In this issue of Blood, Radpour and colleagues uncover a novel mechanism by which leukemia stem cells (LSCs) hijack T helper (Th) cells in the bone marrow of patients with acute myeloid leukemia (AML) to promote their own expansion and contribute to disease progression.1
AML is a clonal hematological malignancy with a heterogeneous cytogenetic and molecular profile that manifests in the abnormal expansion of undifferentiated hematopoietic stem cells and myeloid progenitors.2 Relapse after successful induction chemotherapy remains a major challenge for the management of patients with AML, which is believed to be mediated by therapy-resistant LSCs.3 These undifferentiated plastic cells endowed with a strong capacity for self-renewal are considered to be phylogenetic precursors for AML subclones and reside in low abundance in the bone marrow of patients with AML.4 LSCs have been reported to achieve immune evasion via downregulation of ligands that induce cytotoxicity in lymphocytes or via localization within the regulatory T-cell–rich microenvironment.5,6 Thus, understanding the cellular communication networks in the bone marrow that promote LSC expansion and concomitant immune escape may be key to overcoming the cumbersome treatment resistance seen in people with AML.
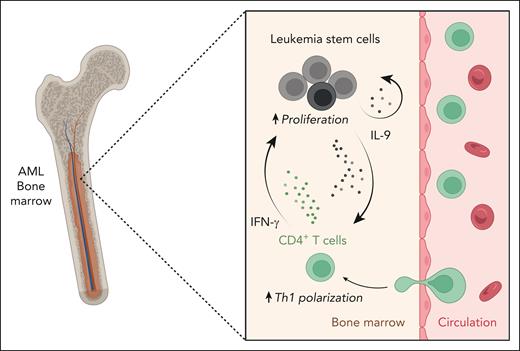
In their work, Radpour et al combined transcriptomic profiling and functional assays to dissect the communication networks in the bone marrow niche of patients with newly diagnosed AML and healthy controls. In stark contrast to the immunologically inactive cytotoxic T-cell compartment of patients with AML, the authors report overt activation and proliferation of Th cells with a pronounced skewing toward Th1 polarization. Using a correlation network analysis and functional validations, the authors demonstrate that the observed Th-cell phenotype could be induced by interleukin-9 (IL-9). The pleiotropic cytokine IL-9 is virtually absent in the bone marrow of healthy individuals. However, in the bone marrow niche of people with AML, the LSCs secrete IL-9, which has been previously reported to stimulate proliferation of LSCs in an autocrine loop.7 In the present work, Radpour and colleagues report a paracrine Th-cell–mediated signaling loop (see figure). They convincingly demonstrate that LSC-derived IL-9 activates CD4+ T cells and induces Th1 polarization via JAK-STAT signaling and concomitant histone H3 methylation that could lead to the epigenetic activation of the T-bet promoter region. These polarized Th1 cells in the bone marrow of patients with AML secrete interferon-γ and tumor necrosis factor, which in turn induces the expansion of LSCs. In addition to the documented autocrine feedback loop of IL-9 in promoting LSC proliferation, the authors thereby reveal a paracrine pro-tumorigenic mechanism of IL-9, in which LSCs hijack CD4+ T cells in the bone marrow niche of patients with AML to promote their own survival and expansion. Last, the authors show that IL-9 expression in LSCs and IL-9 receptor expression as well as tumor necrosis factor and interferon-γ expression in Th cells correlate with reduced overall survival in patients with AML. The authors thereby validate the relevance of the identified IL-9–dependent paracrine communication circuit in promoting disease progression.
IL-9 secretion by LSCs leads to Th1 polarization of bone marrow–infiltrating CD4+ T cells, which in turn stimulates the proliferation of LSCs in patients with AML. In the bone marrow of patients with AML, LSCs secrete IL-9, which skews the Th-cell profile of infiltrating CD4+ T cells toward a Th1 response. These polarized Th1 cells secrete INF-γ and stimulate the proliferation of AML LSCs in the bone marrow, which could contribute to disease progression. IFN-γ, interferon-γ. The figure was created with BioRender.com.
IL-9 secretion by LSCs leads to Th1 polarization of bone marrow–infiltrating CD4+ T cells, which in turn stimulates the proliferation of LSCs in patients with AML. In the bone marrow of patients with AML, LSCs secrete IL-9, which skews the Th-cell profile of infiltrating CD4+ T cells toward a Th1 response. These polarized Th1 cells secrete INF-γ and stimulate the proliferation of AML LSCs in the bone marrow, which could contribute to disease progression. IFN-γ, interferon-γ. The figure was created with BioRender.com.
Radpour et al showcase in an elegant way how tumor-immune communication circuits can be mechanistically disentangled in primary human patient samples and highlight the often counterintuitive and pleiotropic effect of cytokines in the tumor microenvironment. Although the largely enigmatic cytokine IL-9 has been implicated in promoting antitumor immunity in the context of solid tumors, hematological malignancies, particularly AML, appear to thrive in the presence of IL-9.8 Therapeutic anti–IL-9 monoclonal antibodies have been evaluated in clinical trials for the treatment of patients with asthma and demonstrated a favorable safety profile.9 Radpour and colleagues emphasize the importance of IL-9 signaling in promoting disease progression in AML and raise the important question about the extent to which therapeutic blockade of IL-9 could be used to circumvent relapses in patients with AML.
Conflict-of-interest disclosure: F.I. declares no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal