As Star Trek fans and those of us old enough to remember when television had only 3 channels know, whenever the starship Enterprise was in crisis, Captain Kirk would yell down to his chief engineer, Montgomery Scott, “Scotty, we need more power!” There is now a growing realization that multiple myeloma (MM) cells in crisis, whether due to the additional metabolic demands of proliferating or surviving therapeutic assaults, also call on more power from their cellular engines, the mitochondria. In this issue of Blood, Qin et al1 demonstrate that myeloma survival and drug resistance involve a novel role for mitochondrial protein quality control (distinct from the ubiquitin-proteasome pathway) through upregulation of the caseinolytic protease P subunit (CLPP) and demonstrate the antimyeloma efficacy of specifically targeting mitochondrial biology through CLPP inhibition.
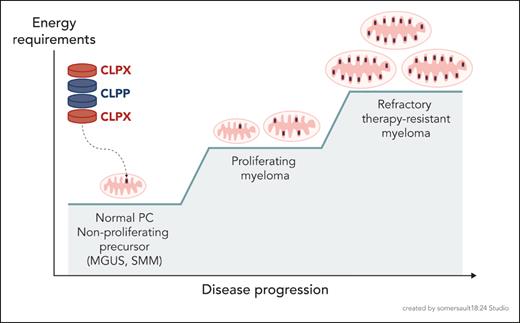
From the perspective that normal plasma cell (PC) biology is the core of myeloma biology,2 it is well established that normal PCs have very high metabolic activity in synthesizing thousands of antibody molecules per second, as evidenced by the high rate of glucose uptake for antibody glycosylation and the essential requirement for induction of the unfolded protein response (UPR) to support this biosynthesis. This activity is critically supported by increased energy production from PC mitochondria through a switch to oxidative phosphorylation of long chain fatty acids3 (as do myeloma cells4), which generates more adenosine triphosphate (ATP) molecules per fuel molecule. However, normal PCs do not proliferate, and myeloma cells do while retaining the energy demands of making the monoclonal antibody. So where do myeloma cells find the additional power to proliferate and with progression to also survive the stress of therapeutic attack? Given the reliance of normal PCs on mitochondria for power, one would predict that malignant transformation to proliferating and treatment-resistant myeloma requires the evolution of more or better mitochondria (see figure). Interestingly, in the myeloma precursor states (monoclonal gammopathy of unknown significance [MGUS], smoldering myeloma [SMM]), the oncogenic events have already occurred, yet these transformed cells do not proliferate, perhaps in part because they have not yet found the additional energy to do so. Thus the ability to generate additional energy may represent the “third hit,” along with proliferation and survival, that is essential for myeloma disease progression. This is consistent with observations that progression from normal PC→MGUS→SMM→MM is significantly associated with increases in mitochondrial DNA and biogenesis signatures,5,6 which has also been seen for other malignancies such as acute myeloid leukemia (AML).7,8 But in addition to more mitochondria, Qin et al and others suggest there are also better mitochondria. What then makes mitochondria “better”?
Evolution of more and better mitochondria to support the increasing energy requirements of MM disease progression.
Evolution of more and better mitochondria to support the increasing energy requirements of MM disease progression.
It would be predicted that “better” mitochondria run at higher metabolic levels, and this would require robust homeostatic mechanisms that increase the baseline tolerances to allow for this. Given the extraordinarily tight integration of protein enzymes in multiple metabolic cycles with the biosynthetic and ATP-generating components of the mitochondria, coordinated enzymatic activity requires equally tight regulation of enzyme concentrations involved, which then involve precise regulation of these enzyme pools through both of the influx and efflux (including degradation) of these proteins.
Qin et al have identified the mitochondrial protease CLPP as a critical component of the efflux arm of mitochondrial protein quality control in myeloma. The CLPP subunit forms 2 stacked heptameric rings that interact with the hexameric ATPases CLPB and CLPX (which facilitate protein unwinding) to form the CLP endopeptidase in a barrel-like structure reminiscent of the proteasome. The CLP endopeptidase is found in the mitochondrial matrix, and in AML it was found that most of the CLPP substrates were components of the respiratory chain or enzymes involved in mitochondrial metabolism.7 What role CLPP plays in myeloma biology is largely unknown, and Qin and colleagues now report that CLPP expression increases with disease progression, although interestingly this was statistically significant only when comparing active (proliferating) myeloma to nonproliferating precursor states (normal PC, MGUS, SMM). High CLPP expression was an adverse prognostic marker, associated with increased disease burden and inferior progression-free and overall survival in patients with newly diagnosed myeloma. Biologically, CLPP inhibition and knockdown caused an increase in reactive oxygen species and decreased mitochondrial membrane potential, resulting in cell cycle arrest and apoptosis in myeloma cells (include drug-resistant lines). CLPP inhibition also caused increased autophagy (likely a compensatory survival response) and mitophagy as evidenced by decreases in electron transport chain components, mitochondrial ATP production, and intracellular ATP levels. Inhibition of CLPP also disrupted mitochondrial metabolism by depleting metabolites in the glycolytic, tricarboxylic acid, and pentose phosphate pathways (PPPs). This translated in vitro to synergy in combination with inhibitors of autophagy, glycolysis and PPPs, and proteasome activity. In vivo, a direct CLPP inhibitor (the β-lactone A2-32-01) had significant in vivo anti-MM efficacy in a xenograft model. However, since A2-32-01 is rapidly hydrolyzed and not translatable to the clinic, the authors took a novel alternative approach based on their insightful observation that the CLPP, CLPB, and CLPX promoters contained multiple E1A-binding protein p300/CBP consensus sites and found that the EP300/CBP bromodomain inhibitor CCS-1477 (inobrodib, which is in phase I and II clinical trials) downregulates CLPP and CLPX expression and decreases myeloma cell viability.
Altogether, this study uncovers previously undefined key role for CLPP in the biology of transformed PCs, and demonstrates the effectiveness of specifically targeting mitochondrial biology in myeloma. The dependence of myeloma cells on high-functioning mitochondria may explain the therapeutic index for CLPP inhibitors, similar to myeloma sensitivity to proteasome inhibitors due to their high level of antibody synthesis. Additionally, inhibiting CLPP may not just be causing a mitochondrial UPR response leading to mitochondrial dysfunction or demise, but may also be disrupting the maintenance of tightly balanced enzyme pools required for integrity of mitochondrial metabolism. This might explain why hyperactivating CLPP activity also causes mitochondrial dysfunction and cell death in AML8 and myeloma9 and is consistent with other studies showing perturbation of mitochondrial proteostasis has antimyeloma activity.10 A need for tight regulation of mitochondrial protein quality control raises an important unanswered question of how CLPP expression and CLP endopeptidase activity are regulated, and as the initial inobrodib studies show, these answers may lead to previously unexplored therapeutic approaches . . . to boldly go where no one has gone before.
Conflict-of-interest disclosure: K.L. reports no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal