In this issue of Blood, Zaiken et al provide compelling evidence that Tet3 deficiency in donor T cells promotes the differentiation of interleukin-4 (IL-4)-producing T follicular helper (TFH) cells and inhibits pathogenic immunoglobulin G2c (IgG2c) class switching, leading to the amelioration of chronic graft-versus-host disease (GVHD) in mice.1
The TET family, comprising TET1, TET2, and TET3, plays a pivotal role in DNA demethylation by oxidizing methylated cytosine (5-mC), which facilitates the expression of target genes. Loss of TET enzymes results in DNA hypermethylation and the suppression of gene expression. TET2 mutations and deletions have been linked to hematological malignancies, possibly through the downregulation of tumor-suppressor genes.
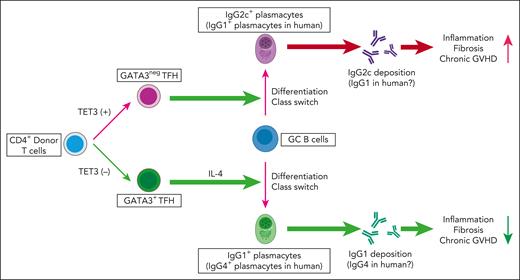
In this study, Zaiken et al demonstrate that Tet3 deficiency in donor T cells is critical for the differentiation of GATA3+ TFH cells after allogeneic hematopoietic cell transplantation. GATA3+ TFH cells drive B-cell class switching toward less inflammatory IgG1 plasmacytes in an IL-4-dependent manner (see figure). This process, in turn, inhibits the differentiation of pathogenic IgG2c producing plasmacytes, significantly reducing IgG2c deposition and fibrosis in chronic GVHD target organs. GATA3+ TFH cells in this study likely represent previously described TFH2 cells, known to express GATA3 and promote IgG1 responses.2 Using a coculture system, Zaiken et al extended their findings to human T cells, showing that TET3 deficiency promotes the differentiation of human GATA3+ TFH, which induces B-cell class switching to IgG4, the human counterpart of mouse IgG1. Both mouse IgG1 and human IgG4 interact with the inhibitory Fc receptor FcγRIIb; therefore, they are less inflammatory than other IgG subtypes.
Schematic overview of the role of T-cell TET3 in TFH-cell differentiation, class switching, and the development of chronic GVHD after allogeneic hematopoietic cell transplantation. The figure was created using BioRender.com.
Schematic overview of the role of T-cell TET3 in TFH-cell differentiation, class switching, and the development of chronic GVHD after allogeneic hematopoietic cell transplantation. The figure was created using BioRender.com.
Zaiken et al highlight that Tet3 deficiency profoundly suppresses lung fibrosis, thus mitigating lung dysfunction caused by chronic GVHD. Lung chronic GVHD, characterized by bronchiolitis obliterans, is associated with the worst prognosis among chronic GVHD-related organ injuries.3
Although novel agents such as belumosudil have shown some promise against early-stage lung chronic GVHD, standard treatments for lung chronic GVHD remain elusive.4 The identification of the critical role of Tet3 in lung fibrosis is an important step toward developing targeted therapies.
Epigenetic modification is a promising therapeutic approach for GVHD treatment. DNA methyltransferase inhibitors such as azacitidine ameliorate GVHD through regulatory T cell–dependent mechanisms,5 and histone deacetylase inhibitors such as vorinostat suppress acute GVHD by enhancing the expression of indoleamine 2,3-dioxygenase.6 Enhancer of zeste homolog 2 (EZH2) inhibitors reduce chronic GVHD severity,7 and these epigenetic modifiers also have antitumor activity, distinguishing them from conventional immunosuppressants. However, the antitumor effects of TET3 inhibition remain poorly understood. Tet inhibition may show antitumor activity,8 but Tet2 and Tet3 double knockout experiments have shown induction of hematopoietic malignancies in mouse models.9 Studying the role of TET3 in hematological malignancies is crucial for advancing TET3-targeting strategies in GVHD prophylaxis and treatment.
Although TET3 inhibition represents a promising therapeutic target for chronic GVHD, significant challenges remain. TET inhibitors have yet to be developed, and thus, Zaiken et al primarily investigated prophylactic Tet3 deficiency using Tet3-deficient donor T cells. Thus, the therapeutic potential for established chronic GVHD has yet to be tested. The molecular targets of Tet3 in TFH cells require further investigation. Tet3 deficiency may lead to DNA hypermethylation and suppress key molecules that regulate TFH differentiation toward GATA3neg TFH cells. However, recent findings suggest that Tet2 deficiency enhances gene expression by affecting RNA 5-mC oxidization.10 Although it remains unknown whether Tet3 deficiency similarly promotes gene expression under specific conditions, a precise evaluation of the epigenetic landscape will be required to identify the molecular targets of Tet3 that regulate TFH differentiation.
In conclusion, this groundbreaking study by Zaiken et al reveals the novel role of Tet3 in TFH differentiation and immunoglobulin-mediated tissue injury in chronic GVHD. This work underscores TET3 as a promising therapeutic target, offering new hope for the treatment and prevention of GVHD.
Conflict-of-interest disclosure: D.H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal