In this issue of Blood, Lin and colleagues1 reevaluated pediatric Langerhans cell histiocytosis (pLCH) diagnostic staging, transitioning staging from a historical focus on survival to prioritizing the risk of treatment failure and LCH-related neurodegeneration (LCH-ND) development. The authors found that the presence of BRAFV600E in pretreatment peripheral blood mononuclear cells (PBMCs) strongly predicted frontline treatment failure and LCH-ND development and developed a new risk score that incorporated lesion location, extent, organ involvement, and PBMC-BRAFV600E status that identified high-risk patients. Their findings suggest a revised pathogenesis model where disease persistence and clinical risks are dictated by PBMC-BRAFV600E status, shifting staging toward a dynamic, risk-adapted strategy that focuses on treatment failure and LCH-ND risk instead of the risk of death.
LCH is a rare, incurable malignancy characterized by the proliferation of Langerhans cells, a type of dendritic cell, in various organs. Affecting both children and adults, LCH has seen a paradigm shift due to recent advancements in understanding its pathobiology. The discovery of BRAFV600E in nearly half of LCH cases, along with other mutually exclusive MAPK pathway gene mutations, has resulted in significantly improved treatment and prognosis.2 LCH is now considered mostly a chronic disease shifting the focus of management toward maximizing survivorship, preserving fertility, ensuring cognitive health, minimizing treatment-related adverse effects, and extending remission duration rather than concentrating solely on overall survival. Consequently, there is a need for a revised staging and prognostic scoring system that incorporates the molecular characteristics and evaluates patients based on clinically relevant end points for a chronic malignancy associated with significant morbidity.
The treatment landscape for LCH reflects the evolving understanding of its molecular underpinnings. In adult LCH, targeted therapy, tailored to the identified molecular alterations, is the predominant approach, especially for multisystem disease, and has produced deep and durable responses.3 Conversely, although fixed-duration chemotherapy remains the primary treatment modality for pLCH, there is increasing exploration of limited-duration combination therapies incorporating targeted agents alongside chemotherapy.4
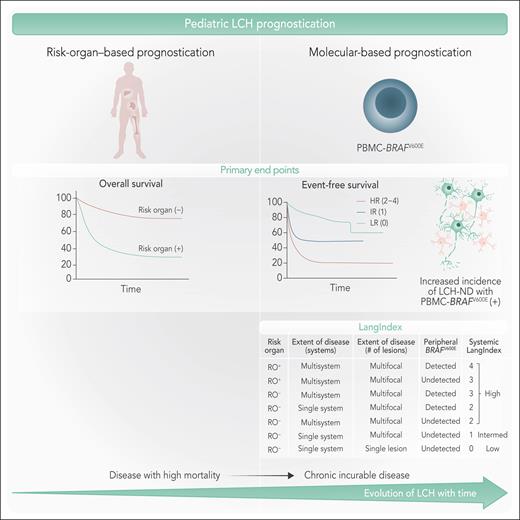
The study by Lin et al offers a compelling reevaluation of risk stratification of predominantly chemotherapy-treated pLCH that identified the prognostic significance of detectable pretreatment BRAFV600E in PBMCs. The proposed staging system, incorporating disease burden, organ involvement, and PBMC BRAFV600E status, offers a more clinically relevant approach to risk stratification. The authors also developed a composite risk score (LangIndex) to predict the frontline treatment failure and the development of LCH-ND in pLCH, a complication with significant clinical implications and still incompletely understood pathophysiology (see figure).
Evolution of prognostic systems in LCH. (Left) Traditional risk-organ–based prognostication categorizes patients based on the presence or absence of risk-organ involvement, affecting overall survival. Patients with risk-organ involvement exhibit poorer survival compared with those without. (Right) Molecular-based prognostication highlights the role of PBMC-BRAFV600E mutation status incorporated in the LangIndex in predicting event-free survival and the development of LCH-ND. The evolution of LCH over time is illustrated as a transition from a high-mortality disease to a chronic, incurable condition with associated morbidity. Professional illustration by Somersault18:24.
Evolution of prognostic systems in LCH. (Left) Traditional risk-organ–based prognostication categorizes patients based on the presence or absence of risk-organ involvement, affecting overall survival. Patients with risk-organ involvement exhibit poorer survival compared with those without. (Right) Molecular-based prognostication highlights the role of PBMC-BRAFV600E mutation status incorporated in the LangIndex in predicting event-free survival and the development of LCH-ND. The evolution of LCH over time is illustrated as a transition from a high-mortality disease to a chronic, incurable condition with associated morbidity. Professional illustration by Somersault18:24.
Several important points highlighted in this study merit further discussion. The study’s strength lies in its large retrospective cohort, allowing for a robust analysis of clinical outcomes and their correlation with molecular markers. This study’s findings must be considered within the established understanding that disease burden and risk-organ involvement significantly correlate with prognostic outcomes in LCH.5,6 Limited only to lesional BRAFV600E-positive pLCH, this study emphasized the correlation between the detection of BRAFV600E in PBMCs and both frontline treatment failure and the development of LCH-ND. However, the independent predictive value of PBMC-BRAFV600E status in developing LCH-ND diminished when disease burden and risk-organ involvement were accounted for. Nonetheless, PBMC-BRAFV600E status remained an independent predictor of frontline treatment failure in pLCH treated with chemotherapy. In addition, the increased detection of PBMC-BRAFV600E in patients with multiorgan/multifocal disease, even in the absence of risk-organ involvement, highlights the persistence of the precursor mutant population reservoir that correlates with disease burden. This finding suggests that PBMC-BRAFV600E could serve as a biomarker for assessing this precursor reservoir, which is predictive of treatment failure. This observation is consistent with previous studies that demonstrate an association between detectable BRAFV600E in the peripheral blood, as circulating tumor DNA or in PBMC, and adverse outcomes in patients with pLCH treated with chemotherapy.7,8 Furthermore, BRAFV600E mutation status has been linked to the development of LCH-ND, which occurs in at least 10% to 20% of patients.9,10
Collectively, these observations raise important questions that need to be addressed through well-designed studies. Key inquiries include the following: (1) does PBMC-BRAFV600E positivity merely reflect disease burden and dissemination in BRAFV600E-positive LCH or does it indicate a distinct biological mechanism that influences LCH-ND development and treatment failure?; (2) what biomarkers can effectively risk stratify non-BRAFV600E LCH?; (3) would incorporating BRAFV600E-targeted therapy into frontline regimens improve event-free survival (EFS) in patients with detectable BRAFV600E in PBMCs?; (4) what are the biological mechanisms by which BRAFV600E contributes to LCH-ND development?; and (5) what is the clinical and prognostic relevance of PBMC-BRAFV600E detection in adult LCH?
The strong association between PBMC-BRAFV600E status and pLCH prognosis necessitates treatment adjustments. MAPK pathway inhibitors offer a potential strategy to reduce treatment failure and improve EFS; however, the long-term toxicity of continuous targeted therapy (BRAFV600E or MEK inhibitors) in pediatric patients requires careful consideration. Combining targeted therapy with chemotherapy to create a fixed-duration regimen, an approach currently under investigation,4 may mitigate the risks associated with continuous treatment. Furthermore, the findings of this study suggest exploring PBMC-BRAFV600E as a potential marker to assess minimal residual disease (MRD) to guide treatment decisions regarding intensification or discontinuation. Future research should, therefore, also investigate treatment modifications to address the inferior prognosis associated with PBMC-BRAFV600E detection and explore the utility of PBMC-BRAFV600E as an MRD assessment tool for tailoring treatment in BRAFV600E-positive pLCH.
In conclusion, this study offers valuable insights into pLCH pathogenesis and risk stratification. It significantly advances the field by shifting the focus from the conventional risk-organ–based mortality staging system to more dynamic assessments centered on the precursor mutant cancer cell population, EFS, and LCH-ND development. However, the current findings apply only to pLCH cases that harbor BRAFV600E in the tumor. In addition, their applicability to adult LCH is limited due to differences in treatment and the less frequent detection of BRAFV600E in PBMC. Therefore, the proposed risk stratification model should not entirely replace risk-organ–based stratification. Instead, it should encourage the incorporation of PBMC-BRAFV600E detection and clinically meaningful end points into existing risk stratification systems. This will pave the way for improved risk stratification of patients and may significantly enhance the efficacy of frontline therapy using molecular medicine.
Conflict-of-interest disclosure: W.O.T. reports receiving research funding from the National Institutes of Health and the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology and royalties from the publication of “Mayo Clinic Cases in Neuroimmunology” (Oxford University Press). J.P.A. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal