In this issue of Blood, Navarro et al1 used recently developed immunocompetent mouse models of multiple myeloma to demonstrate a novel role of glutamine metabolism in the activity of B-cell maturation antigen (BCMA)–targeting chimeric antigen receptor (CAR) T cells.
Many of us heard the adage “you are what you eat” as a child. And, when it comes to multiple myeloma, it turns out that malignant plasma cells like to eat a lot. In particular, myeloma tumors, characterized by markedly high rates of protein synthesis, consume copious amounts of the amino acid glutamine. Indeed, myeloma tumors are strongly dependent on significant intake of glutamine from the tumor microenvironment for survival, more so than other amino acids.2 Blocking glutamine uptake has even been proposed as a potential therapeutic strategy for this disease.2
However, it turns out that malignant plasma cells are not the only heavy consumers of glutamine in the body. Upon activation, T cells also ramp up their protein synthesis needs significantly, thereby becoming heavily dependent on glutamine to achieve either their antitumor or anti-infectious immune potential.3 CAR T cells, which are created by genetically engineering a patient’s own T cells to recognize a tumor surface–expressed antigen, have shown immense promise in the treatment of myeloma.4 Specifically, in the case of CAR T cells that target BCMA, although long-term durable responses have been observed for some patients with myeloma, the majority are unfortunately still destined to relapse.5 Therefore, a key mechanistic question that still remains is why do all patients not achieve durable CAR T-cell responses, and, furthermore, how can this lack of durable response be overcome therapeutically?
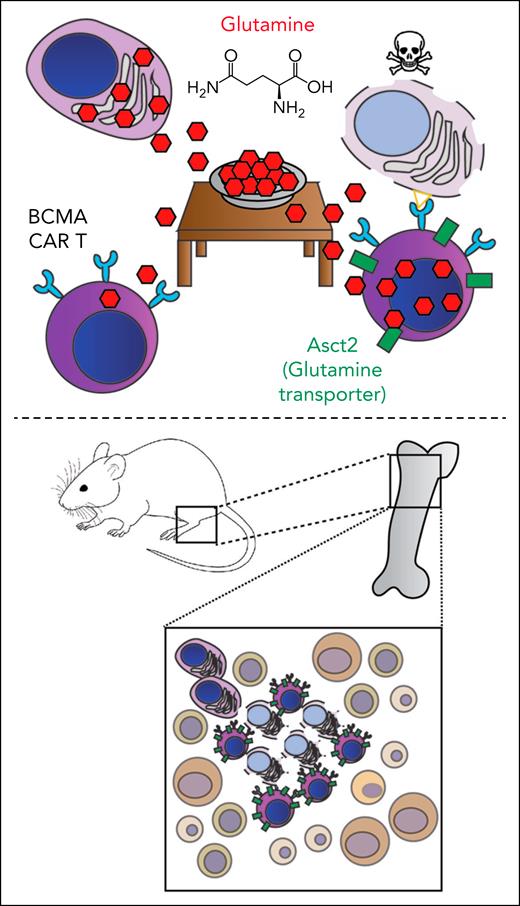
In the study, the authors begin with the insightful observation that BCMA-targeting T cells, like endogenous T cells, also require significant glutamine uptake to achieve their full antitumor potential. However, like an unwelcome houseguest, myeloma tumors are eating most of the available glutamine in the bone marrow, rudely leaving an insufficient glutamine meal for the CAR T cells that are joining them at the tumor microenvironment table (see figure). Navarro et al elegantly demonstrated that overexpression of the glutamine transporter Asct2 in murine CAR T cells that are targeting BCMA led to significantly improved performance, both in vitro and in in vivo mouse models. These Asct2-overexpressing CAR T cells showed improved metabolic profiles and increased cytokine release as underlying mechanisms of improved antitumor efficacy. Finally, they also correlated high ASCT2 expression in human myeloma tumors with poorer outcomes, potentially consistent with myeloma overeating glutamine and outcompeting the surveilling immune system for this key nutrient.5
(Top) Myeloma plasma cells eat significant glutamine within the tumor microenvironment, thereby preventing the full activity of BCMA-targeting CAR T cells unless they are engineered to overexpress the glutamine transporter Asct2. (Bottom) These Asct2-overexpressing CAR T cells are highly active in a fully immunocompetent, genetically engineered mouse model of myeloma.
(Top) Myeloma plasma cells eat significant glutamine within the tumor microenvironment, thereby preventing the full activity of BCMA-targeting CAR T cells unless they are engineered to overexpress the glutamine transporter Asct2. (Bottom) These Asct2-overexpressing CAR T cells are highly active in a fully immunocompetent, genetically engineered mouse model of myeloma.
These observations are significant in not only explaining a potential new mechanism of CAR response and resistance in patients with myeloma but also provide a major advance in the modeling of CAR T-cell activity in the context of an intact tumor immune microenvironment. Essentially, all preclinical CAR T-cell studies in myeloma have been performed in heavily immunocompromised mouse models implanted with human myeloma cell line xenografts and then treated with CAR-engineered human T cells. Although these immunocompromised models are informative to reveal aspects of the antitumor CAR T-cell efficacy (indeed, such preclinical studies have underpinned the successful clinical translation of anti-BCMA CAR T cells), they still leave major gaps in our understanding of how CAR T cells function within an intact immune microenvironment as they necessarily must do in humans.
Therefore, the robust demonstration of BCMA CAR T-cell activity within an immunocompetent mouse model, as shown in the study, stands as a significant accomplishment in the field. Navarro et al took advantage of innovative, genetically engineered mouse models of myeloma that were recently described by members of the authorship team.6 These models represent exciting new options in the field of modeling myeloma therapy with an intact immune microenvironment. Although the study does not delve deeply into the role of this immune microenvironment in CAR T-cell response and resistance, this work sets the stage for future studies to characterize more thoroughly the role of T-regulatory cells, myeloid cells, specific cytokines, and other immune microenvironment components that have been correlated with CAR T-cell outcomes in humans.7 Although such studies will always be limited by the fact that the human and murine immune systems are not identical, much has been learned in other areas of human immunity by using such murine models. The time has now come to explore these dynamics in the context of myeloma CAR T cells.
One question regarding the findings here that requires further investigation is the place of glutamine metabolism within the broader dynamics of myeloma CAR T-cell efficacy. Human correlative studies have suggested that numerous other mechanisms impact the durable response of CAR T cells, ranging from T-cell fitness after manufacturing, T-cell exhaustion dynamics, persistence of CAR T cells in the microenvironment, and more.6 It remains to be seen whether glutamine metabolism plays a key role in all of these mechanisms or only in some. Future work will also be needed to evaluate whether the mechanisms here are unique to BCMA-targeting CAR T cells, whether they apply to other myeloma CAR T-cell targets,8,9 or even beyond myeloma to other cancer indications.
In summary, with their technical advance, Navarro et al have now invited many more investigators to the table of CAR T-cell modeling in an immunocompetent myeloma model. We look forward to feasting on numerous future studies that dissect the mechanisms of CAR T-cell response and resistance, including and beyond glutamine metabolism.
Conflict-of-interest disclosure: A.P.W. reports being an equity holder in and scientific advisory board member for Seen Therapeutics; being an equity holder in Indapta Therapeutics; and receiving licensing fees from Deverra Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal