In this issue of Blood, Kambara et al1 explore the role of oral dysbiosis in exacerbating graft-versus-host disease (GVHD) and assess the efficacy of targeted interventions in mitigating these effects. Their findings underscore the significance of oral health in allogeneic hematopoietic stem cell transplantation (allo-HCT) outcomes and propose oral microbiome modulation as a potential avenue for GVHD prevention and treatment.
The community of microbes present in different regions of the healthy human body consist of distinct populations, reflecting the unique features of each ecological niche.2 Large, multicenter studies have found strong associations between the intestinal microbial composition and GVHD, and murine studies have established causal relationships between gut dysbiotic states and aggravated disease.3,4 Kambara et al questioned whether mucositis-induced oral dysbiosis also contributes to GVHD, suggesting that this ecosystem, too, could serve as a reservoir for pathogens impacting the systemic immune response.
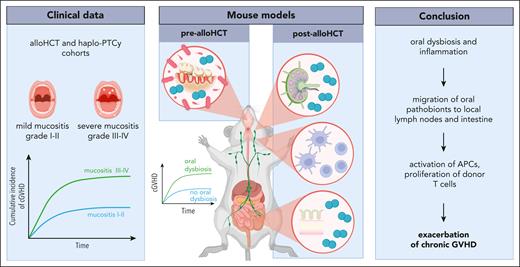
In a cohort of 230 patients who underwent allo-HCT, the authors found an increased cumulative incidence of both moderate-severe acute GVHD (aGVHD) and all chronic GVHD (cGVHD) in the group that experienced more profound oral mucositis (OM), with no differences in GVHD-free and relapse-free survival (GRFS). A subsequent analysis of 71 patients after haploidentical posttransplant cyclophosphamide (haplo-PTCy) HCT, which typically reduces cGVHD risk,5 shared some resemblance: OM severity predicted for cGVHD incidence, but aGVHD was not affected and OM was associated with worse GRFS in this cohort. Although causality cannot be inferred from these findings and confirmation in larger, multicenter cohorts is needed, they do point to a, thus far underappreciated, role of oral dysbiosis as a marker for cGVHD development.
The authors next analyzed the microbial composition in buccal mucosa samples collected from 31 patients before and after allo-HCT, 16 of which developed cGVHD. α-Diversity was significantly decreased at both time points in the cGVHD group, and although no statistically significant shifts in the mean relative abundance of single taxa were identified, Staphylococcaceae and Enterobacteriaceae tended to be more prevalent in patients with cGVHD after transplantation, suggesting that these bacterial profiles might contribute to cGVHD onset.
Next, the authors experimentally induced oral dysbiosis in murine models using oral ligature placement (OLP), a strategy that causes periodontitis in mice and was previously shown to aggravate intestinal inflammation.6 Significant perturbations of the oral microbiome were achieved, with no evident impact on the gut microbial composition or systemic inflammation. Fourteen days after OLP, these mice served as allo-HCT recipients. They exhibited similar clinical scores and survival in an aGVHD model compared with controls, but presented with more severe symptoms and shorter survival when tested in cGVHD and haplo-PTCy models.
To elucidate the mechanisms underlying the exacerbation of cGVHD after induced oral dysbiosis, Kambara et al focused on antigen-presenting cells, finding that dendritic cell numbers and their activation were increased in the cervical lymph nodes (LNs) after OLP, whereas no differences were observed in the peripheral LNs or the spleen. Notably, despite these seemingly localized effects, OLP recipient mice exhibited increased peripheral numbers of donor CD4+/interleukin-17–positive and CD8+/interferon gamma–positive cells compared with allogeneic controls. Microbiome analysis revealed that Enterococcaceae were significantly elevated in the oral cavity and feces before and after allo-HCT, with higher relative and absolute amounts. Enterococcus was identified in the cervical LNs by fluorescence immunostaining and bacterial cultivation. Finally, a repeated oral application of Enterococcus faecalis after targeted antibiotic treatment and OLP lead to more severe cGVHD compared with application of Escherichia coli or placebo, whereas the exposure to either bacterium without ligatures failed to augment cGVHD compared with OLP alone. Previous studies have linked intestinal Enterococcus expansion to higher mortality in patients after allo-HCT.3,7,8
The authors then tested whether the modulation of oral dysbiosis can ameliorate cGVHD. Both the removal of ligatures and the topical application of targeted antibiotics effectively reduced bacterial load, particularly Enterococcaceae, in the oral and gut microbiomes of mice. These interventions led to a marked improvement in cGVHD symptoms and overall survival. Furthermore, the activation of cervical antigen-presenting cells was partially reversed and several cGVHD-related lymphocyte populations decreased in the peripheral LNs, suggesting that the reduced cGVHD severity after dysbiosis correction is, in part, because of a limitation of allogeneic T-cell responses.
Based on their findings, Kambara et al propose that oral dysbiosis leads to an expansion of pathobionts such as Enterococcaceae, which then migrate to the cervical LNs and the gut and facilitate the activation of antigen-presenting cells and allogeneic T cells (see figure). This conclusion expands upon recent findings favoring the “marker hypothesis” over the “expansion hypothesis” to explain the relative enrichment of native oral bacteria in fecal samples observed in various disease states, including allo-HCT. Generally, it is believed that this phenomenon is caused by a reduction of gut microbes, with oral bacteria passing through the intestines without ectopic colonization.9 In the context of a disturbed oral microbiome, however, the current study indicates that pathogenic oral bacteria might be able to establish ectopic colonies in the intestinal tract and affect the immune response.
Exploring the role of the oral microbiome in cGVHD. In a clinical allo-HCT cohort, the cumulative incidence of cGVHD was associated with profound OM. In a mouse model, OLP was used to induce oral inflammation and dysbiosis. Findings from these experiments revealed oral pathobionts in the local LNs and the intestine, as well as the activation of antigen-presenting cells (APCs) and proliferation of donor T cells, likely contributing to cGVHD exacerbation. Figure created with BioRender.com.
Exploring the role of the oral microbiome in cGVHD. In a clinical allo-HCT cohort, the cumulative incidence of cGVHD was associated with profound OM. In a mouse model, OLP was used to induce oral inflammation and dysbiosis. Findings from these experiments revealed oral pathobionts in the local LNs and the intestine, as well as the activation of antigen-presenting cells (APCs) and proliferation of donor T cells, likely contributing to cGVHD exacerbation. Figure created with BioRender.com.
Overall, this study suggests that oral microbiome management, including the reduction of pathogenic bacteria through localized antibiotics or improved oral hygiene, could serve as a therapeutic strategy for reducing cGVHD risk and severity in allo-HCT recipients.
Conflict-of-interest disclosure: R.R.J. reports patent royalties, scientific advisory board membership, and stock options in Seres; scientific advisory board participation with MaaT Pharma; serves on the clinical advisory board of Prolacta; and serves on the scientific advisory board of, and holds stock options in, Postbiotics Plus. M.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal