In this issue of Blood, Kim et al1 present compelling findings from a phase 2 trial exploring the combination of cemiplimab, an anti–programmed death-1 (anti–PD-1) antibody, and isatuximab, an anti-CD38 antibody, in relapsed or refractory (R/R) extranodal natural killer/T-cell lymphoma (ENKTL).
Although l-asparaginase–based chemotherapy remains effective in frontline settings,2 outcomes for patients with R/R disease are dismal, emphasizing the urgent need for innovative therapeutic strategies. Given this background, the results presented by Kim et al are encouraging. The authors demonstrate that CD38 cotargeting can enhance the efficacy of PD-1 blockade, achieving a complete response (CR) rate of 51% and an overall response rate of 65% in 37 treated patients. Importantly, the responses were durable, with a median duration of 21 months. Notably, responders exhibited high programmed death ligand 1 (PD-L1) expression and structural variations in the PD-L1 3'-untranslated region, suggesting a potential biomarker for response. The combination therapy was well tolerated, with no treatment-related deaths. These promising results raise a key question: Can CD38 inhibition unlock the full therapeutic potential of PD-1 blockade in ENKTL?
To contextualize the impact of CD38 inhibition, it is essential to consider prior clinical experience with PD-1 monotherapy. The rationale for PD-1 blockade in ENKTL stems from frequent PD-L1 expression on Epstein-Barr–infected tumor cells3 and the potential to reduce T-regulatory (Treg)-mediated immunosuppression.4 Early reports of pembrolizumab treatment in small cohorts generated much attention, demonstrating remarkable responses in a subset of patients.5 However, larger real-world studies have tempered expectations, showing only modest efficacy, with CR rates ranging from 15% to 35% and limited durability of response.6 In this context, the significantly higher CR rate observed with the cemiplimab-isatuximab combination suggests a possible advantage over PD-1 blockade alone. Although the single-arm design of Kim et al's study precludes direct comparisons, the observed efficacy appears to exceed that of prior PD-1 monotherapy trials. Nevertheless, establishing the true benefit of CD38 inhibition would require a randomized trial directly comparing combination therapy with PD-1 blockade alone.
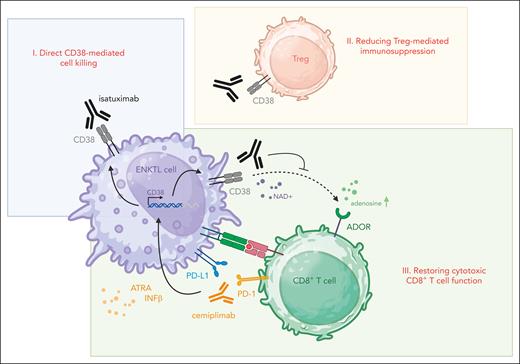
Several mechanisms may explain the synergy between PD-1 and CD38 blockade (see figure): (i) CD38 is frequently upregulated on ENKTL tumor cells,7 making it a plausible direct therapeutic target. However, Kim et al found no correlation between CD38 expression levels and response rates, suggesting that the benefits of isatuximab likely stem from immunomodulation rather than direct tumor killing. (ii) Tregs, which often express CD38, play a crucial role in sustaining an immunosuppressive tumor microenvironment (TME) in ENKTL through their immunoregulatory functions. CD38 blockade may disrupt Treg-mediated suppression, lessening immune inhibition and shifting the TME toward a more immune-permissive state.4 The finding that higher response rates were associated with increased Treg abundance supports this hypothesis. (iii) Another immunoregulatory role of CD38 relates to its effect on CD8+ cytotoxic T cells. PD-1 inhibition can trigger compensatory upregulation of CD38 on tumor cells, which has been linked to the exhaustion of cytotoxic T cells in the surrounding microenvironment. This adaptive resistance mechanism may undermine the efficacy of PD-1 blockade but could potentially be reversed through CD38 inhibition, restoring cytotoxic T-cell activity, and amplifying antitumor immunity.8
Enhancing PD-1 blockade efficacy through CD38 inhibition. The figure illustrates potential mechanisms through which combining the PD-1 inhibitor cemiplimab (orange) with the CD38 monoclonal antibody isatuximab (black) may amplify immune checkpoint blockade in ENKTL. (I) Direct CD38-mediated cell killing: isatuximab induces direct cytotoxicity against ENKTL cells (violet), which have CD38 expression that is further upregulated in response to PD-1 blockade.4 (II) Reducing Treg-mediated immunosuppression: isatuximab eliminates immunosuppressive CD38-positive Tregs (red), promoting a more immune-permissive tumor microenvironment and boosting antitumor immunity.4 (III) Restoring cytotoxic CD8+ T-cell function: PD-1 inhibition with cemiplimab activates cytotoxic T cells (green). However, compensatory upregulation of CD38 in malignant cells, driven by factors like ATRA and IFNβ (black arrows), suppresses cytotoxic T-cell function.8 CD38 plays an important role as an ectoenzyme in metabolizing NAD and generating adenosine, which activates ADORs on cytotoxic T cells, dampening their function (black dotted arrow).4 By blocking CD38, isatuximab disrupts this resistance mechanism (black inhibitor), potentially enhancing and prolonging the efficacy of PD-1 blockade. ADOR, adenosine receptor; ATRA, all-trans retinoic acid; IFNβ, interferon-β; NAD, nicotinamide adenine dinucleotide. Figure created in BioRender (https://BioRender.com/y93h678).
Enhancing PD-1 blockade efficacy through CD38 inhibition. The figure illustrates potential mechanisms through which combining the PD-1 inhibitor cemiplimab (orange) with the CD38 monoclonal antibody isatuximab (black) may amplify immune checkpoint blockade in ENKTL. (I) Direct CD38-mediated cell killing: isatuximab induces direct cytotoxicity against ENKTL cells (violet), which have CD38 expression that is further upregulated in response to PD-1 blockade.4 (II) Reducing Treg-mediated immunosuppression: isatuximab eliminates immunosuppressive CD38-positive Tregs (red), promoting a more immune-permissive tumor microenvironment and boosting antitumor immunity.4 (III) Restoring cytotoxic CD8+ T-cell function: PD-1 inhibition with cemiplimab activates cytotoxic T cells (green). However, compensatory upregulation of CD38 in malignant cells, driven by factors like ATRA and IFNβ (black arrows), suppresses cytotoxic T-cell function.8 CD38 plays an important role as an ectoenzyme in metabolizing NAD and generating adenosine, which activates ADORs on cytotoxic T cells, dampening their function (black dotted arrow).4 By blocking CD38, isatuximab disrupts this resistance mechanism (black inhibitor), potentially enhancing and prolonging the efficacy of PD-1 blockade. ADOR, adenosine receptor; ATRA, all-trans retinoic acid; IFNβ, interferon-β; NAD, nicotinamide adenine dinucleotide. Figure created in BioRender (https://BioRender.com/y93h678).
Supporting the clinical findings in this phase 2 trial, a systematic analysis integrating transcriptomic profiling with immunohistochemical annotations revealed that most ENKTL cases exhibit an immune-evasive phenotype, characterized by high expression of immune checkpoint molecules such as PD-L1.9 In addition, exhaustion of effector T cells within the ENKTL TME has been reported, as evidenced by impaired cytokine production and diminished cytotoxic function.10 These exhausted T cells may persist as dysfunctional cells incapable of mounting effective antitumor responses, even in the presence of PD-1 blockade. Despite the insights just outlined, the intricacies of the ENKTL TME remain incompletely understood. Future studies should integrate high-dimensional profiling techniques, such as single-cell RNA sequencing and spatial transcriptomics, to dissect the TME with greater granularity. These approaches would facilitate the identification of immune signatures predictive of response and reveal additional therapeutic vulnerabilities.
Kim et al have demonstrated that CD38 inhibition enhances the efficacy of PD-1 blockade in R/R ENKTL. The cemiplimab-isatuximab combination revealed encouraging response rates, prolonged remissions, and a favorable safety profile, offering a promising new approach for patients with limited treatment options. Their findings also highlight the clinical relevance of TME modulation, reinforcing the notion that CD38 blockade may help reverse T-cell exhaustion and overcome immune evasion. Although these results mark a significant advance, several critical questions remain unanswered. Randomized trials must confirm the added benefit of CD38 inhibition alongside mechanistic studies that further elucidate the role of the TME in shaping therapeutic outcomes. By integrating clinical and translational research, we can refine patient selection strategies, identify novel combinatorial approaches, and ultimately improve outcomes for patients with this devastating disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal