In this issue of Blood, Ma et al describe the in vivo efficacy of a novel, bivalent CD47 immunotoxin used as targeted therapy for the treatment of T-cell acute lymphoblastic leukemia in a mouse model.1 This study addresses ongoing questions about the best way to target CD47 to maximize antitumor effects and minimize known toxicities such as anemia.
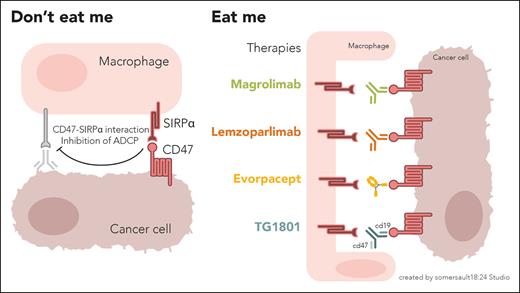
The cell surface protein CD47 has emerged as a promising therapeutic target in multiple cancer types, including acute myeloid leukemia, lung cancer, and gastric cancer.2-4 CD47, which is overexpressed on the surface of cancer cells, interacts with signal regulatory protein alpha (SIRPα) on the surface of macrophages and dendritic cells. This interaction, referred to as the “don’t eat me” signal, prevents tumor cells from being detected by the immune system and allows them to evade phagocytosis (see figure). Given this, many studies and drug development have focused on the immunotherapeutic potential of targeting CD47.5
The “don’t eat me” interaction between CD47 on tumor cells and SIRPα on macrophages (and dendritic cells) allows cancer cells to evade phagocytosis. Drug development has focused on targeting CD47 via various immunotherapeutic mechanisms with the goal of allowing “eat me” signaling, and thus cancer cell recognition and clearance, to occur.
The “don’t eat me” interaction between CD47 on tumor cells and SIRPα on macrophages (and dendritic cells) allows cancer cells to evade phagocytosis. Drug development has focused on targeting CD47 via various immunotherapeutic mechanisms with the goal of allowing “eat me” signaling, and thus cancer cell recognition and clearance, to occur.
However, despite its promise, attempts to target CD47 in clinical trials have shown mixed, and sometimes disappointing, results. Magrolimab is a first-in-class humanized immunoglobulin G4 (IgG4) anti-CD47 monoclonal antibody that disrupts the CD47-SIRPα axis and induces macrophage-mediated phagocytosis. The combination of magrolimab and the hypomethylating agent azacitidine was widely viewed as a potentially paradigm-changing treatment for acute myeloid leukemia, particularly in high-risk disease harboring a TP53 mutation, based on phase 1b data showing higher than expected response rates in this population.2 Unfortunately, the larger, randomized, phase 3, Enhance-2 study of this combination vs azacitidine and venetoclax was halted early after an ad hoc data review indicated a lack of survival benefit in the magrolimab arm. Magrolimab development was also plagued by concerns about safety and anemia-related toxicities because of the increased expression of CD47 on aging red blood cells. This resulted in a partial clinical hold and modification of pretreatment transfusion protocols for patients receiving the agent. Due to lack of efficacy, all ongoing magrolimab studies were ultimately halted and the development program has been discontinued. Full data from Enhance-2 has not yet been published, but speculations about study design and toxicity, and how they potentially contributed to the negative outcome, have been ongoing.
Lemzoparlimab is an IgG4 anti-CD47 antibody that targets a different CD47 epitope than magrolimab and has been shown to exert similar immunotherapeutic effects while sparing red blood cells. It has been studied in the treatment of multiple hematologic malignancies including acute myeloid leukemia, myelodysplastic syndrome, non-Hodgkin lymphoma, and multiple myeloma with favorable early phase trial results.6-8 However, studies of lemzoparlimab outside of China were abruptly stopped in 2022. The reasons for this decision are unclear, and questions over safety and efficacy remain.
Evorpacept, previously ALX148, is one of the only anti-CD47-directed therapies that remains in trials in the United States. This myeloid checkpoint inhibitor is composed of a high-affinity CD47 blocker fused to an inactive IgG Fc region and has been studied alone and in combination with other agents for the treatment of advanced solid tumors and some hematologic malignancies.9 Phase 1 study results were promising for patients with gastric or gastroesophageal junction cancer and squamous cell carcinoma of the head and neck, and pharmacodynamic studies demonstrated a significant increase in tumor-associated macrophages confirming the immunomodulatory mechanism of action. Importantly, hematologic toxicity did not occur. Additional studies in these and other solid tumor patient populations are ongoing.
Finally, CD47 has been used as a target in several different bispecific therapies in development, including those cotargeting CD19, CD20, and PDL1. Data for the phase 1 first-in-human and first-in-class study of TG1801, a bispecific antibody with a CD19 arm and a CD47 arm, with and without ublituximab (an anti-CD20 monoclonal) in the treatment of B-cell lymphoma was presented at the American Society of Hematology Annual Meeting in 2022 and looked promising based on safety and efficacy.10 Unfortunately, the follow-up study was halted due to a strategic decision by the sponsor. There are no other currently enrolling studies of CD47 bispecifics.
The Ma et al study is novel in that it suggests that CD47 remains a viable target for cancers that overexpress CD47 on their cell surface, including T-cell acute lymphoblastic leukemia, but that the mechanism of action may not need to be tied to effects on the immune system. Their bivalent CD47 immunotoxin functions solely as a targeted therapy and exerts potent antitumor effects without inducing phagocytosis. It also does not bind to CD47 expressed on normal red blood cells, therefore not causing anemia as a side effect. The in vivo data presented in this study are compelling, although the ability to translate these results from the mouse model to the bedside remains to be seen.
Ultimately, the Ma et al study provides hope and a potential new path forward for drug development in the CD47-directed therapy space, particularly in hematologic malignancies where new treatments remain desperately needed and prior anti-CD47 therapies have failed. This includes potential utility in diseases such as TP53-mutated acute myeloid leukemia, where therapies that do not rely on a functional apoptosis system to exert their mechanism of action hold the most promise.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal