Key Point
Pegcetacoplan treatment increased Hb levels, reduced hemolysis, and increased FACIT-fatigue scale scores in patients with CAD and wAIHA.
Visual Abstract
Cold agglutinin disease (CAD) and warm antibody autoimmune hemolytic anemia (wAIHA) are rare autoimmune hemolytic anemias characterized by red blood cell destruction, largely attributable to complement activation resulting in intravascular and extravascular hemolysis. Pegcetacoplan is a subcutaneously administered C3-targeted therapy, which may be suitable for treating CAD and wAIHA. In this open-label phase 2 study, analyses were conducted in 2 cohorts, 1 for patients with CAD and the other for those with wAIHA. In each cohort, patients were randomly assigned to receive pegcetacoplan 270 mg/d or 360 mg/d for up to 48 weeks. Safety end points included the incidence and severity of treatment-emergent adverse events (TEAEs) and adverse events of special interest (AESI). Efficacy end points included change from baseline in hemoglobin (Hb), lactate dehydrogenase, absolute reticulocyte count, haptoglobin, indirect bilirubin, and functional assessment of chronic illness therapy (FACIT)-fatigue scale. Thirteen of 13 (100%) and 10 of 11 (91%) patients with CAD and wAIHA, respectively, experienced at least 1 TEAE. Ten patients had at least 1 serious AE; none were considered related to pegcetacoplan. The only treatment-related AESIs were injection site reactions. Pegcetacoplan increased Hb levels, reduced hemolysis, and increased FACIT-fatigue scale scores in the first weeks; at week 48 the median (interquartile range) change from baseline Hb for the CAD and wAIHA total groups was 2.4 (0.90-3.00) and 1.7 g/dL (−1.40 to 2.90), respectively, and improvements in hemolysis and FACIT-fatigue scale scores were maintained. This study demonstrated that pegcetacoplan is generally well tolerated and suggests it can be effective for patients with CAD and wAIHA. This trial was registered at www.ClinicalTrials.gov as #NCT03226678.
Introduction
Autoimmune hemolytic anemias (AIHAs) are a group of rare heterogenous diseases characterized by autologous red blood cell (RBC) destruction by autoantibodies, which may involve complement activation.1,2 The most prevalent forms are cold agglutinin disease (CAD) and warm antibody AIHA (wAIHA), comprising 20% to 25% and 60% to 70% of all AIHAs, respectively.1,2
In CAD, RBC destruction is attributed to cold agglutinins (immunoglobulin M [IgM] autoantibodies) that bind to RBCs at temperatures below core body temperature, activating the classical complement pathway.1,2 Hemolysis results from deposition of C3b, promoting extravascular hemolysis in the liver and, to a lesser extent, from formation of the C5 to C9 final lytic complex, causing intravascular hemolysis.1,2 In wAIHA, 28% to 65% of RBC destruction is estimated attributable to complement being weakly activated by IgG, resulting in opsonization of RBC with C3b.1,2 However, non–complement-mediated mechanisms of IgG-opsonized RBC destruction are predominant, including phagocytosis by macrophages in the spleen, and macrophage-mediated damage to the cell membrane resulting in formation of spherocytes, which are cleared from circulation by the spleen.1
Mild cases of CAD may be managed by protection from cold.2 However, first-line treatment for symptomatic disease has generally been rituximab, because CAD responds to corticosteroids only at high unacceptable doses.3-5 There is also evidence to support use of rituximab in combination with chemotherapeutic agents, such as bendamustine and fludarabine, with increased efficacy but greater toxicity.4,6 Recently, the C1s inhibitor sutimlimab was approved to decrease the need for RBC transfusion because of hemolysis in adults with CAD.7-12
First-line therapy for wAIHA is corticosteroids, with adjunctive rituximab in more challenging cases such as patients with severe disease.3,4,13,14 Second-line therapy is rituximab; and third-line options include splenectomy, azathioprine, cyclosporin, and mycophenolate.3,4
Unmet needs with current treatments include relapse after corticosteroids/rituximab in approximately two-thirds and one-quarter of patients with CAD and wAIHA, respectively,3 along with infections and reduced response to vaccines.15 Additionally, side effects associated with corticosteroids, if high doses are required, preclude them as a long-term strategy.3
Pegcetacoplan is a C3 inhibitor conjugated to a linear polyethylene glycol molecule to increase its half-life.16 Pegcetacoplan binds with high affinity to complement protein C3 and its activation fragment C3b, thereby inhibiting both the C3 and C5 convertases.1,17,18 It is currently licensed for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in Europe17 and the United States.19 Its ability to prevent deposition of C3 breakdown products on the RBC membrane may endow pegcetacoplan with efficacy for treating CAD and wAIHA. The expected pegcetacoplan exposure after treatment with the dosing schedules evaluated in this study for patients with CAD and wAIHA had, at the time of protocol development, been observed to significantly decrease complement-mediated hemolytic activity in both eculizumab-naïve and previously treated patients with PNH16,20 and been deemed well tolerated in preclinical studies, in healthy volunteers, and in patients with PNH (for at least 2 months).17,21
The objective of this open-label, prospective, phase 2 study (ClinicalTrials.gov identifier: NCT03226678) was to assess the safety, efficacy, and pharmacokinetics of pegcetacoplan 270 mg per day (mg/d) and 360 mg/d for patients with a primary diagnosis of CAD regardless of prior treatment history or in patients with a primary diagnosis of wAIHA who relapsed from, did not respond to, or did not tolerate, at least 1 prior wAIHA treatment.
Methods
Study design
This was an open-label, prospective, phase 2 study to assess the safety, tolerability, preliminary efficacy, and pharmacokinetics of pegcetacoplan in patients with CAD or wAIHA for up to 48 weeks of treatment. The study planned to enroll up to 24 patients in 2 cohorts (analyzed separately): up to 12 patients each in the CAD and wAIHA cohorts. The sample size was not based on formal statistical testing or intended to represent real-life relative proportions of patients with CAD and wAIHA but was considered sufficient to obtain useful safety, tolerability, pharmacodynamic, and pharmacokinetic data to assist planning future studies.
Inclusion criteria included age of ≥18 years; weight of <125 kg; primary diagnosis of CAD or wAIHA defined by the presence of hemolytic anemia and positive direct antiglobulin test for CAD (C3) or wAIHA (IgG), respectively; hemoglobin (Hb) of <11 g/dL; documented evidence of, or willing to receive, vaccinations for Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type b; and, for wAIHA, patients should have relapsed from, not responded to, or been unable to tolerate, at least 1 prior wAIHA treatment regimen (full inclusion criteria are provided in supplemental Table 1, available on the Blood website).
Exclusion criteria included prior treatment with rituximab within 90 days; deficiency of iron, folate, or vitamin B12 before pegcetacoplan treatment; abnormal liver function as indicated by elevated aspartate aminotransferase or alanine aminotransferase; elevated bilirubin not due to active hemolysis; and active malignant diseases, or presence or suspicion of active bacterial or viral infection or severe recurrent bacterial infections (full exclusion criteria are provided in supplemental Table 1).
The study protocol, any amendments to the protocol, informed consent form, the investigator’s brochure, and other study-specific information were reviewed and approved by the institutional review board or independent ethics committee before the study commenced. The institutional review boards/independent ethics committees were constituted and operated in accordance with all applicable regulatory requirements.
Study treatment
Patients in the CAD and wAIHA cohorts were randomized open-label 1:1 to receive pegcetacoplan 270 mg or 360 mg once daily by subcutaneous (SC) infusion for 48 weeks. After completion of the core study, patients were eligible to participate in a long-term extension (data not reported here) during which they could continue to receive pegcetacoplan. Patients discontinuing treatment returned to clinical sites for safety follow-up after 6 weeks, with final follow-up 6 weeks later.
Prophylactic antibiotic therapy was prescribed during the study to all patients to minimize potential risk of infection. The primary prophylactic antibiotic was penicillin V, 500 mg twice daily. Patients unable to tolerate penicillin were prescribed erythromycin 500 mg twice daily or azithromycin 500 mg 3 times per week.
Study end points
Safety was the primary end point, including the incidence and severity of treatment-emergent adverse events (TEAEs) after administration of multiple doses of pegcetacoplan. AEs of special interest (AESI) included hemolysis/hemolytic anemia; infection; thrombosis; hypersensitivity; clinically significant decrease in kidney function; and injection site reactions. Compliance, assessed for all patients, was calculated as number of administered study doses divided by total expected study doses, ×100, with days on which patients took incomplete or no doses considered missed.
Efficacy end points included change from baseline (CFB) in Hb, lactate dehydrogenase (LDH), indirect bilirubin, absolute reticulocyte count (ARC), and haptoglobin; number of RBC transfusions received during the study; and CFB in functional assessment of chronic illness therapy (FACIT)–fatigue scale scores (total scores range from 0-52 points, with higher scores indicating less fatigue22) and linear analog scale assessment (LASA), including energy level, ability to perform daily activity, and overall quality of life (QoL; Likert scales running from 0-10 points,23 with higher ratings suggesting higher QoL). In addition, pegcetacoplan serum concentrations were assessed.
Statistical analysis
No formal inferential statistics were applied to the data; therefore, no formal hypothesis testing was done. Safety data were analyzed descriptively using the safety set (all patients who received at least 1 dose of study drug). TEAEs were summarized by cohort, dose group, system organ class, and preferred term, in accordance with the Medical Dictionary for Regulatory Activities version 20.0 (March 2017).
Efficacy outcomes were assessed using descriptive statistics, and CFB in efficacy outcomes were summarized at different time points by cohort and dose group.
Pegcetacoplan trough concentrations were summarized descriptively by cohort, dose group, and study week using descriptive statistics.
Results
Patient population
Among 30 patients screened, 13 with CAD were eligible for study enrollment and composed the intention-to-treat (ITT) analysis population; 10 completed the study per the protocol and entered the long-term extension (Figure 1). Eleven patients with wAIHA were eligible for study enrollment and composed the ITT analysis population; 7 completed the study per the protocol and entered the long-term extension. The safety population was identical to the ITT population. Patient baseline demographic characteristics are listed in Table 1.
Patient disposition. ∗One patient completed scheduled daily pegcetacoplan dosing on day 339, and a follow-up exit visit at day 421 but was withdrawn from study and is therefore listed both as withdrawn and completed; †One patient in the wAIHA 270 mg group and 1 patient in the wAIHA 360 mg group were withdrawn from study by the investigator subsequent to discontinuation of study drug because of a TEAE.
Patient disposition. ∗One patient completed scheduled daily pegcetacoplan dosing on day 339, and a follow-up exit visit at day 421 but was withdrawn from study and is therefore listed both as withdrawn and completed; †One patient in the wAIHA 270 mg group and 1 patient in the wAIHA 360 mg group were withdrawn from study by the investigator subsequent to discontinuation of study drug because of a TEAE.
Patient baseline demographics (ITT population)
| Characteristic . | CAD cohort (N = 13) . | wAIHA cohort (N = 11) . |
|---|---|---|
| Age, mean (SD), y | 72.0 (8.50) | 56.3 (19.32) |
| Sex, n (%) | ||
| Female | 9 (69.2) | 6 (54.5) |
| Male | 4 (30.8) | 5 (45.5) |
| Body weight, median (IQR), kg | 80.6 (68.6-82.0) | 77.0 (71.2-100.1) |
| Body mass index, mean (SD), kg/m2 | 28.6 (4.80) | 29.3 (5.38) |
| Race, n (%) | ||
| Black or African American | 1 (7.7) | 0 |
| White | 12 (92.3) | 11 (100.0) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 2 (15.4) | 6 (54.5) |
| Not Hispanic or Latino | 11 (84.6) | 5 (45.5) |
| Time since diagnosis of CAD or wAIHA, mean (SD), y | 4.8 (5.83) | 3.0 (1.61) |
| Hb levels, median (IQR), g/dL | 9.20 (8.10-9.80) | 9.00 (8.00-10.10) |
| Reticulocyte count, median (IQR), 109/L | 148.1 (103.40-157.28) | 228.2 (106.86-255.00) |
| Cold agglutinin titers, n (%) | n = 8 | n = 2 |
| <1:32 | 0 (0.0) | 2 (100.0) |
| 1:128 | 1 (12.5) | 0 (0.0) |
| 1:256 | 1 (12.5) | 0 (0.0) |
| 1:512 | 1 (12.5) | 0 (0.0) |
| >1:512 | 5 (62.5) | 0 (0.0) |
| Prior treatments | ||
| Number of transfusions per month in the 12 mo before first dose, median (IQR) | 0.08 (0.0-0.2) | 0.00 (0.0-0.2) |
| Number of patients not needing a transfusion in the 12 mo before first dose, n (%) | 6 (46.2) | 6 (54.5) |
| Splenectomy | 0 | 4 (36.4) |
| Rituximab (alone or in combination) | 10 (76.9) | 8 (72.7) |
| Immunosuppressant | 0 | 1 (9.1) |
| Prior medications used by patients in both cohorts, n (%) | ||
| Bacterial vaccines | 11 (84.6) | 11 (100.0) |
| Systemic corticosteroids | 7 (53.8) | 11 (100.0) |
| β-Blocking agents | 5 (38.5) | 6 (54.5) |
| Other antineoplastic agents | 7 (53.8) | 6 (54.5) |
| Drugs for peptic ulcer and gastroesophageal reflux disease | 6 (46.2) | 6 (54.5) |
| Other analgesics and antipyretics | 7 (53.8) | 1 (9.1) |
| Vitamin B12 and folic acid | 10 (76.9) | 5 (45.5) |
| Concomitant medications, n (%) | ||
| Systemic corticosteroids | 6 (42.6) | 9 (81.8) |
| Erythropoiesis-stimulating agents | 1 (7.7) | 0 |
| Characteristic . | CAD cohort (N = 13) . | wAIHA cohort (N = 11) . |
|---|---|---|
| Age, mean (SD), y | 72.0 (8.50) | 56.3 (19.32) |
| Sex, n (%) | ||
| Female | 9 (69.2) | 6 (54.5) |
| Male | 4 (30.8) | 5 (45.5) |
| Body weight, median (IQR), kg | 80.6 (68.6-82.0) | 77.0 (71.2-100.1) |
| Body mass index, mean (SD), kg/m2 | 28.6 (4.80) | 29.3 (5.38) |
| Race, n (%) | ||
| Black or African American | 1 (7.7) | 0 |
| White | 12 (92.3) | 11 (100.0) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 2 (15.4) | 6 (54.5) |
| Not Hispanic or Latino | 11 (84.6) | 5 (45.5) |
| Time since diagnosis of CAD or wAIHA, mean (SD), y | 4.8 (5.83) | 3.0 (1.61) |
| Hb levels, median (IQR), g/dL | 9.20 (8.10-9.80) | 9.00 (8.00-10.10) |
| Reticulocyte count, median (IQR), 109/L | 148.1 (103.40-157.28) | 228.2 (106.86-255.00) |
| Cold agglutinin titers, n (%) | n = 8 | n = 2 |
| <1:32 | 0 (0.0) | 2 (100.0) |
| 1:128 | 1 (12.5) | 0 (0.0) |
| 1:256 | 1 (12.5) | 0 (0.0) |
| 1:512 | 1 (12.5) | 0 (0.0) |
| >1:512 | 5 (62.5) | 0 (0.0) |
| Prior treatments | ||
| Number of transfusions per month in the 12 mo before first dose, median (IQR) | 0.08 (0.0-0.2) | 0.00 (0.0-0.2) |
| Number of patients not needing a transfusion in the 12 mo before first dose, n (%) | 6 (46.2) | 6 (54.5) |
| Splenectomy | 0 | 4 (36.4) |
| Rituximab (alone or in combination) | 10 (76.9) | 8 (72.7) |
| Immunosuppressant | 0 | 1 (9.1) |
| Prior medications used by patients in both cohorts, n (%) | ||
| Bacterial vaccines | 11 (84.6) | 11 (100.0) |
| Systemic corticosteroids | 7 (53.8) | 11 (100.0) |
| β-Blocking agents | 5 (38.5) | 6 (54.5) |
| Other antineoplastic agents | 7 (53.8) | 6 (54.5) |
| Drugs for peptic ulcer and gastroesophageal reflux disease | 6 (46.2) | 6 (54.5) |
| Other analgesics and antipyretics | 7 (53.8) | 1 (9.1) |
| Vitamin B12 and folic acid | 10 (76.9) | 5 (45.5) |
| Concomitant medications, n (%) | ||
| Systemic corticosteroids | 6 (42.6) | 9 (81.8) |
| Erythropoiesis-stimulating agents | 1 (7.7) | 0 |
IQR, interquartile range; SD, standard deviation.
Exposure
In the CAD cohort, 7 and 6 patients were randomized to receive 270 mg/d and 360 mg/d pegcetacoplan, respectively. In the wAIHA cohort, 5 and 6 patients were randomized to receive 270 mg/d and 360 mg/d pegcetacoplan, respectively. The mean (standard deviation) total days dosed was 283.2 (107.80) in the CAD cohort and 236.0 (140.56) in the wAIHA cohort. Compliance for both cohorts was good, with median (interquartile range) compliance of 99.7% (98.2-100) and 100% (99.3-100) in the CAD and wAIHA cohorts, respectively.
Safety
All 13 patients (100%) in the CAD cohort and 10 (90.9%) of 11 patients in the wAIHA cohort experienced at least 1 TEAE (events reported in ≥20% patients, listed in Table 2); the most frequently reported were diarrhea, headache, hypertension, nausea, and vitamin B12 deficiency. With hematopoiesis affecting vitamin B12 levels, vitamin B12 deficiency in some patients with chronic hemolytic disease was expected and recorded as a TEAE, because the study exclusion criteria precluded enrollment of patients with existing vitamin B12 deficiency. The majority of TEAEs were mild or moderate. All injection site reactions were mild, except for 1 event of moderate severity in the CAD cohort.
Summary of TEAEs
| TEAEs, n (%) . | CAD cohort (N = 13) . | Number of events . | wAIHA cohort (N = 11) . | Number of events . |
|---|---|---|---|---|
| Any TEAE | 13 (100.0) | 179 | 10 (90.9) | 138 |
| Reported in ≥20% of patients in respective cohort | ||||
| Diarrhea | 5 (38.5) | 9 | — | — |
| Headache | 4 (30.8) | 6 | — | — |
| Hypertension | 4 (30.8) | 6 | — | — |
| Nausea | 4 (30.8) | 6 | — | — |
| Vitamin B12 deficiency | 4 (30.8) | 4 | — | — |
| Dizziness | 3 (23.1) | 7 | — | — |
| Contusion | 3 (23.1) | 5 | — | — |
| Fatigue | 3 (23.1) | 5 | — | — |
| Constipation | 3 (23.1) | 3 | — | — |
| Decreased appetite | 3 (23.1) | 3 | — | — |
| Fall | 3 (23.1) | 3 | — | — |
| Viral upper respiratory tract infection | 3 (23.1) | 3 | — | — |
| Pyrexia | — | — | 4 (36.4) | 6 |
| Injection site pruritus | — | — | 3 (27.3) | 17 |
| Dyspnea | — | — | 3 (27.3) | 4 |
| Cough | — | — | 3 (27.3) | 3 |
| TEAEs at least possibly related to pegcetacoplan | 9 (69.2) | 27∗ | 8 (72.7) | 34† |
| Serious TEAEs | 5 (38.5) | 8 | 5 (45.5) | 5 |
| Serious TEAEs at least possibly related to pegcetacoplan | 0 | 0 | 0 | 0 |
| TEAESI | 10 (76.9) | 37 | 9 (81.8) | 53 |
| TEAESIs at least possibly related to pegcetacoplan | 5 (38.5) | 6 | 5 (45.5) | 25 |
| TEASIs of infection | 7 (53.8) | 13 | 6 (54.5) | 10 |
| Viral upper respiratory tract infection | 3 (23.1) | 3 | 2 (18.2) | 4 |
| Pneumonia | 2 (15.4) | 2 | 1 (9.1) | 1 |
| Upper respiratory tract infection | 1 (7.7) | 1 | 2 (18.2) | 2 |
| Urinary tract infection | 2 (15.4) | 2 | 0 | 0 |
| Fungal infection | 1 (7.7) | 1 | 0 | 0 |
| Incision site abscess | 1 (7.7) | 1 | 0 | 0 |
| Mastitis | 1 (7.7) | 1 | 0 | 0 |
| Oral candidiasis | 1 (7.7) | 1 | 0 | 0 |
| Oral fungal infection | 1 (7.7) | 1 | 0 | 0 |
| Bronchitis | 0 | 0 | 1 (9.1) | 1 |
| Ear infection | 0 | 0 | 1 (9.1) | 1 |
| Influenza | 0 | 0 | 1 (9.1) | 1 |
| TEAEs leading to study drug discontinuation or study withdrawal | 2 (15.4) | 3 | 3 (27.3) | 3 |
| TEAEs, n (%) . | CAD cohort (N = 13) . | Number of events . | wAIHA cohort (N = 11) . | Number of events . |
|---|---|---|---|---|
| Any TEAE | 13 (100.0) | 179 | 10 (90.9) | 138 |
| Reported in ≥20% of patients in respective cohort | ||||
| Diarrhea | 5 (38.5) | 9 | — | — |
| Headache | 4 (30.8) | 6 | — | — |
| Hypertension | 4 (30.8) | 6 | — | — |
| Nausea | 4 (30.8) | 6 | — | — |
| Vitamin B12 deficiency | 4 (30.8) | 4 | — | — |
| Dizziness | 3 (23.1) | 7 | — | — |
| Contusion | 3 (23.1) | 5 | — | — |
| Fatigue | 3 (23.1) | 5 | — | — |
| Constipation | 3 (23.1) | 3 | — | — |
| Decreased appetite | 3 (23.1) | 3 | — | — |
| Fall | 3 (23.1) | 3 | — | — |
| Viral upper respiratory tract infection | 3 (23.1) | 3 | — | — |
| Pyrexia | — | — | 4 (36.4) | 6 |
| Injection site pruritus | — | — | 3 (27.3) | 17 |
| Dyspnea | — | — | 3 (27.3) | 4 |
| Cough | — | — | 3 (27.3) | 3 |
| TEAEs at least possibly related to pegcetacoplan | 9 (69.2) | 27∗ | 8 (72.7) | 34† |
| Serious TEAEs | 5 (38.5) | 8 | 5 (45.5) | 5 |
| Serious TEAEs at least possibly related to pegcetacoplan | 0 | 0 | 0 | 0 |
| TEAESI | 10 (76.9) | 37 | 9 (81.8) | 53 |
| TEAESIs at least possibly related to pegcetacoplan | 5 (38.5) | 6 | 5 (45.5) | 25 |
| TEASIs of infection | 7 (53.8) | 13 | 6 (54.5) | 10 |
| Viral upper respiratory tract infection | 3 (23.1) | 3 | 2 (18.2) | 4 |
| Pneumonia | 2 (15.4) | 2 | 1 (9.1) | 1 |
| Upper respiratory tract infection | 1 (7.7) | 1 | 2 (18.2) | 2 |
| Urinary tract infection | 2 (15.4) | 2 | 0 | 0 |
| Fungal infection | 1 (7.7) | 1 | 0 | 0 |
| Incision site abscess | 1 (7.7) | 1 | 0 | 0 |
| Mastitis | 1 (7.7) | 1 | 0 | 0 |
| Oral candidiasis | 1 (7.7) | 1 | 0 | 0 |
| Oral fungal infection | 1 (7.7) | 1 | 0 | 0 |
| Bronchitis | 0 | 0 | 1 (9.1) | 1 |
| Ear infection | 0 | 0 | 1 (9.1) | 1 |
| Influenza | 0 | 0 | 1 (9.1) | 1 |
| TEAEs leading to study drug discontinuation or study withdrawal | 2 (15.4) | 3 | 3 (27.3) | 3 |
N, number of patients in safety set; n, number of patients with an event.
Related events were headache (3 unique events); fatigue (2 unique events); and nausea, infusion site pain, injection site erythema, injection site pain, injection site pruritus, injection site reaction, injection site swelling, malaise, contusion, neutrophil count decreased, white blood cell count decreased, decreased appetite, dizziness, nasal congestion, lichenoid keratosis, rash maculo-papular, skin warm, and Raynaud phenomenon (1 unique event each).
Related events included injection site pruritus (3 unique events); injection site erythema and pyrexia (2 unique events each); injection site bruising, fatigue, injection site reaction, edema peripheral, arthropod bite, hyponatremia, flushing, and pallor (1 unique event each).
Nine patients (69.2%) in the CAD cohort (5 and 4 in the 270 mg/d and 360 mg/d groups, respectively) experienced at least 1 AE reported as at least possibly related to pegcetacoplan. The most reported related TEAEs in this cohort were injection site reactions (5 patients; 38.5%). Eight patients (72.7%) in the wAIHA cohort (4 patients in each dosage group) experienced at least 1 AE reported as related to pegcetacoplan. Also in this cohort, the most reported related TEAEs were injection site reactions (6 patients; 54.5%). No patients in either cohort discontinued study drug because of injection site reactions.
Of the overall TEAEs, some were defined as TEAE of special interest (TEAESI). TEAESI are reported regardless of their relationship to the study drug. A total of 10 (76.9%) and 9 (81.8%) patients experienced at least 1 TEAESI in the CAD and wAIHA cohorts, respectively (supplemental Table 2). The most frequently reported TEAESIs were injection site reactions and infections in 6 (46.2%) and 7 patients (53.9%), respectively, in the CAD cohort, and in 6 patients (54.5%) and 6 patients (54.6%), respectively, in the wAIHA cohort. All TEAESIs reported to be at least possibly related to pegcetacoplan were injection site reactions.
Five patients in the CAD cohort reported 8 serious AEs (SAEs): AIHA, multiple injuries due to fall, urinary tract infection, acute cholecystitis, pneumonia, dyspnea, blood calcium increased, and blood creatinine increased. One patient discontinued pegcetacoplan because of worsening AIHA and died 4 weeks after the last dose because of complications from AIHA; this was assessed as unrelated to pegcetacoplan by the investigator and sponsor. The patient had a fall on day 33, with sacrum/L5/pubic ramus fracture and pelvic hematoma, and missed pegcetacoplan dosing. Although pegcetacoplan was continued until day 85, there were treatment gaps on days 68 to 71 and 80 to 82. The worsening hemolysis was attributed to frequent hospitalization, antibiotic use, and the fall with subsequent fractures, all of which can activate the immune/complement system. Reasons for not considering increased dosing of pegacetacoplan in the presence of hemolytic anemia were not reported.
In the wAIHA cohort, 5 SAEs were reported in 5 patients: AIHA (2 events in different patients), hemolysis, squamous cell carcinoma, and pulmonary embolism. No SAEs in either cohort were assessed by the investigator to be related to the study treatment. Serious TEAESIs of AIHA, pneumonia, urinary tract infection, and blood creatinine increased were recorded in the CAD cohort, and serious TEAESIs of hemolysis, AIHA, and pulmonary embolism were recorded in the wAIHA cohort. None of these were deemed to be related to treatment with pegcetacoplan. No events of significant hypersensitivity reactions, such as anaphylaxis, was reported in either cohort.
Two (15.4%) patients in the CAD cohort discontinued study drug or withdrew early from the study because of a TEAE; 1 because of blood calcium and blood creatinine increase, and 1 because of worsening AIHA (in the aforementioned patient who subsequently died). These TEAEs were deemed unrelated to study treatment. In the wAIHA cohort, 3 (27.3%) patients discontinued study drug or withdrew early from the study because of TEAEs of AIHA, hemolysis, and anemia; these were also considered unrelated to pegcetacoplan.
Efficacy: CAD cohort
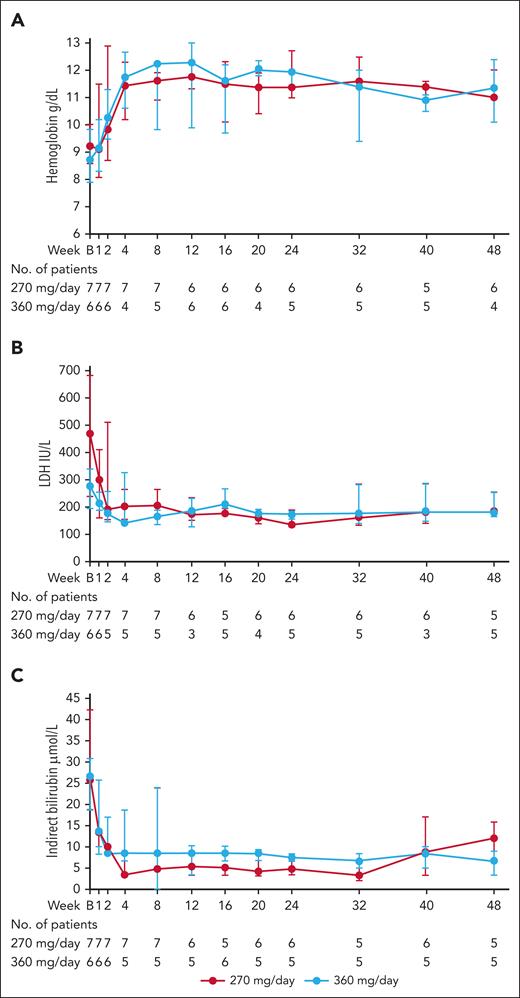
In the CAD cohort, median Hb levels increased during the first 4 weeks and were subsequently maintained, with median CFB of 2.4 g/dL at week 8 (1.7 g/dL and 2.4 g/dL in the 270 mg/d and 360 mg/d groups, respectively) and 2.4 g/dL at week 48 (1.8 g/dL and 2.7 g/dL in the 270 mg/d and 360 mg/d groups, respectively; Figure 2A; Table 3; supplemental Table 3).
Markers of hemolysis over the course of the study for patients with CAD. (A) Median (IQR) Hb levels. (B) median (IQR) LDH levels. (C) median (IQR) indirect bilirubin levels. IQR, interquartile range.
Markers of hemolysis over the course of the study for patients with CAD. (A) Median (IQR) Hb levels. (B) median (IQR) LDH levels. (C) median (IQR) indirect bilirubin levels. IQR, interquartile range.
CFB to week 48 in Hb, hemolytic markers, and QoL in patients with CAD
| Parameter . | Baseline . | Week 48 . | % CFB to week 48 . | CFB to week 48 . |
|---|---|---|---|---|
| Hb, g/dL | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 9.2 (8.10-9.80) | 11.0 (10.10-12.30) | 26.5 (9.78-32.26) | 2.4 (0.90-3.00) |
| Mean (SD) | 8.9 (1.34) | 11.2 (1.14) | 23.5 (18.92) | 2.0 (1.59) |
| Hemolytic markers | ||||
| LDH, IU/L | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 329.0 (232.0-471.0) | 182.0 (167.0-225.0) | −21.9 (−54.3 to −8.1) | −52.0 (−184.0 to −16.0) |
| Mean (SD) | 468.2 (520.2) | 220.8 (109.6) | −29.3 (32.0) | −276.8 (601.5) |
| Indirect bilirubin, μmol/L | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 25.7 (18.81-39.30) | 8.0 (3.42-13.68) | −71.5 (−84.59 to −36.36) | −15.4 (−25.62 to −6.89) |
| Mean (SD) | 31.7 (18.06) | 8.7 (6.26) | −62.5 (29.33) | −16.9 (11.18) |
| ARC, ×109/L | n = 12 | n = 10 | n = 9 | n = 9 |
| Median (IQR) | 148.1 (103.40-157.28) | 79.7 (50.60-95.12) | −51.7 (−67.81 to −34.97) | −94.8 (−109.60 to −51.16) |
| Mean (SD) | 138.6 (64.20) | 80.1 (40.21) | −46.7 (30.34) | −79.4 (55.69) |
| Haptoglobin, g/L | n = 13 | n = 11 | n = 11 | n = 11 |
| Median (IQR) | 0.1 (0.100-0.100) | 0.6 (0.40-0.93) | 320.0 (0.00-550.00) | 0.3 (0.00-0.55) |
| Mean (SD) | 0.2 (0.37) | 0.6 (0.38) | 389.1 (410.37) | 0.4 (0.45) |
| QoL end points | ||||
| FACIT-fatigue scale, score | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 26.0 (21.0-36.0) | 32.5 (32.0-46.0) | 21.2 (4.6-52.4) | 6.0 (2.0-11.0) |
| Mean (SD) | 27.2 (9.93) | 33.3 (13.34) | 21.8 (48.57) | 3.9 (13.57) |
| LASA, score | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 5.0 (4.00-7.00) | 6.5 (5.00-9.00) | 14.6 (0.00-50.00) | 1.0 (0.00-2.00) |
| Mean (SD) | 5.5 (1.85) | 6.8 (1.81) | 20.6 (30.50) | 0.9 (1.45) |
| Parameter . | Baseline . | Week 48 . | % CFB to week 48 . | CFB to week 48 . |
|---|---|---|---|---|
| Hb, g/dL | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 9.2 (8.10-9.80) | 11.0 (10.10-12.30) | 26.5 (9.78-32.26) | 2.4 (0.90-3.00) |
| Mean (SD) | 8.9 (1.34) | 11.2 (1.14) | 23.5 (18.92) | 2.0 (1.59) |
| Hemolytic markers | ||||
| LDH, IU/L | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 329.0 (232.0-471.0) | 182.0 (167.0-225.0) | −21.9 (−54.3 to −8.1) | −52.0 (−184.0 to −16.0) |
| Mean (SD) | 468.2 (520.2) | 220.8 (109.6) | −29.3 (32.0) | −276.8 (601.5) |
| Indirect bilirubin, μmol/L | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 25.7 (18.81-39.30) | 8.0 (3.42-13.68) | −71.5 (−84.59 to −36.36) | −15.4 (−25.62 to −6.89) |
| Mean (SD) | 31.7 (18.06) | 8.7 (6.26) | −62.5 (29.33) | −16.9 (11.18) |
| ARC, ×109/L | n = 12 | n = 10 | n = 9 | n = 9 |
| Median (IQR) | 148.1 (103.40-157.28) | 79.7 (50.60-95.12) | −51.7 (−67.81 to −34.97) | −94.8 (−109.60 to −51.16) |
| Mean (SD) | 138.6 (64.20) | 80.1 (40.21) | −46.7 (30.34) | −79.4 (55.69) |
| Haptoglobin, g/L | n = 13 | n = 11 | n = 11 | n = 11 |
| Median (IQR) | 0.1 (0.100-0.100) | 0.6 (0.40-0.93) | 320.0 (0.00-550.00) | 0.3 (0.00-0.55) |
| Mean (SD) | 0.2 (0.37) | 0.6 (0.38) | 389.1 (410.37) | 0.4 (0.45) |
| QoL end points | ||||
| FACIT-fatigue scale, score | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 26.0 (21.0-36.0) | 32.5 (32.0-46.0) | 21.2 (4.6-52.4) | 6.0 (2.0-11.0) |
| Mean (SD) | 27.2 (9.93) | 33.3 (13.34) | 21.8 (48.57) | 3.9 (13.57) |
| LASA, score | n = 13 | n = 10 | n = 10 | n = 10 |
| Median (IQR) | 5.0 (4.00-7.00) | 6.5 (5.00-9.00) | 14.6 (0.00-50.00) | 1.0 (0.00-2.00) |
| Mean (SD) | 5.5 (1.85) | 6.8 (1.81) | 20.6 (30.50) | 0.9 (1.45) |
Abbreviations are explained in Table 1.
Hemolytic markers were analyzed during the study. At week 8, median CFB in LDH was −89.0 U/L in the overall cohort (−206.0 U/L and −42.0 U/L in the 270 mg/d and 360 mg/d groups, respectively). The reduction was maintained during the study (Figure 2B; Table 3; supplemental Table 3); and at week 48, median CFB was −52.0 (−58.0 U/L and −46.0 U/L in the 270 mg/d and 360 mg/d groups, respectively). These results should be viewed in the light of the higher median LDH level at baseline for the 270 mg /d group (471.0 U/L, with a wide range of 151-2123 U/L) compared with the 360 mg/d group (280.5 U/L; range, 187-348 U/L).
Similar to the results for LDH, early reductions were observed (at week 8) in indirect bilirubin, with median CFB of −22.2 μmol/L (−25.7 μmol/L and −13.6 μmol/L in the 270 mg/d and 360 mg/d groups, respectively), and levels remained lower than baseline throughout the study. At week 48, median CFB was −15.4 (−6.9 μmol/L and −18.8 μmol/L in the 270 mg/d and 360 mg/d groups, respectively; Figure 2C; Table 3; supplemental Table 3).
Likewise, after the first few weeks, ARC had also decreased, and reductions were largely maintained throughout the study, with median CFB of −94.8 × 109/L at week 48 (−73.0 × 109/L and −104.9 × 109/L in the 270 mg/d and 360 mg/d groups, respectively; Table 3; supplemental Table 3). Haptoglobin levels increased compared with baseline at week 8, and a maintained increase at week 48, with median CFB of 0.3 g/L (0.5 g/L and 0.3 g/L in the 270 mg/d and 360 mg/d groups, respectively; Table 3; supplemental Table 3).
Median serum pegcetacoplan concentrations in the CAD cohort are displayed by dosing regimen on a log-linear scale in supplemental Figure 1A. Serum pegcetacoplan concentration appeared to reach steady state around week 8.
At baseline, median FACIT-fatigue scale total score was 26.0 points overall, and 30.0 and 23.5 points in the 270 mg/d and 360 mg/d groups, respectively (Table 3; supplemental Table 3). By week 8, self-reported fatigue and its impact upon daily activities and function had improved, with an increase in median CFB FACIT-fatigue scale total scores of 10.5 points (11.0 and 10.0 points for the 270 mg/d and 360 mg/d groups, respectively). The increase was maintained at week 48, with median overall CFB of 6.0 points, and 4.5 and 9.5 points for the 270 mg/d and 360 mg/d groups, respectively. As expected, QoL improved as impact of fatigue decreased, with median CFB LASA total score at week 8 of 2.0 points (3.0 and 2.0 points for the 270 and 360 mg/d groups, respectively), which was generally maintained, with median CFB at week 48 of 1.0 points (1.0 and 1.0 points for the 270 and 360 mg/d groups, respectively).
Analysis of efficacy in the per-protocol population of the CAD cohort are presented in supplemental Table 4.
Efficacy: wAIHA cohort
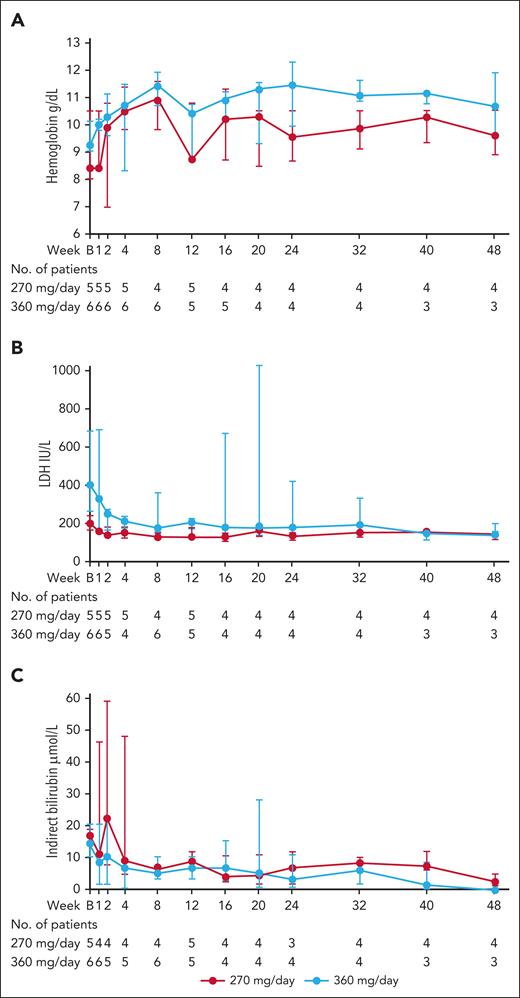
Treatment with pegcetacoplan resulted in increasing median Hb levels until week 8 (median CFB 2.7 g/dL; 1.5 g/dL in the 270 mg/d group and 2.7 g/dL in the 360 mg/d group), after which they plateaued (Figure 3A; Table 4; supplemental Table 5). At week 48, median CFB was 1.7 g/dL (0.2 g/dL in the 270 mg/d group; 1.7 g/dL in the 360 mg/d group).
Markers of hemolysis over the course of the study for patients with wAIHA. (A) Median (IQR) Hb levels. (B) median (IQR) LDH levels. (C) median (IQR) indirect bilirubin levels. IQR, interquartile range.
Markers of hemolysis over the course of the study for patients with wAIHA. (A) Median (IQR) Hb levels. (B) median (IQR) LDH levels. (C) median (IQR) indirect bilirubin levels. IQR, interquartile range.
CFB to week 48 in Hb, hemolytic markers, and QoL in patients with wAIHA
| Parameter . | Baseline . | Week 48 . | % CFB to week 48 . | CFB to week 48 . |
|---|---|---|---|---|
| Hb, g/dL | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 9.0 (8.00-10.10) | 10.6 (9.10-11.00) | 18.9 (−13.33 to 32.22) | 1.7 (−1.40 to 2.90) |
| Mean (SD) | 8.8 (1.67) | 10.3 (1.11) | 13.0 (21.05) | 1.0 (1.92) |
| Hemolytic markers | ||||
| LDH, IU/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 263.0 (173.0-531.0) | 134.0 (121.0-149.0) | −36.0 (−51.1 to 2.1) | −68.0 (−137.0 to 3.0) |
| Mean (SD) | 544.5 (820.05) | 142.1 (27.58) | −30.7 (30.40) | −102.3 (140.87) |
| Indirect bilirubin, μmol/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 15.4 (10.30-20.52) | 1.7 (0.00-5.13) | −81.81 (−100.00 to −62.55) | −10.26 (−15.38 to −5.26) |
| Mean (SD) | 25.7 (27.72) | 2.7 (2.60) | −81.3 (16.12) | −10.3 (4.28) |
| ARC, ×109/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 228.2 (106.86-255.00) | 58.0 (42.00-75.00) | −69.8 (−81.20 to −45.72) | −168.7 (−181.44 to −48.86) |
| Mean (SD) | 226.4 (137.41) | 65.5 (36.82) | −64.0 (18.90) | −138.7 (76.20) |
| Haptoglobin, g/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 0.1 (0.10-0.42) | 0.9 (0.61-1.56) | 510.0 (5.41-1292.86) | 0.5 (0.04-1.46) |
| Mean (SD) | 0.3 (0.28) | 1.0 (0.63) | 586.6 (614.55) | 0.7 (0.68) |
| QoL end points | ||||
| FACIT-fatigue scale, score | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 32.0 (16.0-45.0) | 35.0 (34.0-48.0) | 11.1 (−23.9 to 45.8) | 4.0 (−11.0 to 11.0) |
| Mean (SD) | 31.5 (14.42) | 38.6 (7.25) | 18.3 (43.32) | 2.4 (10.23) |
| LASA, score | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 6.0 (4.00-9.00) | 8.0 (6.00-8.00) | 28.6 (−30.00 to 33.33) | 2.0 (−3.00 to 2.00) |
| Mean (SD) | 6.0 (2.72) | 7.4 (1.13) | 74.4 (189.92) | 0.6 (2.99) |
| Parameter . | Baseline . | Week 48 . | % CFB to week 48 . | CFB to week 48 . |
|---|---|---|---|---|
| Hb, g/dL | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 9.0 (8.00-10.10) | 10.6 (9.10-11.00) | 18.9 (−13.33 to 32.22) | 1.7 (−1.40 to 2.90) |
| Mean (SD) | 8.8 (1.67) | 10.3 (1.11) | 13.0 (21.05) | 1.0 (1.92) |
| Hemolytic markers | ||||
| LDH, IU/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 263.0 (173.0-531.0) | 134.0 (121.0-149.0) | −36.0 (−51.1 to 2.1) | −68.0 (−137.0 to 3.0) |
| Mean (SD) | 544.5 (820.05) | 142.1 (27.58) | −30.7 (30.40) | −102.3 (140.87) |
| Indirect bilirubin, μmol/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 15.4 (10.30-20.52) | 1.7 (0.00-5.13) | −81.81 (−100.00 to −62.55) | −10.26 (−15.38 to −5.26) |
| Mean (SD) | 25.7 (27.72) | 2.7 (2.60) | −81.3 (16.12) | −10.3 (4.28) |
| ARC, ×109/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 228.2 (106.86-255.00) | 58.0 (42.00-75.00) | −69.8 (−81.20 to −45.72) | −168.7 (−181.44 to −48.86) |
| Mean (SD) | 226.4 (137.41) | 65.5 (36.82) | −64.0 (18.90) | −138.7 (76.20) |
| Haptoglobin, g/L | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 0.1 (0.10-0.42) | 0.9 (0.61-1.56) | 510.0 (5.41-1292.86) | 0.5 (0.04-1.46) |
| Mean (SD) | 0.3 (0.28) | 1.0 (0.63) | 586.6 (614.55) | 0.7 (0.68) |
| QoL end points | ||||
| FACIT-fatigue scale, score | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 32.0 (16.0-45.0) | 35.0 (34.0-48.0) | 11.1 (−23.9 to 45.8) | 4.0 (−11.0 to 11.0) |
| Mean (SD) | 31.5 (14.42) | 38.6 (7.25) | 18.3 (43.32) | 2.4 (10.23) |
| LASA, score | n = 11 | n = 7 | n = 7 | n = 7 |
| Median (IQR) | 6.0 (4.00-9.00) | 8.0 (6.00-8.00) | 28.6 (−30.00 to 33.33) | 2.0 (−3.00 to 2.00) |
| Mean (SD) | 6.0 (2.72) | 7.4 (1.13) | 74.4 (189.92) | 0.6 (2.99) |
Abbreviations are explained in Table 1.
Analysis of hemolytic markers revealed an early decrease in LDH values, with median CFB at week 8 of −109.5 U/L (−47.0 U/L and −135.0 U/L in the 270 mg/d and 360 mg/d groups, respectively). The decrease was maintained during the study; and at week 48, median CFB was −68.0 U/L (−61.0 U/L and −137.0 U/L in the 270 mg/d and 360 mg/d groups, respectively; Figure 3B; Table 4; supplemental Table 5).
Reduced hemolysis was also indicated by early decrease in indirect bilirubin, with median CFB of −6.9 μmol/L (−8.6 μmol/L and −4.3 μmol/L in the 270 mg/d and 360 mg/d groups, respectively) at week 8. Overall, indirect bilirubin levels remained lower than baseline throughout the study, with median CFB of −10.3 μmol/L (−11.1 μmol/L and −8.6 μmol/L in the 270 mg/d and 360 mg/d groups at week 48, respectively; Table 4; supplemental Table 5).
Likewise, ARC decreased during the first weeks, with reductions maintained during the study, and median CFB at week 48 of −168.7 × 109/L (−143.7 × 109/L and −168.7 × 109/L in the 270 mg/d and 360 mg/d groups, respectively; Table 4; supplemental Table 5). An increase at week 8 in haptoglobin was then maintained at week 48, with median CFB of 0.5 g/L (0.5 and 0.8 g/L in the 270 mg/d and 360 mg/d groups, respectively; Table 4; supplemental Table 5).
During the study, 4 patients in the wAIHA cohort had RBC transfusions. In total, during 12 months of study, 7 (64.0%) of 11 patients were transfusion free as compared with 6 (55.0%) patients in the 12 months before baseline.
Median serum pegcetacoplan concentrations in the wAIHA cohort are displayed by dosing regimen on a log-linear scale in supplemental Figure 1B. Serum pegcetacoplan concentration appeared to reach steady state between week 4 and week 8.
Patients treated with pegcetacoplan showed a reduction in fatigue and its impact, with a median CFB increase in FACIT-fatigue scale total score of 8.0 points at week 8 (4.5 and 12.5 points for the 270 mg/d and 360 mg/d groups, respectively; Table 4; supplemental Table 5). The increase was maintained by the 360 mg/d group but not the 270 mg/d group, with overall median CFB at week 48 of 4.0 points (−4.0 and 11.0 points for the 270 and 360 mg/d groups, respectively; supplemental Table 4). QoL for patients with wAIHA improved, with median CFB of LASA total score at week 8 of 1.5 points (0.5 and 3.0 points for the 270 mg/d and 360 mg/d groups, respectively). The increase in the 270 mg/d group was maintained through week 24, with median CFB of 1.0 points, which dropped to 0.0 points at week 40. The increase in the 360 mg/d group was maintained through to week 48, with median CFB of 2.0 points (0.5 and 2.0 points for the 270 mg/d group and total wAIHA cohort, respectively; supplemental Table 5).
Analysis of efficacy in the per-protocol population of the wAIHA cohort are presented in supplemental Table 6.
Discussion
This is, to our knowledge, the first study to evaluate the safety and efficacy of the C3 inhibitor pegcetacoplan for treatment of patients with CAD or wAIHA. Pegcetacoplan was generally well tolerated, and the study results suggest that it can be effective in increasing Hb levels, reducing hemolysis, and increasing FACIT-fatigue scale and LASA scores.
The TEAEs reported in the study were mostly mild; none of the 13 SAEs were considered related to pegcetacoplan. The most common TEAEs assessed as possibly related to pegcetacoplan were injection site reactions, consistent with the profile of pegcetacoplan.17 Almost all injection site reactions were recorded as mild, indicating that SC administration of pegcetacoplan was well tolerated. A possible concern with use of complement inhibitors is the potential increased risk of infection with encapsulated bacteria.24 None of the reported infections in this study were considered to be related to pegcetacoplan by the study investigators, considering the available information on the patient clinical situation and the reported infection. There were no cases of meningococcal disease. It should be noted that patients in this study were required to be vaccinated against encapsulated bacteria and received prophylactic antibiotics. Overall, the frequency and pattern of infections was in line with that expected in the study cohorts.
There is growing evidence for use of complement inhibitors to treat AIHAs.1 The C1s inhibitor, sutimlimab,11,12 was recently approved to treat hemolytic anemia in adult patients with CAD.7,8,19 Eculizumab, a C5 inhibitor, has been studied in a phase 2 trial of patients with CAD, demonstrating a reduction in hemolysis but only marginal increases in Hb and no improvement in QoL scores,25 possibly because of its inability to inhibit extravascular hemolysis.1 A few case reports have described successful off-label use of sutimlimab or eculizumab in wAIHA.26,27
Pegcetacoplan administration rapidly increased Hb levels during the first 4 weeks and these were maintained through to week 48 in patients with CAD. There were also improvements in transfusion avoidance during the core study phase compared with the 12 months before baseline. In patients with wAIHA, pegcetacoplan administration produced a rapid increase in Hb levels during the first 8 weeks, maintained to the end of the study. Transfusion avoidance during the core study phase in the wAIHA cohort was similar compared with the 12 months before baseline. Pegcetacoplan is a complement inhibitor and only an estimated 28% to 65% of RBC destruction in wAIHA is complement mediated,1 whereas RBC destruction in CAD is mediated by complement-driven extravascular and intravascular hemolysis.1 However, these data suggest, to our knowledge, for the first time, that complement inhibitor may have some degree of beneficial effect in selected patients with wAIHA. Indeed, in both cohorts, more than half of patients had an improvement of >1.5 g/dL Hb at the end of the study. Given the rapid onset of efficacy, pegcetacoplan may be useful in the acute phase, which is an unmet need associated with risk of thrombosis and mortality.28,29 Predictors of response in wAIHA may be the positivity of the direct antiglobulin test for IgG plus C3d, as well as serum C3 and C4 consumption,2 although further data are needed. Improvement in other hemolytic markers, including both indirect bilirubin (a marker of extravascular hemolysis) and LDH (marker of RBC destruction), was consistent with improvements in Hb.
There were also improvements in patient-reported outcomes, with increases in median FACIT-fatigue scale total scores and LASA scores by week 8 that were generally maintained during the study. A clinically meaningful change in the FACIT-fatigue scale score has previously been estimated as ≥3 points in cancer-related anemia30 and, more recently, to ≥5 points specifically for patients with CAD.31-34 The median improvement in FACIT-fatigue scale score in the overall CAD cohort indicated a clinically meaningful reduction in fatigue according to both these definitions. In the wAIHA cohort, a clinically meaningful improvement in the median FACIT-fatigue scale score was initially attained but not maintained at the end of the study according to the more stringent of these estimates (primarily because of a reduced response in the 270 mg/d group toward the end of the study).
Limitations of this study included the open-label design, lack of a comparator, and the relatively small size of the patient cohorts, which is a common limitation of trials in rare diseases.
This study demonstrated that pegcetacoplan administered SC is generally well tolerated and suggests effectiveness in patients with CAD and wAIHA. Based on the encouraging results of this phase 2 study, particularly in patients with CAD, a phase 3 placebo-controlled study (ClinicalTrials.gov identifier: NCT05096403) has been designed to further assess pegcetacoplan efficacy for patients with CAD, with dosing at 1080 mg twice daily, in line with the pharmacokinetic and safety evidence obtained in this study.
Acknowledgments
Medical writing support was provided by Catherine Hoare (Bioscript Medical Ltd, Macclesfield, United Kingdom), funded by Sobi and Apellis Pharmaceuticals. Apellis and Sobi reviewed and provided feedback on the manuscript.
The PLAUDIT study (ClinicalTrials.gov identifier: NCT03226678) was sponsored by Apellis Pharmaceuticals.
Authorship
Contribution: E.R., B.F., M.S., W.H., S.R.L., and S.S.S.A. were responsible for study conceptualization, investigation, and validation, and review and editing of the manuscript; M.A.-A. was responsible for data curation, formal analysis, study investigation, study methodology, validation, and reviewing and editing of the manuscript; F.V.G. was responsible for study conceptualization, data curation, formal analysis, study investigation, study methodology, validation, and reviewing and editing of the manuscript; and M.A.G. was responsible for study conceptualization, investigation, validation and reviewing and editing of the manuscript.
Conflict-of-interest disclosure: E.R. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Novartis and Apellis Pharma. B.F. has received grants or contracts from Agios; has received consulting fees from Alexion, Janssen, Samsung, and Sobi; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Alexion, Janssen, Samsung, Sanofi, and Sobi. S.R.L. has received research support for this manuscript; and has received grants or contracts from Sobi and Alpine. S.S.S.A. has received research support for this manuscript. F.V.G. received research support from Apellis Pharma for this manuscript; and has stock or stock options from Apellis Pharma. M.A.G has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Ionis/Akcea, Alnylym, Prothena, Sanofi, Janssen, Aptitude Health grants, Ashfield, Juno, Physicians Education Resource, AbbVie, Johnson & Johnson, Celgene, Research to Practice, Sorrento, and i3Health. The remaining authors declare no competing financial interests.
Correspondence: Eloy Roman, Hematology-Oncology, Lakes Research, 5801 NW 151 St, Suite #302, Miami Lakes, FL 33014; email: eroman@lakesresearch.com.
References
Author notes
Sobi and Apellis are committed to responsible and ethical sharing of data for medicines and indications approved by the European Medicines Agency and/or the U.S. Food and Drug Administration, while protecting individual patient integrity and compliance with applicable legislation. Data access will be granted in response to qualified research requests. All proposals requesting data access will need to specify how the data will be used and will need the approval of the trial investigator team before data release. Individual participant data will not be shared. All requests are evaluated by a crossfunctional panel of experts within Sobi and Apellis and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity and commitment to publication of the results. To request access to study data, a data sharing request form (available on www.sobi.com) should be sent to medical.info@sobi.com. Further information on Sobi’s data sharing policy and process for requesting access can be found at: https://www.sobi.com/en/policies. To request access to data or the study protocol, please contact Apellis. The study protocol will be available with no end date.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal