Key Points
We developed a therapeutic cancer vaccine for MM against BCMA.
Our findings revealed BCMA-specific immune response, providing the framework for its clinical evaluation to improve patient outcome.
Visual Abstract
Cancer vaccines are emerging as promising therapies to not only prevent cancer but to treat cancer. Here, we developed a therapeutic vaccine for multiple myeloma (MM) using B-cell maturation antigen (BCMA) protein as a target. Given the remarkable efficacy of COVID-19 messenger RNA (mRNA) vaccines, we first packaged sequence- and base-optimized BCMA mRNA into lipid nanoparticles (LNPs) using next-generation ionizable lipid, enhancing their accumulation in the spleen. A toll-like receptor 3 agonist, polyinosinic:polycytidylic acid [poly(I:C)], was also encapsulated in LNPs to further elicit BCMA-specific immune response. BCMA-mRNA LNPs were internalized by dendritic cells (DCs) in vitro, triggering proliferation and activation of BCMA-specific CD8+ cytolytic T cells (CTLs). Importantly, these CTLs lysed BCMA+ U266 MM cells and CD138+ patient MM cells, without affecting BCMA-knockout U266 or CD138− patient–derived bone marrow cells. Vaccination of C57BL/6J mice with BCMA-mRNA LNPs activated splenic DCs and induced BCMA-specific CTLs, assessed by tetramer staining, which selectively killed murine 5TGM1 BCMA overexpressing MM cells. Finally, vaccination of C57BL/KaLwRijHsd mice bearing BCMA-overexpressing 5TGM1 cells inhibited tumor growth associated with BCMA-specific CD8+ T-cell responses. The combination treatment with poly(I:C) further triggered the immune response induced by BCMA-mRNA LNPs in all instances. Our findings provide the framework for clinical evaluation of BCMA-mRNA LNP vaccines to improve patient outcome in MM.
Introduction
Cancer vaccines are promising therapies to not only prevent cancer (such as the vaccine targeting the oncovirus behind human papillomavirus) but also to treat existing cancer.1-3 Therapeutic cancer vaccines promote uptake of tumor-associated antigens by dendritic cells (DCs; or other antigen-presenting cells [APCs]), which then stimulate T cells to target cancer cells.4,5 Although current immunotherapies such as chimeric antigen receptor T cells and bispecific engagers achieve a high extent and frequency of responses in patients with multiple myeloma (MM), relapses occur because of downregulation, mutation, or genetic loss of target antigens; lack of memory T-cell responses; and/or T-cell exhaustion.6,7 Vaccines can potentially overcome these limitations by inducing memory immune responses against tumor-specific or tumor-associated antigen, and could be used alone or in combination with these T-cell–redirected therapies to enhance their efficacy. Additionally, cancer vaccines can target several antigens at once, potentially overcoming tumor heterogeneity while delaying development of resistance.8,9
Recent advances in messenger RNA (mRNA) vaccines as well as lipid nanoparticle (LNP) delivery systems have markedly enhanced responses, as evidenced by COVID-19 mRNA vaccines. These vaccines deliver mRNA of the target protein to APCs, followed by translation, processing in the immunoproteasome, and presentation of peptides on the cell surface, which, in turn, induce T-cell–mediated antigen-specific adaptive immunity. Ionizable lipid plays the most crucial role in mRNA packaging and cellular delivery among all 4 different lipid components (phospholipid, cholesterol, ionizable lipid, and polyethylene glycol–conjugated lipid) that compose LNPs. Specifically, ionizable lipids remain neutral at physiological pH in the serum but switch to their protonated or positively charged forms at low pH within the endosomal compartment, thereby enhancing mRNA release in the cytosol. Next-generation ionizable lipids such as lipid 5 and lipid H with enhanced biodegradability have been developed by introducing ester bonds and disulfide motifs that accelerate endosomal escape, thereby improving stability while reducing toxicity.10 In addition, recent progress in nanoparticle engineering enables organ-specific delivery of mRNA, which helps to reduce unintended liver accumulation and facilitates activation of immune cells for achieving enhanced therapeutic efficacy. The present study is directed to exploit these advances in mRNA vaccination to develop a therapeutic mRNA vaccine for MM.
Normal and malignant plasma cells express the highly selective marker B-cell maturation antigen (BCMA) on their surface, which has been effectively targeted by chimeric antigen receptor T cells, bispecific T-cell engagers, and immunotoxins.11-15 In this study, we attempted to induce a BCMA-specific adaptive immune response in MM using a BCMA-mRNA vaccine. We first packaged the sequence- and base-optimized BCMA mRNA into LNPs using a next-generation ionizable lipid component, lipid 5, to improve the in vivo delivery and accumulation of mRNA in the spleen, and thereby enhance the induction of in vivo immune responses.16,17 We also evaluated a toll-like receptor 3 (TLR3) agonist, polyinosinic:polycytidylic acid [poly(I:C)] to further elicit an immune response.18 Our study demonstrated that BCMA-mRNA LNP vaccine alone or in combination with poly(I:C) effectively and selectively triggered immune responses and specific cytotoxicity against MM cell lines and autologous patient MM cells in vitro, as well as in vivo in a therapeutic model of murine MM, providing the preclinical rationale for its clinical evaluation to improve patient outcome in MM.
Materials and methods
Production and characterization of LNPs loaded with BCMA mRNA and poly(I:C)
Transfection and translation efficacy of LNPs
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors after written informed consent in accordance with the Declaration of Helsinki and under the approval of the institutional review board of Dana-Farber Cancer Institute. The monocyte-derived DCs (MoDCs) were generated from PBMCs by CD14-positive selection using magnetic beads (Miltenyi Biotec) and cultured for 6 days in differentiation medium, containing granulocyte-macrophage colony-stimulating factor and interleukin-4 (130-094-812; Miltenyi Biotec).22 The MoDCs were incubated with treatment groups: control, free cyanine-5 (Cy5)–tagged hBCMA mRNA, and Cy5-tagged hBCMA mRNA, packaged in LNPs alone or in combination with poly(I:C) LNPs for 1 hour or 2 hours to study cellular internalization using flow cytometry and confocal microscopy. Translation efficacy23 was similarly evaluated using green fluorescent protein (GFP)–encoding mRNA. We used CellProfiler (version 4.2.1) for quantitative analysis of fluorescence intensity for all confocal images.
Evaluation of DC activation, T-cell proliferation, and ELISpot analysis
MoDCs and naïve T cells were isolated from the same PBMCs: MoDCs by CD14-positive selection using CD14 magnetic beads and naïve T cells by negative selection from CD14− fraction using a Naive Pan T-Cell isolation kit (Miltenyi Biotec). The isolated naïve T cells were stored at −80°C. DC activation marker CD80 was evaluated by flow cytometry on CD11c+ DCs after 24-hour treatment. For T-cell proliferation assay conducted at various intervals, T cells were stained with carboxyfluorescein succinimidyl ester (CFSE) cell-proliferation kit (Thermo Fisher Scientific) after coculturing with MoDCs pulsed with control, hBCMA mRNA, hBCMA-mRNA LNPs, and hBCMA-mRNA+poly(I:C) LNPs. Enzyme-linked immunospot (ELISpot) analysis were done as previously described.22,24
Sequence- and base-optimized BCMA mRNA and in vitro tetramer staining
The HLA-A2 binding affinity of different hBCMA peptide sequences was analyzed using an artificial neural network (ANN 4.0) tool. The highest binding affinity peptide, AVFVLMFL, was chosen for hBCMA tetramer development. The APC-conjugated tetramer AVFVLMFL, was obtained from MBL International. Tetramer staining was performed for the detection of AVFVLMFL-specific CD8+ T cells.
Cytotoxicity of CTLs on cancer cells
MoDCs and T cells were isolated from PBMCs from either healthy donors or patients with MM, and CD138+ and CD138− cells were isolated from bone marrow mononuclear cells of patients with MM after informed consent and approval by the institutional review board of the Dana-Farber Cancer Institute. After pulsing with different treatments as described earlier, MoDCs generated from normal donors were cocultured with T cells for 5 days. The CTLs were isolated from the coculture, and cytotoxicity was evaluated 24 hours after incubation with CFSE-prestained U266 wild type (WT), U266 BCMA-knockout (KO) cells, and patient CD138+ and CD138− cells for allogeneic setting using flow cytometry.22,25 For autologous experimental set up, MoDCs and T cells, and CD138+ cells were isolated from the same patient PBMCs and bone marrow, respectively.
Calcein assay
The hBCMA-specific antitumor activity of CTLs in vitro was analyzed on U266-WT and U266-BCMA-KO cells by calcein-release cytotoxicity assay,26 as previously described.
In vivo tissue toxicity
All animal experimental protocols were approved to the relevant regulatory standards of the institutional animal care and use committee at the Dana-Farber Cancer Institute.
In all treatment groups, toxicity in the liver, spleen, lung, kidney, and heart was evaluated by hematoxylin and eosin staining.27
In vivo LNP uptake and DC activation
DiR (1,1'-dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide) dye–loaded LNPs were injected through the IV and intramuscular (IM) routes into C57BL/6J mice28 to assess in vivo internalization by APC cells including macrophages and DCs. DC activation was analyzed by flow cytometry for cell surface expression of CD40 and CD80 on CD11c+ DCs.
In vivo stability and translation efficacy of mRNA-loaded LNPs
The free mRNA encoding firefly luciferase (F-Luc) and F-Luc-mRNA loaded in LNPs were injected into C57BL/6J mice through the IV and IM routes29 to evaluate the in vivo mRNA stability and translation efficacy.
mBCMA-specific CTL by tetramer staining
Splenocytes isolated from immunized C57BL/6J mice were stained for mBCMA-specific tetramer–positive CTLs,30 as described previously.
Immunofluorescence staining of activated splenic T cells
Immunofluorescence staining for CD3+ T cells and CD8+ T cells was used to assess splenic localization of activated cytotoxic effector T cells.
In vivo CTL activity and therapeutic efficacy in mouse model
The anti-MM activities of in vivo mBCMA-specific CTLs were investigated using murine MM cell lines 5TGM1-WT and 5TGM1 BCMA-overexpressing (5TGM1-BCMA-OE),22 and the therapeutic efficacy of the vaccine was evaluated in the tumor-bearing C57BL/KaLwRijHsd mouse model.
Detailed information of materials and all methods is included in the supplemental Methods, available on the Blood website.
Statistical analysis
Images, bar diagrams, and pictorial representations were generated using GraphPad Prism, Origin, BD FACSDiva, Gimp 2.10.30, and ImageJ. Data are presented as mean ± standard deviation. Statistical comparisons between groups were performed using an unpaired Student t test (∗P < .05).
Results
BCMA-mRNA LNPs are stable in serum and show efficient endosomal escape into the cytosol
We packaged BCMA mRNA or poly(I:C) into LNPs, a US Food and Drug Administration–approved delivery mechanism using a next-generation ionizable delivery component.19,31 The lipid components of the LNPs were: heptadecane-9-yl 8-((2-hydroxyethyl)(8-nonyloxy)-8-oxoctyl) amino) octanoate (lipid 5) to ensure efficient encapsulation of mRNA32,33; 1-2-dioleoyl-sn-glycero-3-phosphoethanolamine to facilitate endosomal escape; cholesterol to improve particle stability and tolerability in systemic circulation; and C14-PEG2000 to confer delivery efficacy and prolonged biodistribution34 (Figure 1A).
Synthesis and characterization of mRNA LNPs. (A) The different lipid components used for the packaging of BCMA mRNA and poly(I:C) in LNPs. (B-C) The cryogenic electron microscopy images of the BCMA-mRNA LNPs and poly(I:C) LNPs. (D-E) Particle size analysis performed for BCMA LNPs (82.3 ± 6.75 nm) and poly(I:C) LNPs (117.6 ± 5.86 nm) by dynamic light scattering. (F) The loading percentage of BCMA-mRNA LNPs and poly(I:C) LNPs. (G) The gel electrophoresis shows the stability of free mRNA and mRNA packaged in LNPs after incubation in human serum for 2 hours at 37°C. (H) The switch in surface charge from negative at physiological pH 7.4 to positive at pH 5, mimicking the acidic endosomal environment, confirmed the efficient endosomal escape of the mRNA into cytosol. DOPE, 1-2-dioleoyl-sn-glycero-3-phosphoethanolamine.
Synthesis and characterization of mRNA LNPs. (A) The different lipid components used for the packaging of BCMA mRNA and poly(I:C) in LNPs. (B-C) The cryogenic electron microscopy images of the BCMA-mRNA LNPs and poly(I:C) LNPs. (D-E) Particle size analysis performed for BCMA LNPs (82.3 ± 6.75 nm) and poly(I:C) LNPs (117.6 ± 5.86 nm) by dynamic light scattering. (F) The loading percentage of BCMA-mRNA LNPs and poly(I:C) LNPs. (G) The gel electrophoresis shows the stability of free mRNA and mRNA packaged in LNPs after incubation in human serum for 2 hours at 37°C. (H) The switch in surface charge from negative at physiological pH 7.4 to positive at pH 5, mimicking the acidic endosomal environment, confirmed the efficient endosomal escape of the mRNA into cytosol. DOPE, 1-2-dioleoyl-sn-glycero-3-phosphoethanolamine.
The size of LNPs containing BCMA mRNA or poly(I:C) was determined by dynamic light scattering, Zetasizer, and cryogenic electron microscopy. Additionally, cryogenic electron tomography reflected the spherical morphology of both particles, with a size in the range of 100 nm (Figure 1B-E). A Quant-iT RiboGreen assay found the loading efficiency of the particles to be >96% (Figure 1F). We investigated the stability of the mRNA-loaded LNPs vs naked mRNA in human serum for 2 hours at 37°C. The agarose gel electrophoresis shows a degraded band for free mRNA vs nondegraded band in the well for the packaged mRNA, reflecting the stability of mRNA inside the LNPs (Figure 1G).
Next, we assessed the surface charge of the particles at pH 7.4 and pH 5 to mimic systemic circulation and the endosomal environment, because LNPs will exist in systemic circulation (pH 7.4) until they are internalized inside the cell by endosomal trafficking. The acidic environment (pH 5) of the endosome triggers the LNP capsule to become cationic, thus electrostatically binding to the endosomal membrane and releasing mRNA into the cytosol. As expected, the surface charge of the LNPs converted from negative at physiological pH 7.4 to positive at endosomal pH 5 (Figure 1H).
The LNPs are taken up by MoDCs, and their mRNA cargo is then translated
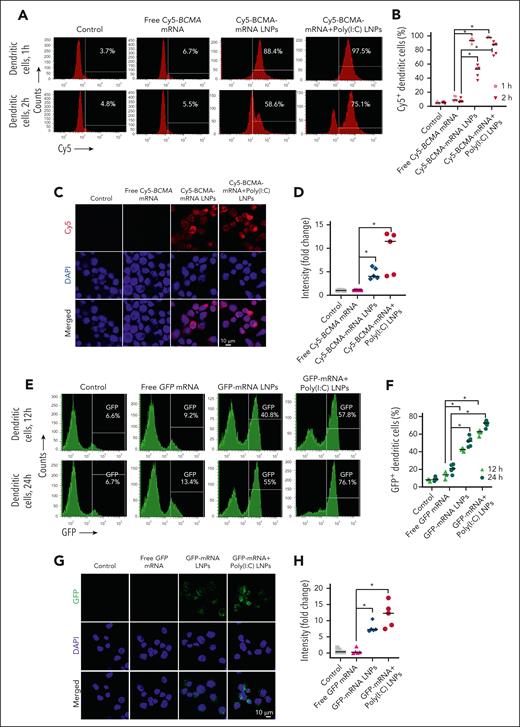
Next, we evaluated whether MoDCs internalize mRNA packaged into LNPs. We treated MoDCs with either control, free Cy5-tagged hBCMA mRNA, Cy5-hBCMA-mRNA LNPs, or Cy5-BCMA-mRNA+poly(I:C) LNPs. After 1 hour of incubation, we observed >80% uptake of mRNA packaged in LNPs alone and in combination with poly(I:C), assessed by flow cytometry (Figure 2A-B). Confocal microscopy confirmed the cytosolic localization of Cy5-tagged BCMA mRNA (Figure 2C-D).
Transfection and translation efficacy of mRNA LNPs in MoDCs. (A-D) MoDCs were treated with free Cy5-tagged BCMA mRNA, Cy5-tagged BCMA mRNA packaged in LNPs, or Cy5-BCMA-mRNA LNPs codelivered with poly(I:C) LNPs. They were evaluated for cellular uptake of Cy5-tagged BCMA-mRNA LNPs using flow cytometry after 1 hour and 2 hours of incubation, and cytosolic localization (red signal) was evaluated by confocal microscopy after 1 hour. Quantification was performed for respective treatment groups using flow cytometry and confocal microscopy. (E-H) MoDCs were treated with free GFP mRNA, GFP-encoding mRNA packaged in LNPs, or GFP-encoding mRNA LNPs codelivered with poly(I:C) LNPs. MoDC uptake of GFP was analyzed by flow cytometry after 12 hours and 24 hours of incubation, and cytosolic expression was evaluated by confocal microscopy after 24 hours of incubation. GFP quantification was determined for respective treatment groups using flow cytometry and confocal microscopy. The data are shown as mean ± standard deviation (SD) from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. DAPI, 4′,6-diamidino-2-phenylindole.
Transfection and translation efficacy of mRNA LNPs in MoDCs. (A-D) MoDCs were treated with free Cy5-tagged BCMA mRNA, Cy5-tagged BCMA mRNA packaged in LNPs, or Cy5-BCMA-mRNA LNPs codelivered with poly(I:C) LNPs. They were evaluated for cellular uptake of Cy5-tagged BCMA-mRNA LNPs using flow cytometry after 1 hour and 2 hours of incubation, and cytosolic localization (red signal) was evaluated by confocal microscopy after 1 hour. Quantification was performed for respective treatment groups using flow cytometry and confocal microscopy. (E-H) MoDCs were treated with free GFP mRNA, GFP-encoding mRNA packaged in LNPs, or GFP-encoding mRNA LNPs codelivered with poly(I:C) LNPs. MoDC uptake of GFP was analyzed by flow cytometry after 12 hours and 24 hours of incubation, and cytosolic expression was evaluated by confocal microscopy after 24 hours of incubation. GFP quantification was determined for respective treatment groups using flow cytometry and confocal microscopy. The data are shown as mean ± standard deviation (SD) from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. DAPI, 4′,6-diamidino-2-phenylindole.
To evaluate their translational efficacy, we packaged mRNA encoding GFP into LNPs and treated MoDCs in serum-containing media. The fluorescence signal increased with time after treatment with packaged GFP mRNA (Figure 2E-F), as confirmed by confocal microscopy (Figure 2G-H). Additionally, flow cytometric analysis showed enhanced BCMA protein expression in MoDCs treated with BCMA-mRNA LNPs alone or in combination with poly:IC (supplemental Figure 1A-B).
hBCMA-mRNA LNPs alone or in combination with poly(I:C) activate MoDCs, which then trigger CTLs in vitro
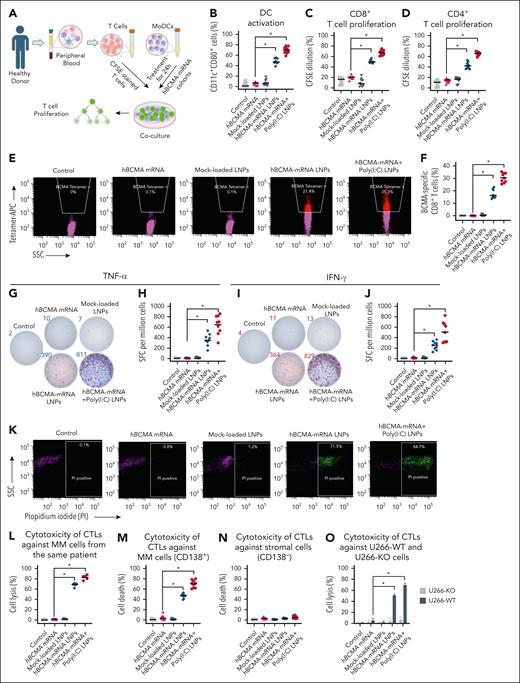
MoDCs and naïve T cells were isolated from healthy donor PBMCs (Figure 3A). MoDCs were then treated for 24 hours before assessing the activation marker CD80 on CD11c+ DCs. Flow cytometry revealed a significant increase of CD11c+ CD80+ cells after hBCMA-mRNA LNPs and hBCMA-mRNA+poly(I:C) LNPs treatments (Figure 3B). Next, we cocultured the treated MoDCs with naïve T cells that had been prestained with CFSE and assessed T-cell proliferation by quantifying the CFSElow population in both CD3+ CD8+ cells and CD3+ CD4+ T cells. We observed significantly enhanced proliferation of CD8+ T cells (Figure 3C; supplemental Figure 1C) and CD4+ T cells (Figure 3D; supplemental Figure 1D) in response to hBCMA-mRNA LNPs alone and in combination with poly(I:C). These CD8+ T cells were specific for hBCMA, as assessed by tetramer staining (Figure 3E-F), and secreted tumor necrosis factor α (Figure 3G-H) and interferon gamma (Figure 3I-J), as determined by ELISpot analysis.
Evaluation of in vitro immune response in primary human T cells and cytotoxic effect of CTLs on cancer cells. (A) Isolation of T cells and MoDCs from the peripheral blood of the same healthy donor. After treatment, MoDCs were cocultured with T cells for 5 days. (B) DC activation marker CD80 was analyzed on CD11c+ MoDCs after treatment with different hBCMA-mRNA cohorts along with irrelevant mRNA or mock-loaded LNP. (C-D) For the T-cell proliferation assay, naïve T cells were prestained with CFSE and cocultured for 5 days with pulsed MoDCs; then the percentage of CFSE-stained CD8+ T cells and CD4+ T cells was determined by flow cytometry. (E-F) The hBCMA-specific CD8+ T-cell percentage was assessed by hBCMA-specific tetramer staining using flow cytometry. The quantification of hBCMA-specific CD8+ T-cell percentage was determined after treatment with different hBCMA-mRNA LNPs, irrelevant mRNA, or mock-loaded LNP. (G-J) ELISpot analysis was performed with the treated MoDCs and T cells coculture to quantify the spot-forming CD8+ T cells for both TNF-α and IFN-γ, dual staining of the same well treated with control and hBCMA-mRNA cohorts. (K-L) CTLs were generated from the 5-day coculture of CD8+ T cells and treated MoDCs from the patient peripheral blood, and their cytotoxicity was evaluated against same patient bone marrow-derived CD138+ MM cells. For flow analysis, CD138+ MM cells were first gated with CD138-APC–positive population followed by MM cell death percentage evaluated by PI staining with respective quantifications. (M-N) CTLs generated from the 5-day cocultures of CD8+ T cells and treated MoDCs isolated from the peripheral blood of healthy donors and the cytotoxicity of CTLs were evaluated against bone marrow–derived CD138+ MM cells and CD138− stromal cells from patients with MM. (O) The cytotoxicity of these isolated CTLs was determined against CFSE-stained U266-WT and U266-BMCA-KO cell lines. The data are shown as mean ± SD from 3 independent experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. IFN-γ, interferon gamma; PI, propidium iodide; SFC, spot-forming cells; SSC, side scatter; TNF-α, tumor necrosis factor α.
Evaluation of in vitro immune response in primary human T cells and cytotoxic effect of CTLs on cancer cells. (A) Isolation of T cells and MoDCs from the peripheral blood of the same healthy donor. After treatment, MoDCs were cocultured with T cells for 5 days. (B) DC activation marker CD80 was analyzed on CD11c+ MoDCs after treatment with different hBCMA-mRNA cohorts along with irrelevant mRNA or mock-loaded LNP. (C-D) For the T-cell proliferation assay, naïve T cells were prestained with CFSE and cocultured for 5 days with pulsed MoDCs; then the percentage of CFSE-stained CD8+ T cells and CD4+ T cells was determined by flow cytometry. (E-F) The hBCMA-specific CD8+ T-cell percentage was assessed by hBCMA-specific tetramer staining using flow cytometry. The quantification of hBCMA-specific CD8+ T-cell percentage was determined after treatment with different hBCMA-mRNA LNPs, irrelevant mRNA, or mock-loaded LNP. (G-J) ELISpot analysis was performed with the treated MoDCs and T cells coculture to quantify the spot-forming CD8+ T cells for both TNF-α and IFN-γ, dual staining of the same well treated with control and hBCMA-mRNA cohorts. (K-L) CTLs were generated from the 5-day coculture of CD8+ T cells and treated MoDCs from the patient peripheral blood, and their cytotoxicity was evaluated against same patient bone marrow-derived CD138+ MM cells. For flow analysis, CD138+ MM cells were first gated with CD138-APC–positive population followed by MM cell death percentage evaluated by PI staining with respective quantifications. (M-N) CTLs generated from the 5-day cocultures of CD8+ T cells and treated MoDCs isolated from the peripheral blood of healthy donors and the cytotoxicity of CTLs were evaluated against bone marrow–derived CD138+ MM cells and CD138− stromal cells from patients with MM. (O) The cytotoxicity of these isolated CTLs was determined against CFSE-stained U266-WT and U266-BMCA-KO cell lines. The data are shown as mean ± SD from 3 independent experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. IFN-γ, interferon gamma; PI, propidium iodide; SFC, spot-forming cells; SSC, side scatter; TNF-α, tumor necrosis factor α.
Importantly, we observed that patient CTLs effectively killed autologous bone marrow–derived CD138+ MM cells, with the greatest cytotoxicity observed after combination treatment with adjuvant poly(I:C) (Figure 3K-L). Next, we evaluated the cytotoxicity of CD8+ T cells generated by coculturing treated MoDCs with T cells from healthy donors, using patient bone marrow–derived CD138+ MM cells and CD138− stromal cells as target populations. Interestingly, hBCMA-specific CTLs specifically lysed CD138+ MM cells (Figure 3M), while sparing CD138− cells (Figure 3N). This cytotoxicity of human CTLs was demonstrated against U266-WT cells but not BCMA-KO cells (P < .05), confirming their specificity (Figure 3O): the percent of U266-WT cell lysis was 69.70% ± 4.32% and 51.30% ± 2.84% for hBCMA-mRNA+poly(I:C) LNPs and hBCMA-mRNA LNPs, respectively, as compared with free mRNA (5.63% ± 4.82%, P < .05), as confirmed by calcein-release assay (supplemental Figure 1E-F). In our experiments, irrelevant mRNA or mock-loaded LNPs had no effect on DC activation, T-cell proliferation, BCMA-specific tetramer positivity on CD8+ T cells, and CTL-mediated cytotoxicity against patient bone marrow–derived CD138+ MM cells, suggesting that hBCMA mRNA is responsible for inducing the immune response.
LNPs effectively deliver their cargo to antigen-presenting cells
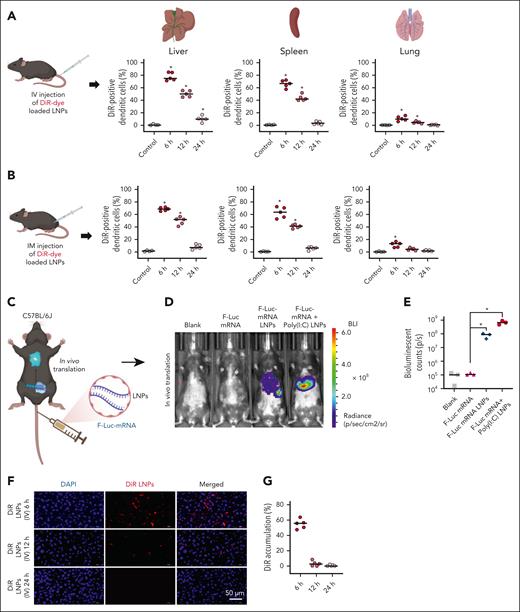
Next, we evaluated the efficacy of mBCMA-mRNA LNPs delivery in vivo using our murine model. We first assessed the effect of LNP injections on major organs (liver, spleen, lung, kidney, and heart) by hematoxylin and eosin staining, and found no significant changes after either IV or IM administration (supplemental Figure 2A-B). We investigated the cellular uptake of DiR dye–loaded LNPs on CD11c+ DCs and F4/80+ macrophages isolated from the spleen, liver, and lung of C57BL/6J mice after IV and IM injection, and observed high rates of DiR dye internalization in both the DCs (Figure 4A-B) and macrophages (supplemental Figure 3A-B) isolated from the liver and spleen 6 hours after IV and IM injection. The DiR dye intensity gradually decreased and was almost completely cleared within 24 hours. We further confirmed the in vivo stability and translational efficacy of mRNA packaged into LNPs using mRNA encoding F-Luc. Six hours after IV (Figure 4C-E) or IM injection (supplemental Figure 3C-D), we observed no signal for empty LNPs and free F-Luc mRNA; in contrast, packaged F-Luc mRNA produced a signal, which was further enhanced by cotreatment with poly(I:C) LNPs.
In vivo distribution of LNPs in major organs and evaluation of in vivo stability and translation efficacy of its packaged mRNA. (A-B) Cellular uptake of DiR-loaded LNPs by DCs isolated from the liver, spleen, and lung of C57BL/6J mice 6 hours, 12 hours, and 24 hours after IV and IM injection. (C-E) The free F-Luc mRNA and F-Luc-mRNA loaded in LNPs were injected into C57BL/6J mice by the IV route to monitor the in vivo stability of mRNA and translation efficacy. Shown is the representative real-time live imaging of animals 6 hours after injection along with quantification of BLI for different treatment groups. (F-G) The in vivo tissue distribution of DiR-loaded LNPs was monitored in the liver, with quantification of liver accumulation of DiR dye at different time points, 6 hours, 12 hours, and 24 hours after IV administration of DiR-loaded LNPs. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. BLI, bioluminescence imaging; DAPI, 4′,6-diamidino-2-phenylindole; DiR, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide.
In vivo distribution of LNPs in major organs and evaluation of in vivo stability and translation efficacy of its packaged mRNA. (A-B) Cellular uptake of DiR-loaded LNPs by DCs isolated from the liver, spleen, and lung of C57BL/6J mice 6 hours, 12 hours, and 24 hours after IV and IM injection. (C-E) The free F-Luc mRNA and F-Luc-mRNA loaded in LNPs were injected into C57BL/6J mice by the IV route to monitor the in vivo stability of mRNA and translation efficacy. Shown is the representative real-time live imaging of animals 6 hours after injection along with quantification of BLI for different treatment groups. (F-G) The in vivo tissue distribution of DiR-loaded LNPs was monitored in the liver, with quantification of liver accumulation of DiR dye at different time points, 6 hours, 12 hours, and 24 hours after IV administration of DiR-loaded LNPs. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. BLI, bioluminescence imaging; DAPI, 4′,6-diamidino-2-phenylindole; DiR, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide.
Consistent with our flow cytometric analysis of DiR dye distribution, histologic studies revealed the highest percentage of DiR dye signal in the liver (Figure 4F-G; supplemental Figure 3E) and spleen at 6 hours after IV and IM injection, which diminished with time (supplemental Figure 4A-B). No DiR signal was observed for the lung, kidney, and heart at any time point (supplemental Figures 5-7). These findings suggest that the LNPs are efficiently cleared from all major organs after delivery into antigen-presenting cells.
In vivo evaluation of mBCMA-specific immunity and therapeutic efficacy of BCMA-mRNA LNP vaccination in a murine tumor model
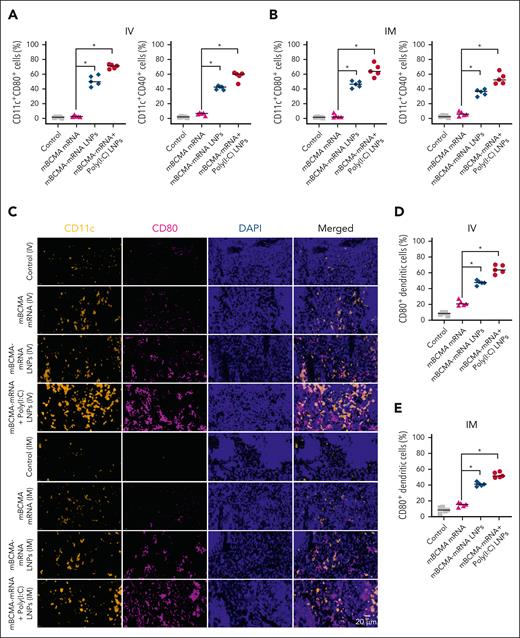
Next, we immunized mice with different treatments by IV or IM route and assessed their impact on DCs by flow cytometric analysis. A significant increase of the activation marker CD80 was induced on DCs in the spleen of mice treated with mBCMA-mRNA LNPs (51.38% ± 7.05% and 46.32% ± 4.59%) and mBCMA-mRNA+poly(I:C) LNPs (70.46% ± 2.86% and 65.44% ± 8.43%) after administration of IV or IM treatment, respectively (Figure 5A-B). This was further confirmed by immunohistochemistry (Figure 5C-E), and expression of another activation marker CD40 (Figure 5A-B; P < .05).
Evaluation of in vivo DC activation after IV and IM injection of mRNA LNPs. (A-B) C57BL/6J mice were treated with free mBCMA mRNA or mRNA LNPs with or without poly(I:C) LNPs by either the IV or IM route. After 24 hours of treatment, the spleens were isolated and splenocytes were analyzed for DC activation markers CD80 or CD40 on CD11c+ DCs. (C-E) Immunofluorescence analysis of the spleen showing activation of CD11c (yellow) and CD80 (magenta) after 24-hour treatment with different groups. The scatter plots represent percentage of CD80+ DCs after IV and IM injections of different treatment groups. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. DAPI, 4′,6-diamidino-2-phenylindole.
Evaluation of in vivo DC activation after IV and IM injection of mRNA LNPs. (A-B) C57BL/6J mice were treated with free mBCMA mRNA or mRNA LNPs with or without poly(I:C) LNPs by either the IV or IM route. After 24 hours of treatment, the spleens were isolated and splenocytes were analyzed for DC activation markers CD80 or CD40 on CD11c+ DCs. (C-E) Immunofluorescence analysis of the spleen showing activation of CD11c (yellow) and CD80 (magenta) after 24-hour treatment with different groups. The scatter plots represent percentage of CD80+ DCs after IV and IM injections of different treatment groups. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. DAPI, 4′,6-diamidino-2-phenylindole.
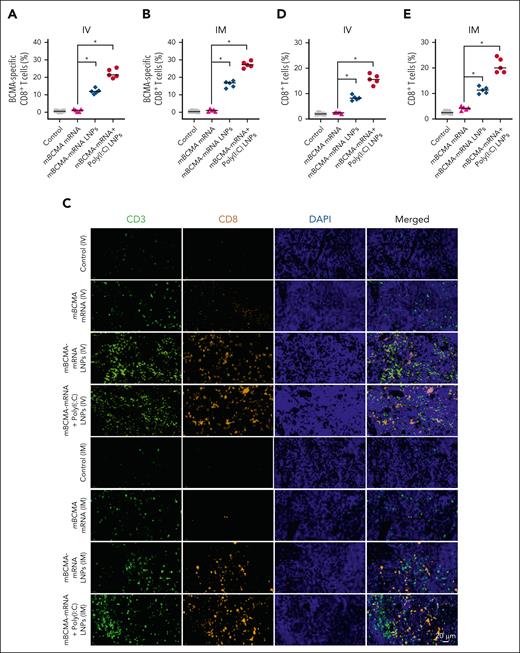
We then evaluated the in vivo antigen-specific T-cell response in C57BL/6J mice either 5 days after IV injection; or 7 days after the first IM injection, with a second IM injection occurring 3 to 4 days after the first injection following the Curevac therapeutic cancer vaccination protocol. The splenocytes were isolated, and the CD8+ T cells were analyzed for mBCMA specificity using tetramer staining by flow cytometry. The treatments with mBCMA-mRNA LNPs and mBCMA-mRNA+poly(I:C) LNPs elicited immune responses, with increased mBCMA-tetramer–positive CD8+ T cells observed after both IV and IM vaccination (Figure 6A-B). In addition, there was a significant accumulation of CD3+ and CD8+ T cells infiltrating into the spleen in mice treated with mBCMA-mRNA LNPs alone or in combination with poly(I:C) (Figure 6C-E).
Analyzing BCMA-specific tetramer+ CD8+ T cells and infiltration of T cells in splenic tissue. C57BL/6J mice received different treatment groups by either IV or IM injection. Five days after a single IV dose, and 7 days after the first IM dose in a schedule of 2 doses separated by 3 days, the mice were euthanized, and their splenocytes were stained to assess the BCMA-specific tetramer+ CD8+ T cells by flow cytometry. (A-B) Percentage of BCMA-specific tetramer–positive cells analyzed on splenocytes after IV and IM injections in different treatment groups. (C) The spleens were further analyzed for infiltrating CD3+ and CD8+ T cells by immunofluorescence staining. The representative images demonstrate the infiltrating CD3+ (green) and CD8+ (yellow) T cells in splenic tissue. (D-E) The percentage of CD8+ T cells infiltrating into the spleen after different treatments is shown after IV and IM injection. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. DAPI, 4′,6-diamidino-2-phenylindole.
Analyzing BCMA-specific tetramer+ CD8+ T cells and infiltration of T cells in splenic tissue. C57BL/6J mice received different treatment groups by either IV or IM injection. Five days after a single IV dose, and 7 days after the first IM dose in a schedule of 2 doses separated by 3 days, the mice were euthanized, and their splenocytes were stained to assess the BCMA-specific tetramer+ CD8+ T cells by flow cytometry. (A-B) Percentage of BCMA-specific tetramer–positive cells analyzed on splenocytes after IV and IM injections in different treatment groups. (C) The spleens were further analyzed for infiltrating CD3+ and CD8+ T cells by immunofluorescence staining. The representative images demonstrate the infiltrating CD3+ (green) and CD8+ (yellow) T cells in splenic tissue. (D-E) The percentage of CD8+ T cells infiltrating into the spleen after different treatments is shown after IV and IM injection. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. DAPI, 4′,6-diamidino-2-phenylindole.
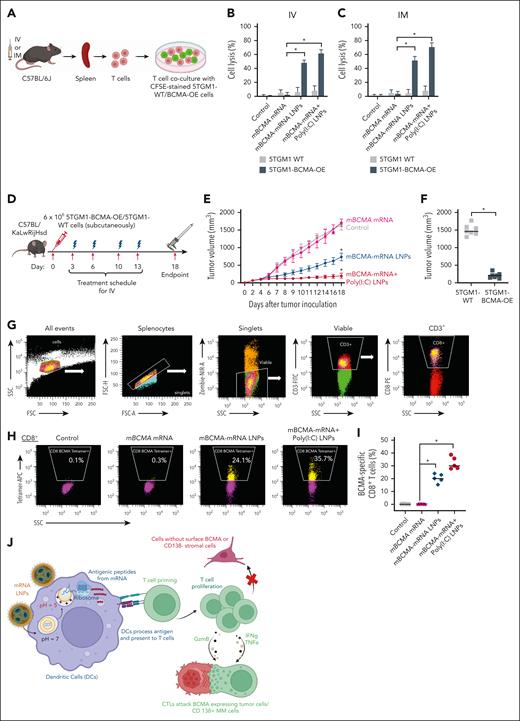
Importantly, we found that the splenic T cells isolated after IV and IM treatment were able to kill the BCMA-overexpressing murine MM cell line 5TGM1-BCMA-OE but not the 5TGM1-WT cells (Figure 7A-C), which have no surface mBCMA expression (supplemental Figure 8). In all instances, the adjuvant poly(I:C) LNPs further elicited the immune response (P < .05) induced by BCMA-mRNA LNPs.
Specific cytotoxicity of in vivo CTLs on murine tumor cells and effect of therapeutic vaccination on tumor growth and immune responses in the C57BL/KaLwRijHsd murine model. (A-C) The spleens were isolated from treated C57BL/6J mice 5 days after a single IV dose, and 7 days after the first IM dose in a schedule of 2 doses separated by 3 days, for different treatment groups. CD8+ T cells were isolated from splenocytes and cultured with CFSE prestained 5TGM1-WT and 5TGM1-BCMA-OE cells to evaluate cytotoxicity. After 24 hours of incubation, cell death percentage was evaluated by PI using flow cytometry. (D) Experimental workflow for in vivo therapeutic vaccination. C57BL/KaLwRijHsd mice were subcutaneously inoculated with 6 × 106 5TGM1-BCMA-OE cells and randomized into 4 treatment groups once the tumor reached 60 to 80 mm3. Mice received different mBCMA-mRNA LNPs twice weekly for 2 weeks by IV injections. The spleens were removed for analysis 5 days after the final treatment. (E) A significant reduction in tumor volume was observed in groups treated with BCMA-mRNA LNPs and BCMA-mRNA+poly(I:C) LNP, compared with control and free mRNA. (F) The treatment with mBCMA-mRNA+poly(I:C) LNP selectively inhibited growth of 5TGM1-BCMA-OE but not 5TGM1-WT tumors. (G-I) After treatment, splenocytes were isolated and stained for flow cytometry to assess the BCMA-specific tetramer positivity on CD8+ T cells. The representative plots and scatter plots demonstrate increased BCMA-specific tetramer+ CD8+ T cells in treated groups. (J) mRNA LNP vaccine’s mechanism of action. LNPs can efficiently deliver mRNA to antigen-presenting cells, such as DCs. Activated DCs can present processed antigens to educate T cells for efficient priming and amplification of T cells specific to MM cells. Cytotoxic T cells specifically target BCMA-expressing tumor cells or CD138+ MM cells but do not target cells without BCMA surface expression. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. PI, propidium iodide.
Specific cytotoxicity of in vivo CTLs on murine tumor cells and effect of therapeutic vaccination on tumor growth and immune responses in the C57BL/KaLwRijHsd murine model. (A-C) The spleens were isolated from treated C57BL/6J mice 5 days after a single IV dose, and 7 days after the first IM dose in a schedule of 2 doses separated by 3 days, for different treatment groups. CD8+ T cells were isolated from splenocytes and cultured with CFSE prestained 5TGM1-WT and 5TGM1-BCMA-OE cells to evaluate cytotoxicity. After 24 hours of incubation, cell death percentage was evaluated by PI using flow cytometry. (D) Experimental workflow for in vivo therapeutic vaccination. C57BL/KaLwRijHsd mice were subcutaneously inoculated with 6 × 106 5TGM1-BCMA-OE cells and randomized into 4 treatment groups once the tumor reached 60 to 80 mm3. Mice received different mBCMA-mRNA LNPs twice weekly for 2 weeks by IV injections. The spleens were removed for analysis 5 days after the final treatment. (E) A significant reduction in tumor volume was observed in groups treated with BCMA-mRNA LNPs and BCMA-mRNA+poly(I:C) LNP, compared with control and free mRNA. (F) The treatment with mBCMA-mRNA+poly(I:C) LNP selectively inhibited growth of 5TGM1-BCMA-OE but not 5TGM1-WT tumors. (G-I) After treatment, splenocytes were isolated and stained for flow cytometry to assess the BCMA-specific tetramer positivity on CD8+ T cells. The representative plots and scatter plots demonstrate increased BCMA-specific tetramer+ CD8+ T cells in treated groups. (J) mRNA LNP vaccine’s mechanism of action. LNPs can efficiently deliver mRNA to antigen-presenting cells, such as DCs. Activated DCs can present processed antigens to educate T cells for efficient priming and amplification of T cells specific to MM cells. Cytotoxic T cells specifically target BCMA-expressing tumor cells or CD138+ MM cells but do not target cells without BCMA surface expression. The data are shown as mean ± SD from at least 3 independent biological experiments. Statistical analysis was performed using an unpaired Student t test: ∗P < .05. PI, propidium iodide.
We further investigated the therapeutic effect of mBCMA-mRNA LNPs vaccination using the C57BL/KaLwRijHsd mouse model. Mice were inoculated with 6 × 106 5TGM1-BCMA-OE cells, and once tumors reached 60 to 80 mm3, IV vaccination was initiated twice weekly for 2 weeks (Figure 7D). Tumor burden was monitored throughout the study, and significant tumor growth inhibition was observed in mice treated with mBCMA-mRNA LNPs and mBCMA-mRNA+poly(I:C) LNPs (Figure 7E). In contrast, injections with free mRNA had no significant effect compared with control. Interestingly, treatment with mBCMA-mRNA+poly(I:C) LNPs suppressed tumor growth only in 5TGM1-BCMA-OE tumor-bearing mice but not in those with 5TGM1-WT tumor, indicating a BCMA-specific immune response (Figure 7F). 5TGM1-WT cells have very low level of mBCMA expression, as shown in our previous RNA-sequencing data and supported by Bennett et al22,35 (supplemental Figure 8). To evaluate the underlying mechanisms, spleens were collected 5 days after the final injection for flow cytometric analysis. mBCMA-specific tetramer response was then assessed in CD3+ T cells and CD8+ cells (Figure 7G). As expected, the mBCMA-mRNA+poly(I:C) LNPs treatment triggered the highest percentage of tetramer-positive CD8+ T cells (Figure 7H-I), indicating a mBCMA-specific immune response associated with inhibition of in vivo tumor growth.
Discussion
Here, we developed a therapeutic cancer vaccine for MM using LNPs that contain BCMA mRNA. The first human application of an mRNA vaccine for melanoma prolonged progression-free survival.36 Additionally, a similar study using 20 predicted neoantigens to develop a personalized cancer vaccine demonstrated an antigen-specific T-cell response in high-risk patients with melanoma.37 The most recent mRNA vaccine to undergo a phase 3 clinical trial, considered the first personalized mRNA vaccine for melanoma and using a combination of mRNA-4157 and programmed cell death protein 1, efficiently reduced the risk of distant metastasis and prolonged survival.38 BioNTech has also designed an innovative mRNA vaccine capable of targeting several tumor-associated antigens for prostate cancer, human papillomavirus–related cancers, non-small cell lung cancer, and melanoma that has been considered for a phase 2 clinical trial.39,40 Currently, BioNTech is working on developing a personalized mRNA vaccine in a United Kingdom trial, aiming to treat 10 000 patients with cancer by 2030.41 A recent approach in combining mRNA-based neoantigen immunotherapy with chemotherapy showed significant potential in delaying the recurrence of pancreatic ductal adenocarcinoma.42 In a similar way, Merck and Moderna evaluated a combination of mRNA-4157 and pembrolizumab for patients with melanoma and observed potentially reduced death and recurrence risk.38 Despite significant progress in developing cancer vaccines stimulated by the clinical efficacy of the mRNA vaccine during the COVID-19 pandemic, there are still many challenges.
In this study, we exploited the latest advances to design our mRNA vaccine. The first US Food and Drug Administration–approved small interfering RNA drug packaged in LNPs used MC3 as an ionizable lipid delivery component; however, adverse side effects occurred with repeated doses because of its long tissue half-life in the liver.43 Biodegradable lipids, such as lipid 5, lipid H, and ALC-0315 have been designed for effective in vivo mRNA delivery and pharmacokinetic efficacy. Moderna and Pfizer used LNPs for their COVID-19 mRNA vaccines, mRNA-1273 and BNT162b, which have the ionizable lipids ALC-0315 and lipid H (SM-102), respectively, resulting in efficient payload delivery and quick elimination from the liver and blood.10 Sabnis et al found a sixfold increase in the uptake and endosomal escape of lipid 5–based LNPs, with a half-life of 5.8 hours and high accumulation in the spleen but not in other major organs.31 In this study, we used lipid 5 for mRNA packaging and evaluated tissue distribution of LNPs loaded with DiR dye after vaccination in C57BL/6J mice. Indeed, accumulation in the spleen was increased to levels similar to those observed in the liver. Importantly, in this model, there were no adverse effects of vaccination in the liver, lung, spleen, and kidney, supporting its potential clinical utility. Interestingly, an enlarged spleen volume with enhanced splenic lymphocyte count was observed after systemic delivery of BCMA-mRNA LNP and combination treatment with poly(I:C), indicating a selective accumulation of mRNA into the spleen (supplemental Figure 9).
We first conducted the quality control studies of LNPs and compared different cohorts in terms of their ability to trigger a selective BCMA CTL response in vitro. Neither control nor free hBCMA mRNA treatment led to uptake by MoDCs whereas BCMA-mRNA LNP as well as BCMA-mRNA+poly(I:C) LNP treatment triggered MoDC uptake, evidenced by flow and confocal analysis. Once the LNPs were internalized, we showed translation using a GFP mRNA construct, and for BCMA expression, it was evidenced by flow analysis. Activation of DCs, as assessed by flow cytometry, was triggered in a similar pattern. In aggregate, these studies confirmed that uptake and translation of hBCMA mRNA as well as DC activation were all induced to further extent when poly(I:C) adjuvant was combined with hBCMA-mRNA treatment.
Next, we characterized the selective immune response triggered in vitro by these activated DCs. Although CD4+ T-cell proliferation was increased after treatment, the predominant response was CD8+ hBCMA tetramer–positive CTLs, triggered to a greater extent after treatment with hBCMA-mRNA+poly(I:C) LNPs. Importantly these CD8+ CTLs killed U266 MM cells and patient CD138+ MM cells but did not lyse U266 lacking BCMA and CD138− bone marrow cells, suggesting a favorable therapeutic index. Studies in 5 patients demonstrated the killing of autologous MM cells after hBCMA-mRNA+poly(I:C) LNPs treatment, further supporting its potential clinical application.
We then investigated DC activation as well as immune response in the C57BL/6J murine model. As observed in vitro, DC activation, evidenced by flow cytometry and immune multiplexing as well as mBCMA-specific CD8+ T cells, were both induced to the greatest extent by mBCMA-mRNA+poly(I:C) LNPs. Importantly, T-cell infiltration was significantly increased after treatment, and these T cells demonstrated cytotoxicity against 5TGM1-BMCA-OE but not 5GTM1-WT cells, which lack or only weakly express BCMA. These studies confirmed induction of mBCMA-selective responses in vivo.
To further assess the relevance of each of these treatments in vivo, we used a murine 5TGM1 MM model. In a therapeutic model, we first injected 5TGM1-BCMA-OE cells subcutaneously into C57BL/KaLwRijHsd mice, followed by IV treatment with control and mBCMA LNPs to investigate induction of mBCMA-specific immune response as well as the impact on tumor growth. Importantly, mBCMA-mRNA LNPs induced mBCMA tetramer–positive CD8+ CTL response, associated with inhibition of tumor growth.
Most vaccines are preferably administered by IM injection; however, the IV route of administration can induce the greatest immune response.10 Thus, we here compared the efficacy of our vaccine administered by both IV and IM routes. We observed a significant increase in mBCMA-specific CD8+ T cells 5 days after a single mBCMA-mRNA+poly(I:C) LNP vaccination with IV injection. In contrast, no significant increase was observed after a single IM injection; however, an IM booster immunization administered after a 3- to 4-day interval triggered a significant increase in mBCMA-specific CD8+ T cells. Therefore, we found that either the IV or IM route can induce an immune response to this MM vaccine.
Finally, the adjuvant poly(I:C) can bind to the TLR3 receptor, exclusively localized to the early endosomes in DCs and macrophages, thereby inducing the maturation of these antigen-presenting cells, which are crucial for the adaptive immune response.44,45 The TLR3 agonist, poly(I:C), can also target CD8+ T cells and augment their antigen-specific responses. Thus, this adjuvant plays a critical role in coordinating both innate and adaptive immunity through secreting proinflammatory molecules and cytokines.46 In our study, we confirmed that adding adjuvant enhanced the selective immune response of BCMA-mRNA vaccination, increasing its therapeutic potential. Although the present study was focused on adaptive immune response, we also observed presence of serum anti-mBCMA antibody 4 weeks after combination treatment with mBCMA-mRNA+poly(I:C) LNPs (supplemental Figure 10).
In summary, we developed an MM therapeutic cancer vaccine with BCMA-mRNA LNPs that can efficiently deliver the mRNA to APCs, in which BCMA antigens are processed and presented on the surface to prime BCMA-specific T cells for an effective immune response against MM cells with BCMA surface expression (Figure 7J). Our study provides the framework for clinical evaluation of BCMA-mRNA vaccination, alone or combined with poly(I:C), to improved patient outcome in MM. Moreover, analogous mRNA vaccination against immunogenic neoantigens determined by sequencing samples from patients with MM may lead to the development of personalized vaccines in MM, as well.
Acknowledgments
The authors gratefully acknowledge Robert Langer and group members, Department of Chemical Engineering, Koch Institute, Massachusetts Institute of Technology, for their initial help in lipid nanoparticle synthesis and characterizations. They thank Christina Usher, science writer, Dana-Farber Cancer Institute, for editing the manuscript and for insightful comments, and Marc Delcommenne, Director, MBL International, for analyzing peptide sequences for tetramer and development of tetramer for this study. They thank Elinor Grant Sterner and Kun Huang, Molecular Imaging Core Facility, Dana-Farber Cancer Institute, for their help in confocal microscopy imaging and quantifications.
This work was supported by the National Cancer Institute, National Institutes of Health (grants P01CA155258-10 and P50CA100707) (K.C.A.), a Paula and Rodger Riney Foundation grant (K.C.A.), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation grant (K.C.A.). A.G. is supported by an individual start-up grant from the Italian Association for Cancer Research (project number 27750); a Fondazione Piemontese per la Ricerca sul Cancro “5xmille” 2021 Ministry of Health project (EMAGEN-FaBer); and the Italian Ministry of Health, Ricerca Corrente 2025.
Graphics were created with BioRender.com.
Authorship
Contribution: D.D., T.H., and K.C.A. conceived and designed the research studies; D.D., Y.S., and K.C.A. wrote the manuscript; D.D., J.L., K.W., and A.S. performed experiments, data analysis, and interpretation of experiments under supervision of T.H., A.G., and K.C.A. A.R. and Y.S. helped in experimental settings; X.L. helped during some of the experimental procedures; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: K.C.A. is a consultant to Pfizer, Janssen, AstraZeneca, and Genentech-Roche; and serves as a board member/founder of C4 Therapeutics, Dynamic Cell Therapies, OncoPep, Starton, Window, and Predicta. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02215; email: kenneth_anderson@dfci.harvard.edu.
References
Author notes
J.L. and K.W. contributed equally to this study.
Data are available from the corresponding author, Kenneth C. Anderson (kenneth_anderson@dfci.harvard.edu), on request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal