On page 1616, in the abstract, the sentence “Afib was observed in 11 patients (pts) on BR (3%) and 67 pts on ibrutinib (18%)” should read “Afib was observed in 6 patients (pts) on BR (3%) and 66 pts on ibrutinib (18%).”
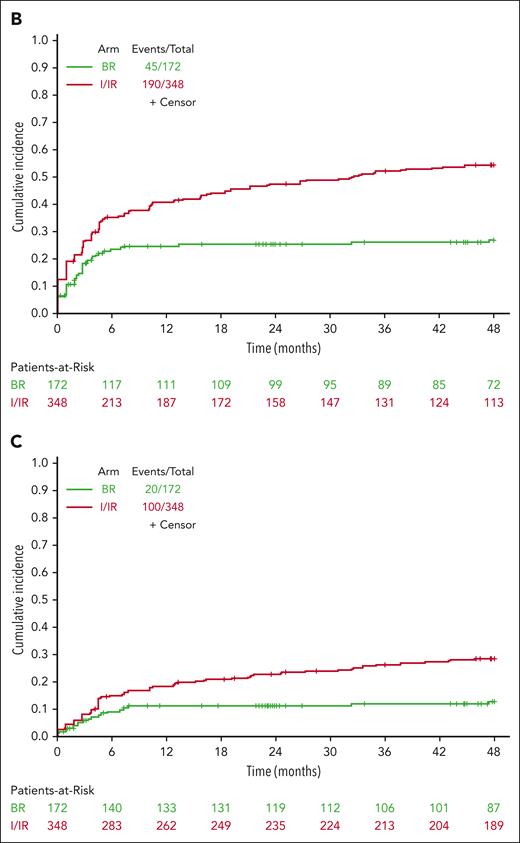
On page 1623, in the paragraph that begins “For hypertension,” the sentences “Hypertension (any grade) was reported in 47 patients on BR (27%) and 198 patients on ibrutinib (55%) during treatment (Figure 4B). Grade 3 or higher hypertension (requiring >1 medication for management, systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥100 mm Hg) was reported in 21 patients on BR (12%) and 103 patients on ibrutinib (29%) during treatment (Figure 4C)” should read “Hypertension (any grade) was reported in 45 patients on BR (26%) and 190 patients on ibrutinib (55%) during treatment (Figure 4B). Grade 3 or higher hypertension (requiring >1 medication for management, systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥100 mm Hg) was reported in 20 patients on BR (12%) and 100 patients on ibrutinib (29%) during treatment (Figure 4C).”
On page 1623, in the paragraph that begins “Secondary cancers,” the sentence “New primary malignancies were reported in 14 patients on arm 1 (BR), 14 on arm 2 (I), and 17 on arm 3 (IR)” should read “New primary malignancies were reported in 15 patients on arm 1 (BR), 14 on arm 2 (I), and 17 on arm 3 (IR).”
On pages 1624-1625, in Figure 4B and 4C, the reported numbers for patients on bendamustine plus rituximab (BR), ibrutinib (I), and ibrutinib plus rituximab (IR) were incorrect. The corrected Figure 4B and 4C are shown below.
Cumulative incidence of AEs. Extended follow-up demonstrates continued incidence of all-grade atrial fibrillation (A), all-grade hypertension (B), and grade 3 or higher hypertension (C) for patients treated with ibrutinib compared with those treated with bendamustine plus rituximab.
Cumulative incidence of AEs. Extended follow-up demonstrates continued incidence of all-grade atrial fibrillation (A), all-grade hypertension (B), and grade 3 or higher hypertension (C) for patients treated with ibrutinib compared with those treated with bendamustine plus rituximab.
On page 1625, in the second footnote of Table 2, the sentence “Other causes of death on the BR arm include chronic kidney disease (1) and site unable to provide further information (1)” should read “Other causes of death on the BR arm include DLBCL (1) and site unable to provide further information (1).”

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal