In this issue of Blood, Roman et al present the results of PLAUDIT, a phase 2 study of pegcetacoplan in primary cold agglutinin disease (CAD) and warm autoimmune hemolytic anemia (wAIHA), which found that pegcetacoplan was a safe and potentially effective therapeutic option in CAD.1
CAD is a clonal B-cell lymphoproliferative disorder linked to risk of thrombosis and early mortality. CAD is mediated by IgM autoantibodies (cold agglutinins) binding to erythrocytes in acral circulation, where body temperature is in the antibody’s thermal binding range. Cold agglutinins activate the classic complement pathway. Proximal complement activation leads to C3 cleavage and formation of C3 convertase on C3-opsonized surfaces. With the increased surface density of C3b, formation of C5 convertase initiates terminal complement, eventually forming the membrane attack complex (MAC) (see figure).2,3 MAC formation on erythrocytes causes intravascular hemolysis, whereas C3b remaining bound to erythrocytes leads to extravascular hemolysis.4 Hemolysis is predominately extravascular in chronic CAD, with intravascular hemolysis associated with more severe disease and exacerbations. Conversely, in wAIHA, autoantibodies are typically polyclonal IgG panagglutinins, which engage Fc-γ receptors on splenic macrophages, leading to extravascular hemolysis. Although IgM strongly activates complement, certain subclasses of IgG also fix complement, thus complement-mediated intravascular and extravascular hemolysis are mechanisms of erythrocyte destruction in wAIHA.
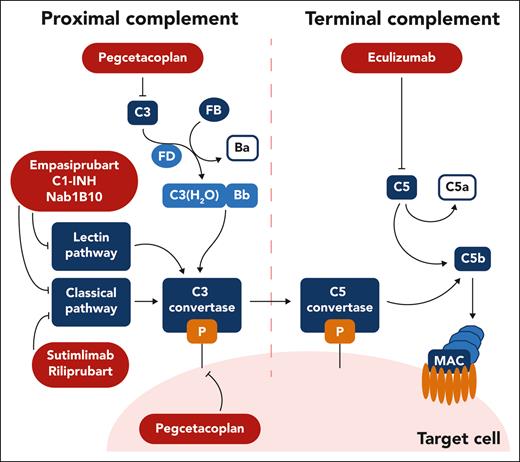
Schema of complement therapeutic targets under study for autoimmune hemolytic anemia. Complement inhibitors are summarized according to their target and the step of the complement pathway it involves. Ecullizumab inhibits C5; pegcetacoplan inhibits C3 and C3 convertase activity; sutimlimab and riliprubart inhibit C1s of the classic pathway; empasiprubart and Nab1B10 inhibit C2 targeting the classic and lectin pathway; C1-INH inhibits C1r, C1s, and mannan-binding lectin serine proteases 1 and 2, targeting the classic and lectin pathway. Professional illustration by Somersault18:24.
Schema of complement therapeutic targets under study for autoimmune hemolytic anemia. Complement inhibitors are summarized according to their target and the step of the complement pathway it involves. Ecullizumab inhibits C5; pegcetacoplan inhibits C3 and C3 convertase activity; sutimlimab and riliprubart inhibit C1s of the classic pathway; empasiprubart and Nab1B10 inhibit C2 targeting the classic and lectin pathway; C1-INH inhibits C1r, C1s, and mannan-binding lectin serine proteases 1 and 2, targeting the classic and lectin pathway. Professional illustration by Somersault18:24.
Current apparoches to treatment of CAD focus on complement inhibition, because conventional treatment (mainly symptomatic and pharmacologic with rituximab) offers low remission rates and short reponse duration, linked to poor quality of life.5 Addition of cytotoxic agents (eg, bendamustine, fludarabine) leads to improved response rate and duration; however, use of cytotoxic agents is associated with lower tolerability, especially in unfit patients.
Complement inhibitors and their targets under study in AIHAs are shown (see figure). Eculizumab is the first-in-class complement inhibitor targeting terminal complement activation, which was approved for paroxysmal nocturnal hemoglobinuria (PNH) in 2007. In a prospective phase 2 study of 13 patients with CAD, eculizumab reduced hemolysis and transfusion dependency but had no impact on quality of life, and median increase in hemoglobin was <1 g/dL. Eculizumab is administered IV every 2 weeks and does not address extravascular hemolysis. Sutimlimab is a first-in-class humanized monoclonal antibody that binds to C1s and inhibits classic complement activation. It received approval in 2022 for CAD based on improvements in hemoglobin (sustained increase in hemoglobin by ≥2 g/dL in responders), hemolysis markers, fatigue, and quality of life demonstrated in the CADENZA and CARDINAL trials.6-8 Sutimlimab is administered IV every 2 weeks. Outside of case reports, complement inhibitors have not been studied in wAIHA.
Roman et al report in this issue the phase 2 study of pegcetacoplan, a subcutaneous C3 complement inhibitor. C3 inhibition targets proximal complement activation and, in particular, C3-mediated extravascular hemolysis. The safety and efficacy of this approach led to approval of pegcetacoplan in PNH in 2021.9 In this open-label, single-arm, prospective study, investigators aimed to assess safety, efficacy, and pharmacokinetics of pegcetacoplan over 48 weeks in primary CAD regardless of prior treatments and in relapsed or refractory wAIHA. Among 30 patients screened (24 receiving ≥1 dose), 10 completed 270- and 360-mg daily dosing in CAD (5 patients each), whereas 4 (270 mg) and 3 (360 mg) participants completed dosing in wAIHA. Participants had a baseline hemoglobin ∼9 g/dL in both cohorts and ∼50% received transfusions in the prior year (range, 0-2 transfusions). Injection site reactions were the only treatment-emergent adverse event (TEAE) considered related to pegcetacoplan. No serious adverse events were assessed as treatment related. TEAE leading to drug discontinuation or study withdrawal occurred in 2 of 13 participants with CAD and 3 of 11 participants with wAIHA. In wAIHA, pegcetacoplan increased hemoglobin during the first 8 weeks, maintained up to week 48, but did not improve transfusion avoidance or quality of life and fatigue scores, with lesser effects seen in the lower dosing group. Nevertheless, response in select patients, especially in the acute phase, suggest that it may be useful to further study this approach in wAIHA, potentially aiming to identify whether a subset of patients with both C3+ and IgG+ benefit.
Encouraging results were observed in CAD, with increase in hemoglobin from week 4 that was maintained until week 48 (median hemoglobin, 11 g/dL, a >2-g/dL increase from baseline), improvements in hemolytic markers, fatigue, and quality of life, and transfusion avoidance compared with 12 months prior. These results prompted an ongoing phase 3 placebo-controlled trial (NCT05096403) in a larger population of patients with CAD, incorporating detailed hemolytic markers, such as C3 deposition. Pegcetacoplan is administered using the twice-weekly dosing already approved for PNH.
Complement inhibition has increasingly become the standard of care for CAD since the approval of sutimlimab. Unfortunatley, its availability is limited even in countries with access to other complement inhibitors. In clinical practice, sutimlimab is often used in patients refractory to or who cannot receive rituximab-based regimens or as a temporizing measure to allow time for B-cell–depleting therapies. The current study suggests that pegcetacoplan may have similar efficacy to sutimlimab and offers a self-administered approach. Further studies are needed to confirm the findings reported here. Although hemolysis markers stabilized after 2 weeks of pegcetacoplan, onset of action may be longer than sutimlimab because of subcutaneous administration. Currently under development, a second-generation classic complement inhibitor, riliprubart, selectively inhibits only the activated form of C1s. It has shown safety, tolerability, and activity in a phase 1b study of patients with CAD. Other complement inhibitors targeting the classic and lectin pathway are also under study for AIHAs, including empasiprubart and Nab1B10 that inhibit C2, whereas C1-INH inhibits C1r, C1s, and mannan-binding lectin serine proteases 1 and 2 (see figure).10
In conclusion, complement inhibition represents a significant advance in the treatment of CAD. The approved and under development treatments have differences in dosing schedules, mechanisms of actions, availability in the clinic, and patient or disease characteristics. The difference should lead to a future of personalized treatment of CAD with shared physician and patient decision-making.
Conflict-of-interest disclosure: E.G. has consulted for AstraZeneca, Sanofi, Sobi, and Omeros Pharmaceuticals, Inc. G.F.G. has consulted for Apellis and Alexion Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal