In this issue of Blood, Flamann et al1 elucidate mechanisms of impaired macrophage-mediated clearance of alloreactive T cells that accumulate in the inflamed gastrointestinal tract of patients (and animals) with graft-versus-host disease (GVHD).
GVHD is a significant barrier to allogeneic hematopoietic transplantation (alloHCT) and the leading cause of nonrelapse mortality.2 Although adaptive and innate immune cells play a role in GVHD pathogenesis,3 T cells are the “generals” that command coordinated destruction of host tissues. Therapies targeting T-cell, B-cell, and macrophage biology have proven efficacy in acute4 and chronic5 GVHD. The authors observed that, in patients with acute GVHD, circulating CD3+ T cells, including the gut-homing subset, have higher CD47 expression compared with T cells isolated from autoHCT recipients. Moreover, ileal biopsies from patients with acute GVHD show higher CD47 expression on T cells compared with alloHCT recipients without GVHD. To reveal the mechanisms behind this observed increase in CD47 surface expression on T cells isolated from patients with GVHD, Flamann et al performed high-resolution mapping on in vitro–stimulated T cells. They demonstrated that T-cell receptor engagement promotes increased CD47 expression on activated T cells through NF-κB binding to CD47 promoter regions. The resulting increased CD47 (ligand) expression on alloreactive T cells sends the “don’t eat me” signal to “Pac-Man” macrophages expressing the signal regulatory protein α receptor. Through traditional flow cytometry and phagocyte adhesion flow analyses the authors established that overexpression of CD47 allows alloreactive T cells to escape antibody-dependent cellular phagocytosis.
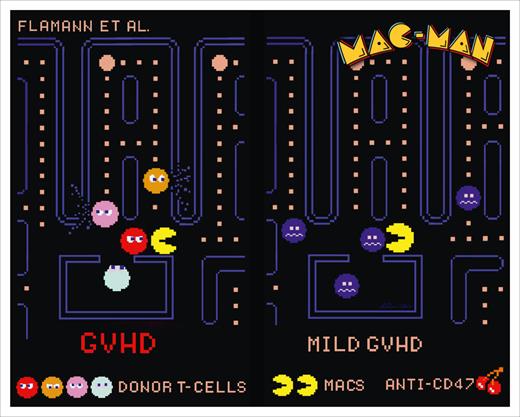
In parallel animal modeling experiments, Flamann et al probe and further delineate mechanisms of phagocyte dysregulation that are also at play in 2 major histocompatibility mismatch mouse models of GVHD. First, they show that, similar to human circulating T cells in patients with GVHD, donor-derived T cells isolated from the spleens and lamina propria of mice with GVHD have higher CD47 expression compared with syngeneic counterparts. Phagocytosis of such murine alloreactive T cells expressing high levels of CD47 on the cell surface by macrophages is impaired. Phagocytosis is then restored when grafts containing CD47 KO donor T cells are used (see figure) leading to reduced GVHD clinical severity and improved survival. In addition, macrophage depletion abrogates the positive effects of using CD47-deficient grafts, implicating “Mac-Man” macrophages in this phagocytosis axis. Finally, the authors test systemic administration of anti-CD47 antibody provided daily on days +4, +5, and +6 after HCT in the acute GVHD model. The treated animals survived longer, had improved intestinal histology scores with increased frequency of phagocytosed T cells in situ, and had reduced systemic levels of proinflammatory cytokines. These experiments provide rationale for further clinical development of anti–CD47-targeted therapies for GVHD.
“Mac-Man” macrophages can eat (clear) alloreactive CD47-overexpressing T cells that are preferentially found in patients and animals with GVHD upon the systemic administration of anti-CD47 antibody that leads to milder GVHD. Figure provided by Andreas Beilhack in homage to the digital art of Toru Iwatani, the original creator of Pac-Man.
“Mac-Man” macrophages can eat (clear) alloreactive CD47-overexpressing T cells that are preferentially found in patients and animals with GVHD upon the systemic administration of anti-CD47 antibody that leads to milder GVHD. Figure provided by Andreas Beilhack in homage to the digital art of Toru Iwatani, the original creator of Pac-Man.
CD47 targeting represents a promising clinically relevant therapeutic strategy, especially given that several antibodies are currently available for clinical testing. An additional benefit of targeting CD47 to address GVHD is that it has the potential to synergize with antitumor targeting because leukemic cells have also been shown to overexpress CD47.6 A key consideration for GVHD therapies is the potential negative impact on graft-versus-leukemia,7 which can be avoided when the therapeutic target is shared by the pathogenic immune cells driving GVHD and the malignant cells. Flamman et al illuminate such an approach, highlighting the need to test this therapeutic target clinically in patients undergoing alloHCT, especially for malignant indications, where synergy between anti-GVHD and pro–graft-versus-leukemia effects is critical.
Conflict-of-interest disclosures: N.P.B. has received (preclinical) research funding from Incyte.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal