In this issue of Blood, Koehn et al demonstrate that VISTA (V-type immunoglobulin domain-containing suppressor of T-cell activation) is briefly upregulated on donor T cells after allogeneic hematopoietic stem cell transplantation (HSCT), and its pinpoint targeting by anti-VISTA monoclonal antibody (mAb) selectively depletes alloreactive donor T cells, suppressing graft-versus-host disease (GVHD) while preserving bystander T cells and the graft-versus-leukemia (GVL) effect in mice.1
T-cell function is dependent upon a variety of inducible structures that have stimulatory or inhibitory effect. VISTA (also known as PD-1H) is an immune checkpoint molecule with homology to programmed death-1 (PD-1) and negatively regulates T-cell function.2 Among all other negative checkpoint regulators, VISTA, expressed on naive T cells, is the earliest checkpoint regulator critical for steady-state maintenance of peripheral tolerance3 (see figure). In contrast, cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibits T-cell activation at the priming stage, whereas PD-1 inhibits T cells at the effector stage.
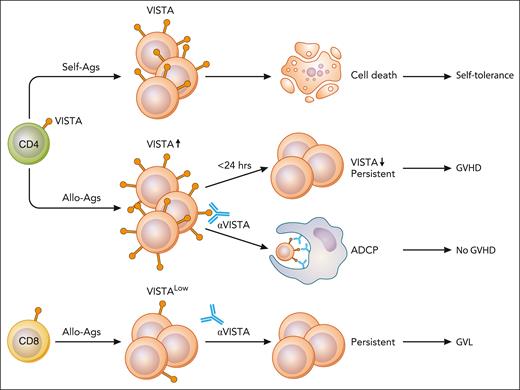
Self-antigen (self-Ags) stimulation induces VISTA expression on CD4+ T cells and induces self-tolerance.3 Alloantigen (allo-Ags) stimulation induces transient expression of VISTA, which is lost by 24 hours and induces GVHD. Anti-VISTA mAb given during this 24-hour time window deletes alloreactive T cells via antigen-dependent cellular phagocytosis (ADCP). CD8+ T cells are spared, and GVL is preserved. Illustration from NIAID NIH BIOART Source (bioart.niaid.nih.gov/bioart/). Professional illustration by Patrick Lane, ScEYEnce Studios.
Self-antigen (self-Ags) stimulation induces VISTA expression on CD4+ T cells and induces self-tolerance.3 Alloantigen (allo-Ags) stimulation induces transient expression of VISTA, which is lost by 24 hours and induces GVHD. Anti-VISTA mAb given during this 24-hour time window deletes alloreactive T cells via antigen-dependent cellular phagocytosis (ADCP). CD8+ T cells are spared, and GVL is preserved. Illustration from NIAID NIH BIOART Source (bioart.niaid.nih.gov/bioart/). Professional illustration by Patrick Lane, ScEYEnce Studios.
In allogeneic HSCT, initial studies showed that a PD-1H/VISTA agonistic mAb inhibited donor T-cell activation and suppressed GVHD.4,5 The study by Koehn and colleagues demonstrated a novel mechanism of VISTA targeting-mediated suppression of GVHD. A key observation is that VISTA is upregulated on CD4+ T cells rapidly after allogeneic HSCT, but then is lost by 24 hours. In a murine model, anti-VISTA mAb (8G8) was given during this 24-hour time window, resulting in a profound depletion of alloreactive donor CD4+ T cells via Fc receptor (FcR)-dependent phagocytosis (see figure). Thus, alloantigen stimulation via T-cell receptor (TCR) briefly upregulates VISTA expression on CD4+ T cells and makes them vulnerable to anti-VISTA-mediated deletion at that time. Importantly, VISTA-mediated depletion was more profound in alloreactive T cells with upregulated VISTA expression than in bystander T cells with low VISTA expression.
Selective depletion of alloreactive T cells by anti-VISTA mAb is reminiscent of posttransplant high-dose cyclophosphamide (PTCy), which also selectively eliminates alloreactive donor T cells, while preserving bystander T cells. PTCy-induced deletion of alloreactive T cells is inhibited by calcineurin inhibitor (CI) given before PTCy because CI inhibits the activation of alloreactive T cells.6 This is the rationale for delayed administration of CI after PTCy. The same appears to be true for VISTA. Peritransplant administration of CI blunted VISTA upregulation after TCR engagement and abrogated the tolerogenic effects of anti-VISTA mAb. Therefore, it is reasonable to combine anti-VISTA mAb with delayed administration of CI or use the anti-VISTA mAb with rapamycin, which does not interfere with calcineurin pathway.
However, there is a significant difference in the targets of GVHD prophylaxis using PTCy vs anti-VISTA therapy. PTCy targets both CD4+ and CD8+ T cells, while anti-VISTA therapy exclusively targets CD4+ T cells. Recent studies investigating the leukemia immune microenvironment have shown that a cytotoxic CD8+ T-cell signature in the bone marrow is associated with continuing remission after allogeneic HSCT in patients with acute myelogenous leukemia (AML).7 Therefore, anti-VISTA treatment that spares CD8+ T cells could maintain GVL effects, representing a promising strategy for the separation of GVL from GVHD.
Thus, anti-VISTA therapy has many features suggesting that it should be evaluated clinically; however, many clinical questions remain. Particularly, the kinetics of VISTA expression in both CD4+ and CD8+ donor T cells in human HSCT needs to be clarified to determine the timing of administration and to confirm selective depletion of CD4+ T cells while sparing CD8+ T cells responsible for GVL. This is technically a difficult task. It is also unclear whether FcR-dependent phagocytic activity is preserved immediately after myeloablative conditioning in humans. Since VISTA is expressed predominantly within hematopoietic compartment with highest expression within the myeloid lineages,2 donor cell engraftment needs to be monitored closely. Finally, human AML cells highly express VISTA, which helps explain immune evasion in AML.8 Blocking cell surface VISTA on AML cells by anti-VISTA blocking mAb should also inhibit AML progression by promoting T-cell activity. Taken together, targeting VISTA to block cellular signaling or to deplete cells represents a novel and promising strategy for leukemia immunotherapy.
Conflict-of-interest disclosure: T.T. declares consultancy for Kyowa Kirin, Meiji Seika Pharma, Takeda, Roche Diagnostics, and Nippon Shinyaku; honoraria for Nippon Shinyaku, Daiichi Sankyo, Novartis, Otsuka, Genmab, Janssen, Pfizer, Kyowa Kirin, Asahi Kasei Pharma, Meiji Seika Pharma, Takeda, Gilead, Symbio, MSD, Bristol Myers Squibb, Sanofi, Nippon Kayaku, AbbVie, Chugai, AstraZeneca, and Astellas; and research funding from Daiichi Sankyo, Otsuka, Kyowa Kirin, Asahi Kasei Pharma, LUCA Science, Pharma Essentia Japan, Sumitomo Pharma, JCR Pharma, Chugai, and Astellas.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal