In this issue of Blood, Xue et al1 provide evidence for the role of neutrophil gelatinase-associated lipocalin (NGAL) in coagulation and hemostasis, uncovering a novel link between the innate immune system and thrombosis. NGAL, conventionally known as an inflammatory mediator, is revealed here to have a significant impact on thrombosis, thus expanding our understanding of how inflammatory pathways contribute to both thrombosis and hemostasis.
NGAL is a protein primarily secreted by the immune cells, including neutrophils, macrophages, and dendritic cells, in response to inflammation. It is detectable in the blood and other body fluids, including urine. It is widely recognized as a biomarker for acute kidney injury, chronic kidney disease, and dialysis-related complications. In addition to kidney-related conditions, NGAL levels are elevated in patients with cardiovascular diseases. It is expressed in the heart tissue and atherosclerotic plaques, with plasma levels notably higher in conditions such as coronary artery disease and both acute and chronic heart failure. Although increased serum NGAL levels in these patients are often linked to renal dysfunction, many studies have also observed elevated NGAL concentrations in individuals with cardiovascular diseases even when kidney function is unaffected.2-4
Thrombosis and inflammation are distinct yet closely interconnected physiological processes. Inflammation can activate the coagulation system as part of the immune response to pathogens, involving innate immune cells and platelets in a process called immunothrombosis. This prothrombotic state is associated not only with acute infections but also with chronic cardiovascular conditions, including chronic inflammation (eg, in atherosclerosis), plaque rupture (eg, in myocardial infarction and stroke), and blood flow stasis (eg, in venous thromboembolism). Emerging evidence supports targeting inflammation to prevent cardiovascular events, a theory not yet fully integrated in current therapeutic strategies.5,6
Although the role of NGAL in inflammation is well documented, its involvement in coagulation, hemostasis, and thrombosis, along with the underlying mechanisms, remains unclear. In this study, Xue et al carried out elegant in vitro, in vivo, and ex vivo models using mice and human specimens to explore the role of NGAL in these processes. Their findings provide strong evidence that NGAL is a mediator of hypercoagulability, promoting clot formation through multiple mechanisms.
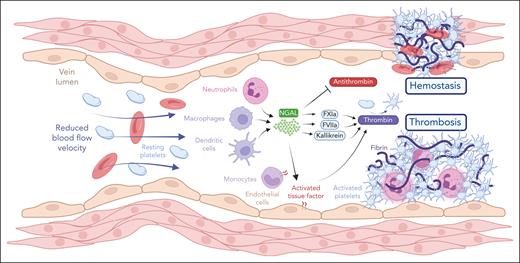
Xue et al observed upregulation of NGAL levels in patients with deep vein thrombosis compared with those with ST-elevation myocardial infarction and healthy individuals. This increase suggests that NGAL may play a role in hypercoagulability, a condition predisposing individuals to thrombosis. Mechanistically, the authors observed that NGAL amplifies coagulation by enhancing the activities of thrombin, kallikrein, factor XIa (FXIa), and FVIIa while inhibiting antithrombin. This dual action of promoting clotting factors and impeding inhibitors suggests a role of NGAL in generating hypercoagulability. Furthermore, NGAL promotes thrombin-induced platelet aggregation, a key step in clot formation, without affecting other platelet-stimulating agents such as adenosine 5′-diphosphate or collagen. Moreover, treatment with NGAL or its overexpression decreases clot reaction and kinetic time while increasing clot firmness, as revealed by thromboelastography. These findings indicate that NGAL promotes rapid and robust clot formation. Interestingly, NGAL enhances tissue factor expression and activity on endothelial and monocyte surfaces, initiating and sustaining the clotting cascade. However, how NGAL affects tissue factor activity and expression is unclear. Furthermore, the authors observed that NGAL binds directly to thrombin, kallikrein, FXIa, FVIIa, and antithrombin, modulating these factors within clots, though the mechanisms involved remain uncertain. Xue et al found that NGAL-thrombin/kallikrein/FXIa/FVIIa/antithrombin complexes are present in the thrombi, indicating a close association of NGAL with active clotting. Notably, NGAL inhibition through monoclonal antibodies prolonged clotting times (activated partial thromboplastin time and prothrombin time) and significantly reduced plasma thrombin activity, suggesting that NGAL shifts the hemostatic balance toward thrombosis. Moreover, in knockout mice, NGAL deficiency causes a hemophilia-like bleeding phenotype, with prolonged bleeding times and delayed arterial occlusion after vascular injury. In 2 inflammation models induced by bacterial lipopolysaccharide or carrageenan, NGAL promotes thrombosis. Its deficiency reduces inflammatory responses and clot formation, supporting a role of NGAL in the interaction between inflammation and coagulation.
This study provides new insights into the critical role of NGAL in the coagulation system, emphasizing its ability to link inflammatory responses with thrombotic processes (see figure). Although previous research has primarily focused on the involvement of NGAL in immune responses and cardiovascular diseases, this study reveals its central role in coagulation and hemostasis. NGAL enhances tissue factor activity, potentiates thrombin generation, and influences platelet aggregation, positioning it as a novel mediator between inflammation and thrombosis. However, the complement system plays a key role in inflammation and thrombosis.7,8 Whether and how NGAL interacts with the complement system remains unclear. In addition, the role of NGAL in neutrophil extracellular trap (NET) formation is not well understood. NETs amplify inflammation and thrombosis by creating a scaffold that supports clot formation and captures platelets. Further studies are necessary to determine whether NGAL directly affects NET formation or whether this relationship is reciprocal. Another critical aspect of this study is the use of significantly higher NGAL concentrations in the in vitro experiments compared with levels observed in healthy individuals and patients with thrombosis. Although Xue et al offered valuable insights into the role of NGAL in thrombosis, the underlying triggers for NGAL release in thrombotic processes remain unclear. Further studies are essential to elucidate these questions.
NGAL, usually recognized for its role in inflammation, is now highlighted as a key mediator in thrombosis and hemostasis. In deep vein thrombosis and hemostatic processes, NGAL drives hypercoagulability and promotes clot formation by binding to and enhancing the activities of thrombin, kallikrein, FXIa, and FVIIa while simultaneously inhibiting antithrombin. In addition, NGAL facilitates thrombin-induced platelet aggregation and boosts both the expression and activity of tissue factor on endothelial and monocyte surfaces, thereby initiating and sustaining the clotting cascade. See Figure 6K in the article by Xue et al that begins on page 975. Figure created with BioRender.com.
NGAL, usually recognized for its role in inflammation, is now highlighted as a key mediator in thrombosis and hemostasis. In deep vein thrombosis and hemostatic processes, NGAL drives hypercoagulability and promotes clot formation by binding to and enhancing the activities of thrombin, kallikrein, FXIa, and FVIIa while simultaneously inhibiting antithrombin. In addition, NGAL facilitates thrombin-induced platelet aggregation and boosts both the expression and activity of tissue factor on endothelial and monocyte surfaces, thereby initiating and sustaining the clotting cascade. See Figure 6K in the article by Xue et al that begins on page 975. Figure created with BioRender.com.
The identification of NGAL as a key mediator in coagulation and thrombosis highlights its therapeutic potential. Because NGAL promotes hypercoagulability through various mechanisms, it could serve as a valuable target for managing clotting disorders, particularly in conditions where inflammation and thrombosis coexist, such as cardiovascular disease, autoimmune disorders, and certain cancers. Moreover, elevated NGAL levels in cases of deep vein thrombosis suggest its potential as a biomarker for predicting and monitoring thrombotic events, offering clinicians a new tool for early intervention in high-risk patients.
In conclusion, this study establishes NGAL as a novel target in the pathophysiology of thrombosis and inflammation, providing a foundation for further research into NGAL-based therapies. By continuing to explore the molecular interactions of NGAL and its impact on clotting pathways, researchers may develop new treatments aimed at reducing thrombotic risk while managing inflammation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal