In this issue of Blood, Onsaker and colleagues provide further evidence that links histo-blood group ABO system transferase (BGAT) with the elevated risk of venous thromboembolism (VTE) in individuals with non-O blood groups (see figure).1
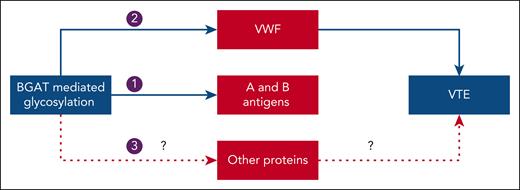
Potential mechanisms linking histo-blood group ABO system transferase with venous thromboembolism. Arrow 1: Histo-blood group ABO system transferase (BGAT) adds N-acetylgalactosamine or galactose sugar moiety to transform the H antigen on red cells to A or B antigens, respectively. Arrow 2: BGAT-mediated glycosylation of von Willebrand factor (VWF) leads to elevated levels of VWF and factor VIII in non-O blood groups, thereby increasing the risk of VTE. Arrow 3: BGAT-mediated glycosylation of other proteins could also increase the risk of VTE by unknown mechanisms.
Potential mechanisms linking histo-blood group ABO system transferase with venous thromboembolism. Arrow 1: Histo-blood group ABO system transferase (BGAT) adds N-acetylgalactosamine or galactose sugar moiety to transform the H antigen on red cells to A or B antigens, respectively. Arrow 2: BGAT-mediated glycosylation of von Willebrand factor (VWF) leads to elevated levels of VWF and factor VIII in non-O blood groups, thereby increasing the risk of VTE. Arrow 3: BGAT-mediated glycosylation of other proteins could also increase the risk of VTE by unknown mechanisms.
The presence of a non-O blood group was identified as a potential risk factor for VTE in the 1960s.2 Since then, several epidemiology studies have reported associations between ABO blood group or ABO genotype and VTE. In meta-analyses of these studies, non-O blood groups (A, AB, and B) were found to be associated with a 2- to 3-fold higher risk of VTE when compared with the O blood group, with the highest risk observed with the A1 and B haplotypes.3-5
The H antigen, found on the surface of red blood cells, is the precursor to the A and B antigens, which are formed when a specific sugar molecule is added through a reaction catalyzed by a glycosyltransferase. A- and B-glycosyltransferases (jointly known as BGAT) add either an N-acetylgalactosamine or d-galactose sugar moiety to transform the H antigen to A or B antigens, respectively (see figure). In contrast, the H antigen is unmodified in individuals with the O blood group because the O allele codes for a nonfunctional protein. The ABO antigens are not only found on red blood cells, but they are also expressed in various tissues (including endothelial cells) and found in a wide range of glycolipids and glycoproteins, of which von Willebrand factor (VWF) is an example. BGAT is expressed in the same tissues as the ABO antigens, where it mediates posttranslational glycosylation of proteins. BGAT is also present as a soluble form in plasma where it can be readily measured. Plasma levels are thought to be a surrogate for intracellular BGAT expression.
Compared with individuals with O blood group, those with non-O blood groups have higher levels of factor (F)VIII and its carrier protein, VWF. Both VWF and FVIII are procoagulant proteins that undergo posttranslational modifications by glycosyltransferases. In individuals with non-O blood groups, the addition of A or B antigens by BGAT to VWF protects VWF from proteolysis and reduces its clearance.6 Accordingly, the posttranslational glycosylation of VWF by BGAT results in increased levels of VWF and FVIII in non-O blood groups, thereby providing a plausible explanation for the elevated VTE risk (see figure). The possibility also exists that BGAT could contribute to the increased risk of thrombosis by glycosylation of other proteins (see figure). Until recently, there was a lack of robust evidence associating BGAT with an increased VTE risk, and it was unclear whether such an association is mediated entirely or partially through VWF or FVIII.
In a previous proteome-wide discovery study of >7000 human proteins, Onsaker and colleagues identified plasma BGAT as a novel protein biomarker associated with VTE.7 In their current study, they build on these earlier findings. Thus, in the same case-cohort nested within the Trøndelag Health Study (HUNT 3), they investigated: 1) the effect of ABO haplotype on plasma BGAT levels; 2) the association between plasma BGAT levels and the incidence of first VTE; and 3) whether or not the association between BGAT and VTE is mediated solely through its effects on VWF or FVIII. The HUNT 3 source cohort consisted of 46 513 participants, who at baseline did not have a history of VTE, cancer, or abdominal aortic aneurysm. Cases included the 294 participants who developed a first episode of VTE within the first 5 years of follow-up. At baseline, the participants had a mean age of 68 years. Of the participants with VTE, 113 (38%) presented with deep vein thrombosis and 118 (62%) with pulmonary embolism. Among the VTE cases, there were 115 (39%) unprovoked events and 179 (61%) provoked events. Controls included 1066 participants who were randomly sampled from the source cohort at baseline and weighted for age and sex distributions of VTE cases.
As expected, the results showed that the ABO haplotypes strongly modulated the level of BGAT, a finding consistent with an earlier study by a different group.8 Onsaker and colleagues also found that high plasma BGAT levels were associated with a 2- to 4-fold increased risk of a first VTE, with a higher risk observed for unprovoked events. These findings extend those of a separate proteome-wide association study, which previously identified ABO gene–encoded protein as a potential risk factor for VTE.9 The novel finding of the current study is that, even after controlling for VWF or FVIII levels, the risk of VTE associated with BGAT remained statistically significant, although it was attenuated. This latter finding supports the notion that BGAT may also contribute to an increased risk of VTE through mechanisms that are independent of VWF or FVIII. The authors’ findings are consistent with earlier studies that also demonstrated how the association between non-O blood groups and an increased risk of VTE persisted despite controlling for VWF and FVIII levels.10 Therefore, the evidence appears convincing that higher BGAT levels are associated with an increased VTE risk and that part of this increase in risk is independent of VWF/FVIII.
The strengths of the work by Onsaker et al include its relatively large sample size and prospective design that mitigates the risk of reverse causation. Although the observational nature of the study precludes definite conclusions on causality, the validity of the authors’ conclusion that BGAT is a potential cause of the increased risk of VTE associated with the non-O blood groups is supported by the strength of the association and the consistency of the findings linking ABO blood group with VTE in epidemiological studies. The case for a VWF/FVIII-independent effect of BGAT is biologically plausible because BGAT can also mediate posttranslational glycosylation of other proteins. Therefore, on balance, their findings support the hypothesis that the increased risk of VTE associated with non-O blood groups is contributed to by BGAT acting through VWF/FVIII or other unknown proteins. What remains unsolved are the precise biological mechanisms responsible for the increase in VTE risk.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal