In this issue of Blood, Chiang et al evaluate the performance of a novel in vitro assay to quantify cytotoxic lymphocyte (CTL) exocytosis, an immunologic function that is defective in certain subtypes of primary (ie, familial) hemophagocytic lymphohistiocytosis (pHLH). Their analysis supports further exploration as to how clinical laboratories might integrate this assay into the diagnostic workup for patients with suspected pHLH.1
pHLH is a hyperinflammatory disorder caused by biallelic loss-of-function mutations in genes required for CD8 T-cell and natural killer (NK)-cell cytotoxicity. These cytotoxic effectors eliminate infected or malignant cells by releasing lytic granules containing perforin and granzymes at the site of target cell contact. Perforin forms a pore on the target cell membrane, enabling entry of granzymes, which then mediate target cell apoptosis.2 Many of the genes mutated in pHLH encode cytoplasmic proteins necessary for the assembly, transport, and release of lytic granules. Accordingly, patients with pHLH exhibit impaired lymphocyte exocytosis, resulting in reduced or absent cytotoxicity. In these individuals, immune cells become activated in response to various triggers, but they are unable to eradicate the trigger, resulting in persistent immune cell stimulation.3
HLH is most commonly diagnosed using the HLH-2004 diagnostic criteria, which consist of a constellation of clinical and laboratory findings, one of which is functional impairment in NK-cell cytotoxicity.4 Historically, an in vitro assay based on chromium-51 release has been the gold standard to measure NK-cell cytotoxicity. In this assay, cells from the K562 human leukemia cell line are labeled with radioactive chromium-51 and cocultured with NK cells. NK cells with intact cytotoxic function induce K562 apoptosis, releasing chromium-51 into the supernatant, where the radioactivity can be quantified. When K562 cells are cocultured with NK cells with impaired cytotoxicity, chromium release is reduced or absent.5 Although this assay can effectively identify patients with pHLH, it has a number of caveats. In particular, its reliance on radioactivity poses safety concerns for personnel, and as such, this assay is unavailable at many institutions. Further, the assay requires a relatively large number of NK cells, which may be challenging to obtain in young infants, individuals with severe hyperinflammation, or patients who have received immunosuppressive therapy. These factors can decrease the frequency of NK cells in the peripheral blood and lead to false-positive results.6
As an alternative, Chiang et al evaluated the utility of quantifying CTL exocytosis. Exocytosis assays are known to have similar sensitivity and specificity relative to cytotoxicity assays, but do not use radioactivity and require a smaller number of lymphocytes.6 These assays are traditionally performed by coculturing NK cells with K562 target cells and measuring the surface expression of CD107a, a protein on the lytic granule inner membrane. During exocytosis, lytic granules fuse with the plasma membrane exposing CD107a on the surface of the lymphocyte,7 where it can be detected via flow cytometry. Lymphocyte CD107a expression can thus be used as a surrogate of cytotoxic function.
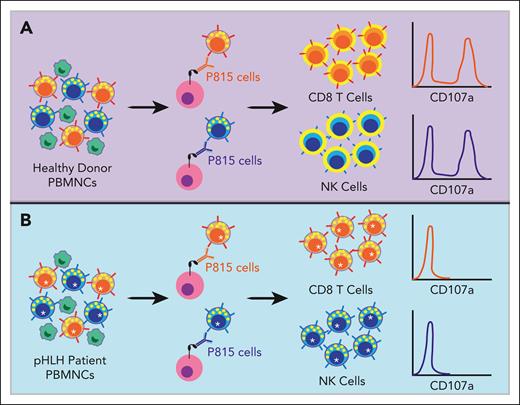
In the present article, Chiang et al compared the standard K562 exocytosis assay with a similar assay involving the P815 murine mastocytoma cell line. P815 cells express fragment crystallizable gamma receptor II (FcγRII) on their surface, which binds the Fc portion of murine immunoglobulin G antibodies. In this assay, P815 cells are cultured with antibodies against CD3 or CD16 and peripheral blood mononuclear cells (PBMNCs) from patients with suspected pHLH. The Fc portion of the antibodies binds the Fc receptors on P815 cells, leaving the epitope-specific portion free to engage the T-cell receptor on CD8 T cells or CD16 on NK cells, respectively. This so-called redirected cytotoxicity assay activates the CTLs, leading to their degranulation and translocation of CD107a to the cell surface (see figure).8 When comparing the P815 with the K562-induced degranulation assay, the authors found superior performance of the P815 assay with decreased inter- and intraindividual variability across cells from healthy donors. The P815 assay was also less subject to variability caused by decreased numbers of CTLs within PBMNCs or delays in sample transport. When the authors analyzed cells from healthy donors and patients with pHLH, they found higher sensitivity, specificity, and overall accuracy for the P815 assay relative to the K562 assay. Further, when the CD8 T- and NK-cell P815 exocytosis assays were combined, the accuracy in detecting pHLH was highest at 99.3%. Based on these findings, the authors advocate for replacing the K562 assay with the more accurate P815 assay for the diagnosis of patients with suspected pHLH or other primary immunodeficiencies associated with defective lymphocyte exocytosis.
Schematic of the P815 exocytosis assay. The P815 assay is performed by coculturing patient-derived PBMNCs with P815 cells and anti-CD3 or anti-CD16 antibodies. The Fc portion of the antibodies binds the FcγRII on the P815 cells. The epitope-specific portion of the anti-CD3 antibody then binds to the T-cell receptor on CD8 T cells, and the anti-CD16 antibody binds to CD16 on NK cells. This serves to “redirect” the CTL toward the P815 target cell. In PBMNCs from healthy donors with intact lymphocyte exocytosis (A), this process causes the CD8 T and NK cells to degranulate, resulting in lytic granule fusion with the lymphocyte plasma cell membrane and translocation of CD107a protein from the inner membrane of the lytic granule to the surface of the lymphocyte, where it can be detected via flow cytometry. In contrast, PBMNCs from patients with pHLH (B) harbor loss-of-function mutations in genes required for lymphocyte exocytosis (denoted with an asterisk). Although these pHLH CD8 T and NK cells are activated by the anti-CD3 and -16 antibodies, respectively, they are unable to effectively degranulate and CD107a is not detected on the cell surface.
Schematic of the P815 exocytosis assay. The P815 assay is performed by coculturing patient-derived PBMNCs with P815 cells and anti-CD3 or anti-CD16 antibodies. The Fc portion of the antibodies binds the FcγRII on the P815 cells. The epitope-specific portion of the anti-CD3 antibody then binds to the T-cell receptor on CD8 T cells, and the anti-CD16 antibody binds to CD16 on NK cells. This serves to “redirect” the CTL toward the P815 target cell. In PBMNCs from healthy donors with intact lymphocyte exocytosis (A), this process causes the CD8 T and NK cells to degranulate, resulting in lytic granule fusion with the lymphocyte plasma cell membrane and translocation of CD107a protein from the inner membrane of the lytic granule to the surface of the lymphocyte, where it can be detected via flow cytometry. In contrast, PBMNCs from patients with pHLH (B) harbor loss-of-function mutations in genes required for lymphocyte exocytosis (denoted with an asterisk). Although these pHLH CD8 T and NK cells are activated by the anti-CD3 and -16 antibodies, respectively, they are unable to effectively degranulate and CD107a is not detected on the cell surface.
Prompt and accurate diagnosis of HLH allows for early initiation of therapy, which decreases morbidity and mortality.9 Functional analysis of CTLs is an important component of the diagnostic workup, but existing assays pose significant logistical limitations. Chiang et al provide compelling data regarding the utility of the P815 cell assay for measuring CD8 T- and NK-cell exocytosis, thus circumventing some of the existing challenges. However, several key questions remain. In particular, although these assays do not rely on radioactivity, the flow cytometry-based readout necessitates technical expertise that may not exist in many clinical laboratories. In the current article, the assay was performed on fresh blood following overnight shipment. Thus, it will be important to determine whether the assay can be reliably performed on older samples or those in which cells have been viably frozen. The introduction of new clinical tests is also costly and time-consuming and requires establishing reference ranges and standardization across laboratories. Together, these factors may pose obstacles to widespread implementation. Finally, additional studies are needed to evaluate how hyperinflammation or exposure to immunosuppressive therapy might impact assay performance and whether or how the resulting data should be integrated into existing HLH diagnostic criteria.
In summary, the work by Chiang et al represents an important first step toward the validation and implementation of the P815-based assay of CD8 T- and NK-cell functional activity for the diagnosis of patients with pHLH. Further studies to prospectively evaluate the performance of the P815 assay and navigate the logistical barriers to implementation will facilitate its future clinical utility.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal