced murine TRALI. Wild-type BALB/c male mice were treated with mAbs against MHC 1 (anti-MHC 1: mAb 34-1-2S) for TRALI induction as indicated. In the prophylactic approach, anti-C7 (dose: 1 mg/200 μL in PBS) was injected before TRALI induction with mAb 34-1-2S (4.5 mg/kg). In the therapeutic approach, TRALI was first induced with mAb 34-1-2S (4.5 mg/kg), then treated with anti-C7 (dose: 1 mg/200 μL in PBS) after TRALI. PBS was treated as a control. Rectal temperatures (A) and lung W/D weight ratios (B) were measured as described earlier (n = 5 in each cohort). Statistical analysis was performed with 1-way analysis of variance with Bonferroni correction for multiple comparisons. Each dot represents 1 mouse and error bars represent the standard deviations. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01.
All these results demonstrated the critical role of the MAC, rather than C5a, as demonstrated by specific C7 inhibition in antibody-mediated murine TRALI. These findings strengthen the feasibility and further exploration of inhibiting complement activation as a treatment strategy for patients with TRALI.20,21
Acknowledgments
The authors thank Gregor Bein from the Institute for Clinical Immunology, Transfusion Medicine, and Hemostaseology, Justus Liebig University, for providing the doctoral position in his department. The authors thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of the manuscript.
D.C. is a PhD candidate at the Justus Liebig University, and this work is submitted in partial fulfillment of the doctoral requirements of the PhD thesis.
This work was supported by the National Natural Science Foundation of China (82270237 and 82470231), the Basic and Applied Basic Research Foundation of Guangdong (2023A1515010924 and 2025A1515010465), Science and Technology Projects in Guangzhou (2023A03J0552, 202201010013, 2025A03J3369, and 2025A03J3453), and the Key Medical Disciplines and Specialties Program of Guangzhou (2025-2027).
Authorship
Contribution: D.C., R.K., S.S., and Y.F. conceived of and designed the research; D.C., X.X., W.X., X.Y., Y.L., J.H., Y.X., J.L., H.R., and S.L. performed experiments; W.M.Z. and T.M.W. supplied anti-C7 monoclonal antibody (clone 73D1) and C5aR2 agonist (P32) and accompanied the experiments in this regard, respectively; D.C., S.S., H.L., X.X., and W.X. analyzed data; D.C., R.K., S.S., and Y.F. wrote the manuscript; and W.M.Z., B.P.M., and T.M.W. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yongshui Fu, Institute of Blood Transfusion, Guangzhou Blood Centre, 31 Lu Yuan Rd, Guangzhou 510095, China; email: fuyongshui@sina.com; Sentot Santoso, Institute for Clinical Immunology, Transfusion Medicine and Hemostaseology, Justus Liebig University Giessen, Langhansstr 7, 35385 Giessen, Germany; email: sentot.santoso@immunologie.med.uni-giessen.de; and Rick Kapur, Sanquin Blood Supply Foundation, Department Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; email: r.kapur@sanquin.nl.
References
Author notes
D.C., H.L., and X.X. contributed equally to this study.
Data are available on request from the corresponding authors, Yongshui Fu (fuyongshui@sina.com), Sentot Santoso (sentot.santoso@immunologie.med.uni-giessen.de), and Rick Kapur (r.kapur@sanquin.nl).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal