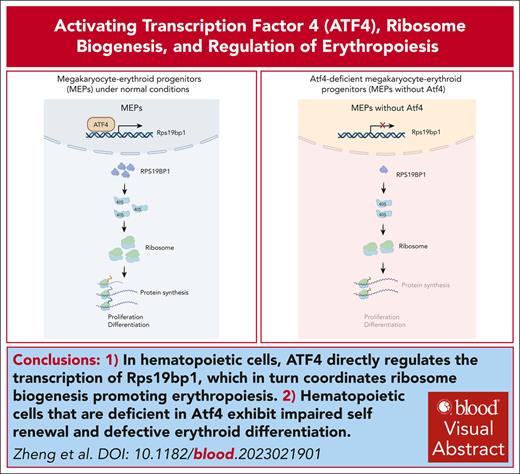
Key Points
Atf4–deficient hematopoietic cells exhibited hematopoietic stem cells function and erythroid differentiation defects.
ATF4 directly regulates the transcription of Rps19bp1 to coordinate ribosome biogenesis and promote erythropoiesis.
Visual Abstract
Hematopoietic differentiation is controlled by intrinsic regulators and the extrinsic hematopoietic niche. Activating transcription factor 4 (ATF4) plays a crucial role in the function of fetal and adult hematopoietic stem cell maintenance. However, the precise function of ATF4 in the bone marrow (BM) niche and the mechanism by which ATF4 regulates adult hematopoiesis remain largely unknown. Here, we used 4 cell-type-specific mouse Cre lines to achieve conditional knockout of Atf4 in Cdh5+ endothelial cells, Prx1+ BM stromal cells, Osx+ osteoprogenitor cells, and Mx1+ hematopoietic cells and uncovered the role of Atf4 in niche cells and hematopoiesis. Intriguingly, depletion of Atf4 in niche cells did not affect hematopoiesis; however, Atf4-deficient hematopoietic cells exhibited erythroid differentiation defects, leading to hypoplastic anemia. Mechanistically, ATF4 mediated direct regulation of Rps19bp1 transcription, which is, in turn, involved in 40 S ribosomal subunit assembly to coordinate ribosome biogenesis and promote erythropoiesis. Finally, we demonstrate that under conditions of 5-fluorouracil–induced stress, Atf4 depletion impedes the recovery of hematopoietic lineages, which requires efficient ribosome biogenesis. Taken together, our findings highlight the indispensable role of the ATF4-RPS19BP1 axis in the regulation of erythropoiesis.
Introduction
The production of mature blood and immune cells is maintained by a rare population of hematopoietic stem cells (HSCs) located in the bone marrow (BM).1 HSC function and hematopoiesis are governed by the complex interplay between extrinsic signals from the microenvironment and intrinsic programs encompassing transcription factors (TFs), noncoding RNAs, and epigenetic modifications.2,3 Furthermore, the HSC niche formed by various populations, such as mesenchymal stromal cells (MSCs), endothelial cells (ECs), and osteoblasts, provides crucial signals and interactions that directly regulate HSC functions.4-9 Dysregulation of this process can lead to severe hematopoietic failure and/or hematologic malignancy.
Erythropoiesis encompasses 2 primary phases. In the early phase, HSCs differentiate to generate erythroid progenitors.10 This is followed by erythroid terminal differentiation, which involves a series of morphological and biochemical changes in erythroblasts that culminate in the production of functional red blood cells (RBCs). Critical TFs and other regulatory factors contribute collaboratively to this process.2,11-15 Investigations of the signals and regulatory networks governing erythropoiesis are required to elucidate the biology of erythroid cells and to identify potential therapeutic approaches to erythroid-related disorders.
Activating transcription factor 4 (ATF4) is induced by signals such as endoplasmic reticulum stress and oxidative stress.16 Studies in the Atf4-knockout mouse model have demonstrated that ATF4 plays a pivotal role in multiple biological processes, including fetal liver hematopoiesis, HSC maintenance, bone formation, and tumorigenesis.17-21 ATF4 depletion induces partial perinatal lethality and impaired hematopoiesis in the fetal liver, resulting in severe anemia and abnormal erythropoiesis in E15.5 mouse embryos.21 We previously showed that ATF4 functions in a cell-extrinsic manner to mediate HSC expansion and maintenance in murine fetal liver by upregulating Angptl3 in niche cells.17 Sun et al also reported the role of ATF4 in the regulation of adult HSC aging.19 Atf4 has also been shown to regulate erythropoiesis via the HRI-elF2aP-ATF4 axis.22 This signaling pathway is essential for terminal erythropoiesis under conditions such as iron/heme deficiency, environmental stresses (eg, oxidative stress), and pathological conditions of β-thalassemia.22-24 ATF4 is highly expressed in proerythroblasts and basophilic erythroblasts.25 During iron deficiency, ATF4-target genes are highly activated to maintain mitochondrial function, redox homeostasis, and facilitate erythroid differentiation.23 Additionally, the HRI-elF2aP-ATF4 axis is involved in controlling the expression of fetal globin through the regulation of MYB or BCL11A.26,27 These observations highlight the pleiotropic role of ATF4 in stress-induced erythropoiesis. Intriguingly, Atf4–/– embryos also develop transient fetal anemia even under iron sufficiency, suggesting a potential role in erythroid development.17 Thus, in this study, we investigated whether ATF4 regulates adult HSC function and erythropoiesis at a steady state as well as the underlying mechanisms.
Methods
Mice
Atf4fl/fl mice were generated by Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China. Cdh5-CreER mice were generated by the Biocytogen Co Ltd (Beijing, China). C57BL/6, B6.SJL, Col2.3-GFP, and β-actin-GFP mice (aged 6-8 weeks) were maintained in the animal facility of the State Key Laboratory of Experimental Hematology (SKLEH; Tianjin, China). All animal experimental protocols were approved by the institutional animal care and use committee of SKLEH.
Colony formation assay
Murine BM cells or sorted Lin–cKit+Sca-1+ (LKS+) cells were cultured in M3434 medium (Stemcell Technologies) for 7 to 10 days to generate BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-GEMM colonies. Murine BM, cKit+, or megakaryocyte-erythroid progenitor (MEP) cells were cultured in M3334 medium (Stemcell Technologies) for 48 hours to generate CFU-E colonies or in M3436 medium (Stemcell Technologies) for 10 to 14 days to generate BFU-E colonies.
BM transplantation
Competitive BM transplantation assay
BM cells (1.5 × 106) from conditional knockout or wild-type (WT) mice (aged 8 weeks) were transplanted with CD45.1+ competitive BM cells (1 × 106) into lethally irradiated CD45.1+ recipients.
Secondary transplantation experiment
BM cells (1.5 × 106) from the primary recipients were transplanted into lethally irradiated CD45.1+ recipients.
Reciprocal transplantation experiment
CD45.1+ BM cells (1 × 106) were transplanted into CD45.2+ conditional knockout or WT mice.
In each experiment, peripheral blood reconstitution was monitored every 4 weeks.
Statistical analysis
All data are presented as the means obtained from 3 independent biological experiments ± standard deviation. The 2 groups were compared using an unpaired 2-tailed Student t test. Multiple groups were compared using analysis of variance with Bonferroni correction. P value <.05 were considered to indicate statistical significance. Statistical analyses and graphs were generated using GraphPad Prism v8.0 software.
Protocol approval and additional methods
The protocols used for animal experiments were approved by the Institutional Animal Care and Use Committee of SKLEH. Additional methods are provided in the supplemental Methods, available on the Blood website.
Results
ATF4 depletion in MSCs does not affect hematopoiesis
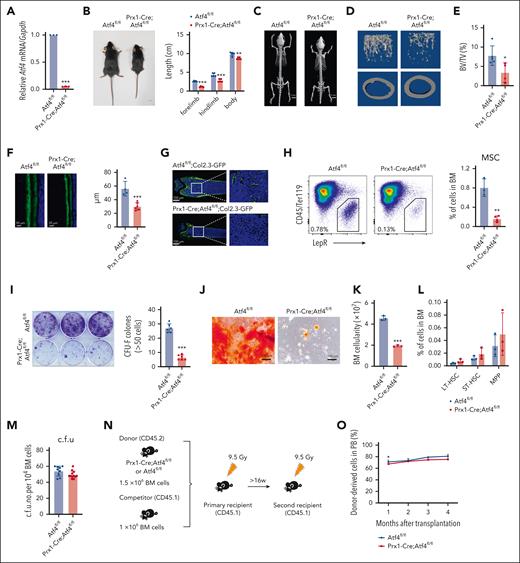
To investigate whether depletion of Atf4 in niche cells affects hematopoiesis, we crossed Atf4fl/fl mice (fl/fl) with Prx1-Cre mice28 (supplemental Figure 1A). The Atf4 knockout efficiency (95%) was validated using quantitative real-time polymerase chain reaction (qRT-PCR) (Figure 1A). ThePrx1-Cre;Atf4fl/fl mice had shortened limbs and a reduced body size (Figure 1B). Microcomputed tomography confirmed limb defects and revealed a lower trabecular bone number, volume, and mineral density in Prx1-Cre;Atf4fl/fl mice (Figures 1C-E; supplemental Figure 1B). The bone formation rate was also reduced after Atf4 deletion in the MSCs (Figure 1F). These results were further confirmed in Prx1-Cre;Atf4fl/fl;Col2.3-GFP mice (Figure 1G). Moreover, Atf4 deletion reduced BM MSC numbers (Figure 1H) and impaired their CFU-F activity (Figure 1I), as well as differentiation toward osteoblasts and adipocytes (Figure 1J; supplemental Figure 1C). These findings indicated that ATF4 is required for MSC function and differentiation.
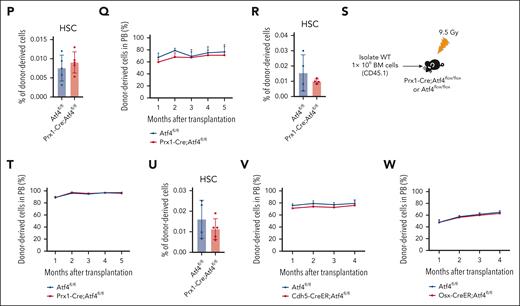
Atf4 deficiency alters MSC function but not HSC maintenance. (A) qRT-PCR analysis of Atf4 expression in CD45–Ter119–LepR+ cells from Prx1-Cre;Atf4fl/fl and Atf4fl/fl mice (n = 3 mice). (B) Gross phenotype of Atf4fl/fl and Prx1-Cre;Atf4fl/fl mice at 7 weeks of age (left) and body, forelimb, and hindlimb length measurements (right). ∗∗∗P < .001; ∗∗P < .01; n = 5. (C) Microcomputed tomography (micro-CT) image showing the limb phenotype of Atf4-mutant vs WT mice. (D-E) Micro-CT analysis showing decreased bone volume fraction in Atf4 mutants; BV/TV, bone volume per total volume (n = 5 mice). (F) Representative calcein double-labeling images (left) with quantification of trabecular bone formation in the femur metaphysis (right) (n = 4-6 mice). (G) Representative femur sections from Prx1-Cre;Atf4fl/fl;Col2.3-GFP and Atf4fl/fl;Col2.3-GFP mice showing the decrease in the number of Col2.3-GFP+ osteoblasts (n = 3 mice). (H) Representative flow cytometry dot plots of CD45–Ter119–LepR+ cells (left) and corresponding quantitative data showing the decreased frequency of CD45–Ter119–LepR+ cells in Prx1-Cre;Atf4fl/fl mice at 7 weeks of age (right) (n = 3-4 mice). (I) Representative images of CFU-F colonies formed when cells were cultured from enzymatically dissociated BM (left) and corresponding quantitative data (right); n = 3 mice from at least 3 independent experiments. (J) Representative images of CFU-F differentiating into osteoblasts after culture in differentiation medium for 2 to 3 weeks, showing the reduced osteogenic potential of Atf4-depleted MSCs. (K) BM cell numbers of Prx1-Cre;Atf4fl/fl and Atf4fl/fl mice (n = 3 mice). (L) Percentages of the indicated cell populations in Prx1-Cre;Atf4fl/fl and Atf4fl/fl mice BM (n = 3 mice). (M) Results from colony formation assays using 104 BM cells cultured for 10 to 14 days in a complete methylcellulose-based medium (n = 3 mice). (N) Experimental workflow of competitive transplantation. (O) Percentage of CD45.2+ donor cells in the peripheral blood (PB) of recipient mice at the indicated time points after competitive BM transplantation (n = 4-8 mice). (P) Percentage of CD45.2+ donor-derived HSCs in the BM of recipients (n = 5 mice). (Q) Percentage of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after secondary BM transplantation (n = 4-7 mice). (R) Percentage of CD45.2+ donor-derived HSCs in the BM of recipients after the secondary BM transplantation (n = 4 mice). (S) Experimental workflow of the assay in which 106 BM cells were transplanted from donor mice (CD45.1) into irradiated Prx1-Cre;Atf4fl/fl or Atf4fl/fl mice. (T) Percentage of CD45.1+ donor cells in the PB of recipient mice at indicated time points after BM transplantation. The donor cells were CD45.1+ (n = 5 mice). (U) Percentage of CD45.1+ donor-derived HSCs in the BM of recipients (n = 4-5 mice). (V-W) Percentage of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after competitive BM transplantation (n = 4-6 mice). Data represent mean ± standard deviation (SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test.
Atf4 deficiency alters MSC function but not HSC maintenance. (A) qRT-PCR analysis of Atf4 expression in CD45–Ter119–LepR+ cells from Prx1-Cre;Atf4fl/fl and Atf4fl/fl mice (n = 3 mice). (B) Gross phenotype of Atf4fl/fl and Prx1-Cre;Atf4fl/fl mice at 7 weeks of age (left) and body, forelimb, and hindlimb length measurements (right). ∗∗∗P < .001; ∗∗P < .01; n = 5. (C) Microcomputed tomography (micro-CT) image showing the limb phenotype of Atf4-mutant vs WT mice. (D-E) Micro-CT analysis showing decreased bone volume fraction in Atf4 mutants; BV/TV, bone volume per total volume (n = 5 mice). (F) Representative calcein double-labeling images (left) with quantification of trabecular bone formation in the femur metaphysis (right) (n = 4-6 mice). (G) Representative femur sections from Prx1-Cre;Atf4fl/fl;Col2.3-GFP and Atf4fl/fl;Col2.3-GFP mice showing the decrease in the number of Col2.3-GFP+ osteoblasts (n = 3 mice). (H) Representative flow cytometry dot plots of CD45–Ter119–LepR+ cells (left) and corresponding quantitative data showing the decreased frequency of CD45–Ter119–LepR+ cells in Prx1-Cre;Atf4fl/fl mice at 7 weeks of age (right) (n = 3-4 mice). (I) Representative images of CFU-F colonies formed when cells were cultured from enzymatically dissociated BM (left) and corresponding quantitative data (right); n = 3 mice from at least 3 independent experiments. (J) Representative images of CFU-F differentiating into osteoblasts after culture in differentiation medium for 2 to 3 weeks, showing the reduced osteogenic potential of Atf4-depleted MSCs. (K) BM cell numbers of Prx1-Cre;Atf4fl/fl and Atf4fl/fl mice (n = 3 mice). (L) Percentages of the indicated cell populations in Prx1-Cre;Atf4fl/fl and Atf4fl/fl mice BM (n = 3 mice). (M) Results from colony formation assays using 104 BM cells cultured for 10 to 14 days in a complete methylcellulose-based medium (n = 3 mice). (N) Experimental workflow of competitive transplantation. (O) Percentage of CD45.2+ donor cells in the peripheral blood (PB) of recipient mice at the indicated time points after competitive BM transplantation (n = 4-8 mice). (P) Percentage of CD45.2+ donor-derived HSCs in the BM of recipients (n = 5 mice). (Q) Percentage of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after secondary BM transplantation (n = 4-7 mice). (R) Percentage of CD45.2+ donor-derived HSCs in the BM of recipients after the secondary BM transplantation (n = 4 mice). (S) Experimental workflow of the assay in which 106 BM cells were transplanted from donor mice (CD45.1) into irradiated Prx1-Cre;Atf4fl/fl or Atf4fl/fl mice. (T) Percentage of CD45.1+ donor cells in the PB of recipient mice at indicated time points after BM transplantation. The donor cells were CD45.1+ (n = 5 mice). (U) Percentage of CD45.1+ donor-derived HSCs in the BM of recipients (n = 4-5 mice). (V-W) Percentage of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after competitive BM transplantation (n = 4-6 mice). Data represent mean ± standard deviation (SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test.
We next explored the effects of MSC dysfunction induced by Atf4 depletion on BM hematopoiesis. Compared with littermate controls, Prx1-Cre;Atf4fl/fl mice had half the number of whole BM cells (Figure 1K), whereas the frequencies of hematopoietic stem and progenitor cells (HSPCs) and lineage cells were similar (Figure 1L; supplemental Figure 1D-E). Prx1-Cre;Atf4fl/fl mice also showed normal blood cell counts (supplemental Figure 1F). BM cells from the 2 groups yielded comparable numbers of colonies (Figure 1M) and competitive BM transplantation assays showed that repopulation ability, lineage regeneration, and HSC output were similar (Figure 1N-P; supplemental Figure 1G-K). MSC-specific Atf4 deletion affected neither HSC self-renewal in secondary transplantation assays (Figure 1Q-R) nor hematopoietic reconstitution, lineage regeneration, and HSC output in reciprocal transplantation (Figure 1S-U; supplemental Figure 1L-M). Moreover, Prx1-Cre;Atf4fl/fl mice showed normal hematopoiesis under 5-fluorouracil (5-FU) stress (supplemental Figure 1N-R). These findings indicate that Atf4 loss in MSCs spares hematopoiesis and HSC function in adult mice.
ATF4 depletion in ECs and osteoprogenitors does not affect hematopoiesis
To test whether Atf4 deletion in ECs and osteoprogenitors affects the adult BM niche and hematopoiesis, we generated Cdh5-CreER;Atf4fl/fl and Osx-CreER;Atf4fl/fl mice, respectively. Atf4 deletion did not affect the frequency of relevant niche cells (supplemental Figure 2A-B). Moreover, conditional knockout mice had normal blood cell counts (supplemental Figure 2C-D), BM HSPC frequencies (supplemental Figure 2E-F), and BM cell colony-forming abilities (supplemental Figure 2G-H). BM cells from conditional knockout mice and control mice also had similar levels of long-term multilineage reconstitution in competitive reconstitution assays (Figure 1V-W; supplemental Figure 2I-J). These results indicate that, unlike in the fetal liver, Atf4 depletion in MSCs, ECs, or osteoprogenitors of adult mice has a minor effect on BM hematopoiesis and HSC maintenance.
ATF4 depletion from hematopoietic cells impairs HSC self-renewal
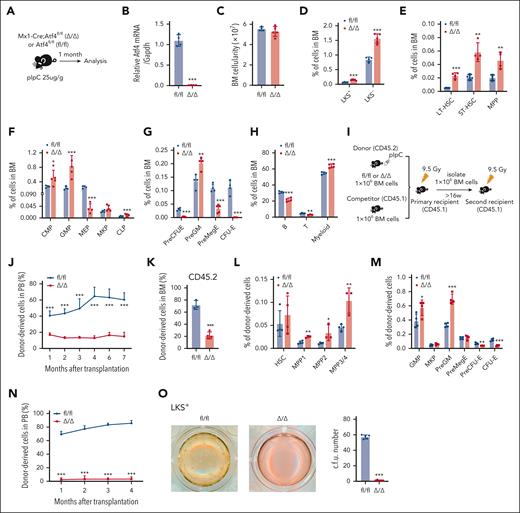
To investigate whether ATF4 regulates adult murine hematopoiesis and HSC function in a cell-intrinsic manner, we generated Mx1-Cre;Atf4fl/fl (Δ/Δ) mice. Atf4 deletion was induced by polyinosinic-polycytidylic acid (pIpC) treatment, and the mice were euthanized 1 month later (Figure 2A-B). Although there was no difference in the number of total nucleated BM cells between the 2 groups (Figure 2C), the frequencies of most HSPC subsets (including long-term HSC [LT-HSC], short-term HSC, multipotent progenitor [MPP], common myeloid progenitor [CMP], granulocyte-monocyte progenitor [GMP], and PreGM subsets) were increased, whereas those of erythroid progenitor cells (EPCs, including MEP, PreCFU-E, and CFU-E cells) were decreased in Δ/Δ mice (Figure 2D-G; supplemental Figure 3A). Furthermore, Atf4 deficiency skewed differentiation toward the myeloid lineage, which was evidenced by a reduction in T- and B-cell proportions (Figure 2H).
ATF4 depletion in hematopoietic cells impairs HSC self-renewal. (A) Experimental workflow showing that Mx1-Cre;Atf4fl/fl (Δ/Δ) and Atf4fl/fl (fl/fl) mice were given pIpC and euthanized for analysis 1 month later. (B) qRT-PCR analysis of Atf4 expression in BM cells of fl/fl and Δ/Δ mice (n = 4 mice). (C) BM cell numbers in fl/fl and Δ/Δ mice (n = 4-5 mice). (D-H) Percentages of indicated cell populations in the BM of fl/fl and Δ/Δ mice (n = 4-5 mice). (I) Experimental workflow for serial and competitive transplantation. (J) Percentage of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after competitive BM transplantation (n = 5-8 mice). (K) Percentage of CD45.2+ donor-derived cells in the BM of recipients (n = 3-4 mice). (L-M) Percentages of different CD45.2+ donor–derived cell populations in the BM of recipients (n = 3-4 mice). (N) Percentages of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after secondary BM transplantation (n = 7 mice). (O) Results from CFU colony assays performed using 150 LKS+ cells from fl/fl and Δ/Δ mice cultured for 10 to 14 days in complete methylcellulose-based medium (n = 4-5 wells for 3 independent experiments). Data represent mean ± standard deviation (SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test.
ATF4 depletion in hematopoietic cells impairs HSC self-renewal. (A) Experimental workflow showing that Mx1-Cre;Atf4fl/fl (Δ/Δ) and Atf4fl/fl (fl/fl) mice were given pIpC and euthanized for analysis 1 month later. (B) qRT-PCR analysis of Atf4 expression in BM cells of fl/fl and Δ/Δ mice (n = 4 mice). (C) BM cell numbers in fl/fl and Δ/Δ mice (n = 4-5 mice). (D-H) Percentages of indicated cell populations in the BM of fl/fl and Δ/Δ mice (n = 4-5 mice). (I) Experimental workflow for serial and competitive transplantation. (J) Percentage of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after competitive BM transplantation (n = 5-8 mice). (K) Percentage of CD45.2+ donor-derived cells in the BM of recipients (n = 3-4 mice). (L-M) Percentages of different CD45.2+ donor–derived cell populations in the BM of recipients (n = 3-4 mice). (N) Percentages of CD45.2+ donor cells in the PB of recipient mice at the indicated time points after secondary BM transplantation (n = 7 mice). (O) Results from CFU colony assays performed using 150 LKS+ cells from fl/fl and Δ/Δ mice cultured for 10 to 14 days in complete methylcellulose-based medium (n = 4-5 wells for 3 independent experiments). Data represent mean ± standard deviation (SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test.
We evaluated the impact of ATF4 deficiency on HSC function in vivo using competitive BM transplantation assays (Figure 2I). Atf4 depletion severely impaired BM repopulation ability (Figure 2J-K). Furthermore, the frequencies of donor–derived MPPs, GMPs, and PreGM subsets were significantly higher in Atf4–deleted donor cells, whereas those of EPCs (including PreCFU-E and CFU-E) were lower, which was consistent with the phenotype observed at steady state (Figure 2L-M). Secondary BM transplantation experiments further confirmed that Atf4 deletion inhibited the HSC self-renewal capacity (Figure 2N). To verify that cell-specific Atf4 deletion leads to HSC dysfunction, we performed transplantation assays in which BM cells from Δ/Δ and fl/fl mice were cotransplanted with competitor BM cells into lethally irradiated recipients. pIpC–induced Atf4 deletion (supplemental Figure 3B) rapidly reduced the donor–derived cell repopulation ability and increased the frequency of most donor–derived HSPC subsets while decreasing EPC numbers (supplemental Figure 3C-F). These data confirm that Atf4 deletion severely impaired HSC self-renewal ability. In vitro colony formation assays to evaluate the function of HSPCs showed that LKS+ cells, whole BM cells, and LT-HSCs from the Atf4-depleted group formed only a few scattered colonies (Figure 2O; supplemental Figure 3G-H). These results demonstrate that Atf4 deletion leads to HSC functional defects.
ATF4 depletion in hematopoietic cells causes severe macrocytosis
Atf4 loss not only impaired the function of adult HSCs but also led to a significant decrease in EPC numbers compared with control mice (Figure 2D-G,L; supplemental Figure 3F). In addition, Δ/Δ mice had severe anemia, with fewer RBCs and lower hemoglobin levels (Figure 3A-B). The mean corpuscular volume was increased after Atf4 deletion, suggesting that Atf4-deficient mice developed macrocytosis (Figure 3B). Crucially, >70% of the Δ/Δ mice died from anemia (Figure 3C). These findings show that Atf4 loss in the hematopoietic cells of adult mice represses erythropoiesis.
Mx1-Cre;Atf4fl/fl mice experience severe macrocytosis. (A) Representative images of femurs and PB smears. (B) Routine blood parameters of fl/fl and Δ/Δ mice (n = 4-5 mice). (C) Survival curves of fl/fl and Δ/Δ mice treated with 25 μg/g pIpC delivered on alternate days for a total of 3 doses. (D) Percentages of terminal erythroid cell populations in BM (n = 3-4 mice). (E) Representative flow cytometry dot plots of CD44 expression vs forward scatter (FSC), after gating on TER119+ cells (left) and quantification of terminally differentiated erythroid subsets in fl/fl and Δ/Δ mice (right) (n = 4-5 mice). (F) Quantitative results from BFU-E colony formation assays performed using 3 × 103 cKit+ BM cells from fl/fl and Δ/Δ mice cultured in MethoCult SF M3436 methylcellulose-based medium for 10 to 14 days (n = 4 mice). (G) Quantitative results (left) from CFU-E colony formation assays performed using 3 × 103 cKit+ BM cells from fl/fl and Δ/Δ mice cultured in MethoCult SF M3334 methylcellulose-based medium for 48 hours and representative images from 3 independent experiments (right). Scale bar, 25 μm (n = 4 mice). (H) Representative flow cytometry dot plots (bromodeoxyuridine [BrdU]/Hoechst) of MEP cells (left) and a graph showing the percentage of BrdU+ MEP cells (right) (n = 3-4 mice). (I) Percentages of MEP cells in different cell cycle phases (n = 3-4 mice). (J) Quantitative results (left) from CFU-E colony formation assays performed using 500 fresh BM MEP sorted from fl/fl and Δ/Δ mice and cultured in MethoCult SF M3334 methylcellulose-based medium for 48 hours (right) (n = 3 mice). (K) Quantitative results (left) from BFU-E colony formation assays performed using 1000 fresh BM MEP cells sorted from fl/fl and Δ/Δ mice and cultured in MethoCult SF M3436 methylcellulose-based medium for 2 weeks (right), (n = 3 mice). Data represent the mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test. HGB, hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume.
Mx1-Cre;Atf4fl/fl mice experience severe macrocytosis. (A) Representative images of femurs and PB smears. (B) Routine blood parameters of fl/fl and Δ/Δ mice (n = 4-5 mice). (C) Survival curves of fl/fl and Δ/Δ mice treated with 25 μg/g pIpC delivered on alternate days for a total of 3 doses. (D) Percentages of terminal erythroid cell populations in BM (n = 3-4 mice). (E) Representative flow cytometry dot plots of CD44 expression vs forward scatter (FSC), after gating on TER119+ cells (left) and quantification of terminally differentiated erythroid subsets in fl/fl and Δ/Δ mice (right) (n = 4-5 mice). (F) Quantitative results from BFU-E colony formation assays performed using 3 × 103 cKit+ BM cells from fl/fl and Δ/Δ mice cultured in MethoCult SF M3436 methylcellulose-based medium for 10 to 14 days (n = 4 mice). (G) Quantitative results (left) from CFU-E colony formation assays performed using 3 × 103 cKit+ BM cells from fl/fl and Δ/Δ mice cultured in MethoCult SF M3334 methylcellulose-based medium for 48 hours and representative images from 3 independent experiments (right). Scale bar, 25 μm (n = 4 mice). (H) Representative flow cytometry dot plots (bromodeoxyuridine [BrdU]/Hoechst) of MEP cells (left) and a graph showing the percentage of BrdU+ MEP cells (right) (n = 3-4 mice). (I) Percentages of MEP cells in different cell cycle phases (n = 3-4 mice). (J) Quantitative results (left) from CFU-E colony formation assays performed using 500 fresh BM MEP sorted from fl/fl and Δ/Δ mice and cultured in MethoCult SF M3334 methylcellulose-based medium for 48 hours (right) (n = 3 mice). (K) Quantitative results (left) from BFU-E colony formation assays performed using 1000 fresh BM MEP cells sorted from fl/fl and Δ/Δ mice and cultured in MethoCult SF M3436 methylcellulose-based medium for 2 weeks (right), (n = 3 mice). Data represent the mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test. HGB, hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume.
To investigate how ATF4 is involved in terminal erythroid differentiation, we characterized the stages of erythropoiesis in the BM using flow cytometry. Atf4 deletion markedly impaired each stage of the terminal erythroid differentiation (Figure 3D). In both groups, Pro, Baso, Poly, and Ortho erythroblasts within the nucleated erythroblast population were present in a ratio of 1:2:4:8 (Figure 3E). This finding suggests that the blockade of erythroid differentiation induced by Atf4 deletion occurs at the progenitor stage.
We next performed in vitro erythroid colony formation assays (CFU-E and BFU-E) to evaluate the function of EPCs. cKit+ cells from Δ/Δ mice generated fewer and smaller colonies (Figure 3F-G). In addition, fewer Atf4-depleted MEPs entered the cell cycle than WT MEPs (Figure 3H-I). Similarly, MEPs from Δ/Δ mice generated fewer erythroid colonies (Figure 3J-K). We also observed extramedullary hematopoiesis and blocked erythroid commitment in the spleen of Δ/Δ mice (supplemental Figure 4). These data suggest that erythroid commitment is blocked by Atf4 loss.
ATF4 loss alters lineage commitment in HSPCs
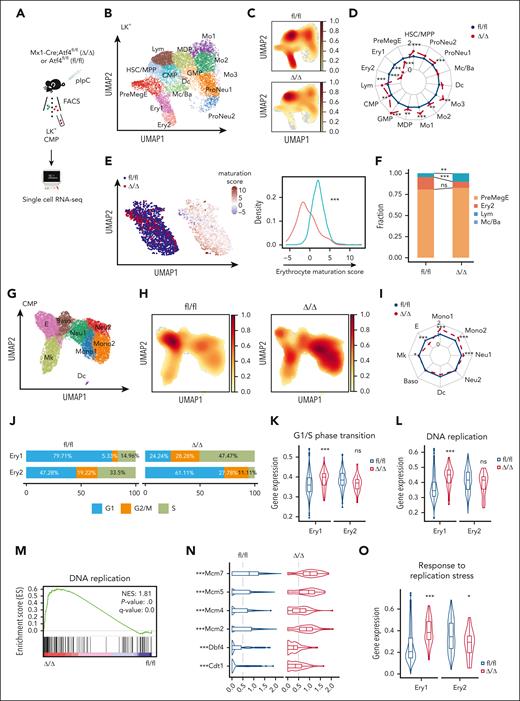
To decipher the effect of ATF4 on the transcriptional profiles of HSPC subsets, we performed single-cell RNA sequencing (scRNA-seq) of fluorescence activated cell sorting (FACS)–sorted BM Lin–cKit+ (LK+) cells from Δ/Δ and fl/fl mice 4 weeks after Atf4 deletion (Figure 4A). We also enriched LK+ cells from the BM of secondary recipient mice for additional scRNA-seq analysis (supplemental Figure 5A-B). Uniform manifold approximation and projection analysis and feature gene expression identified 15 distinct clusters (Figure 4B; supplemental Figure 5C; supplemental Table 1). In accordance with the flow cytometry analysis (Figure 2E-G), the frequencies of transcriptomically defined EPCs (including the Ery1 and Ery2 subsets) were markedly lower in Δ/Δ mice than in controls, confirming the impairment of erythropoiesis (Figure 4C-D). However, Atf4 deletion increased the number of other HSPC subsets, revealing a shift in transcriptional priming during hematopoiesis (Figure 4C-D). Transcriptional analysis of EPCs in Atf4-deficient cells further supported the impaired terminal maturation of these cells (Figure 4E). FateID analysis showed that Atf4-depleted HSCs/MPPs exhibited reduced differentiation into the erythroid lineage, suggesting that transcriptional priming had already occurred in HSCs/MPPs (Figure 4F; supplemental Figure 5D). After transplantation, the Δ/Δ group also had lower numbers of transcriptomically detected Ery1 and Ery2 subsets (supplemental Figure 5E-F).
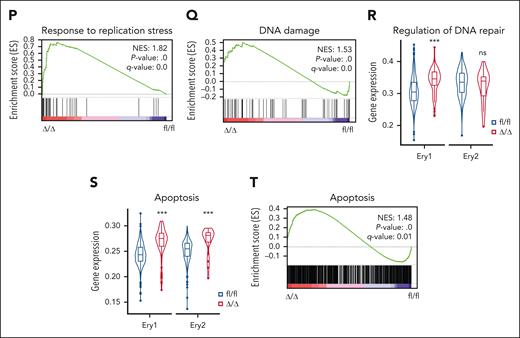
ATF4 loss alters transcriptional lineage commitment in HSPCs. (A) Experimental workflow showing the strategy used to sort cells from the BM of fl/fl and Δ/Δ mice (n = 3 mice) for scRNA-seq. (B) Uniform manifold approximation and projection (UMAP) visualization of 14 189 BM lin–cKit+ (LK+) cells categorized into 15 clusters from the fl/fl and Δ/Δ mice. (C) UMAP plot showing the cell subset distribution frequencies within the fl/fl and Δ/Δ BM LK+ cell populations. (D) Transcriptome cluster frequency changes in Atf4–depleted LK+ cells relative to the control. ∗∗P < .01; ∗∗∗P < .001; χ2 test. (E) UMAP plots colored according to genotype (fl/fl: blue, 1544 cells; Δ/Δ: red, 117 cells; left). The fourth principal component, which had the strongest correlation with erythrocyte maturation, was used to construct an erythrocyte maturation score. Each dot represents a single cell. Distribution of cells according to erythrocyte maturation scores (right). ∗∗∗P < .001; Wilcoxon rank-sum test. (F) Barplot showing the fate bias of single HSC/MPPs computed by FateID. ns, not significant; ∗∗P < .01; ∗∗∗P < .001; 2-sided Fisher exact test. (G) UMAP visualization of 19 706 CMP cells from fl/fl and Δ/Δ mice categorized into 8 clusters. (H) UMAP plot showing the cell subset distribution frequencies in the fl/fl and Δ/Δ CMP cell populations. (I) Transcriptome cluster frequency changes in Atf4-depleted CMP cells relative to the control. ∗P < .05; ∗∗∗P < .001; χ2 test. (J) Cell cycle distributions of Ery1 and Ery2 in fl/fl and Δ/Δ LK+ cells. (K) Violin plots showing the expression of the G1/S phase transition gene set in Ery1 and Ery2 cells from fl/fl and Δ/Δ group. (L) Violin plots showing the expression level of the DNA replication gene set in Ery1 and Ery2 cells from fl/fl and Δ/Δ groups. ns, not significant; ∗∗∗P < .001; Wilcoxon rank-sum test. (M) Gene set enrichment analysis (GSEA) of DNA replication in Ery1 cells from the fl/fl and Δ/Δ groups. (N) Expression levels of representative genes related to DNA replication in Ery1 subsets within fl/fl and Δ/Δ LK+ cell populations. (O). Violin plots showing the expression of the replication stress gene set in Ery1 and Ery2 cells from fl/fl and Δ/Δ groups. (P) GSEA of replication stress in Ery1 cells from the fl/fl and Δ/Δ groups. (Q) GSEA of DNA damage in Ery1 cells from fl/fl and Δ/Δ groups. (R) Violin plots showing the expression of the DNA repair gene set in Ery1 and Ery2 cells from the fl/fl and Δ/Δ groups. (S) Violin plots showing the expression of the apoptosis gene set in Ery1 and Ery2 from the fl/fl and Δ/Δ groups. (T) GSEA of apoptosis in Ery1 cells from the fl/fl and Δ/Δ groups. ns, not significant; ∗P < .05; ∗∗∗P < .001; Wilcoxon rank-sum test.
ATF4 loss alters transcriptional lineage commitment in HSPCs. (A) Experimental workflow showing the strategy used to sort cells from the BM of fl/fl and Δ/Δ mice (n = 3 mice) for scRNA-seq. (B) Uniform manifold approximation and projection (UMAP) visualization of 14 189 BM lin–cKit+ (LK+) cells categorized into 15 clusters from the fl/fl and Δ/Δ mice. (C) UMAP plot showing the cell subset distribution frequencies within the fl/fl and Δ/Δ BM LK+ cell populations. (D) Transcriptome cluster frequency changes in Atf4–depleted LK+ cells relative to the control. ∗∗P < .01; ∗∗∗P < .001; χ2 test. (E) UMAP plots colored according to genotype (fl/fl: blue, 1544 cells; Δ/Δ: red, 117 cells; left). The fourth principal component, which had the strongest correlation with erythrocyte maturation, was used to construct an erythrocyte maturation score. Each dot represents a single cell. Distribution of cells according to erythrocyte maturation scores (right). ∗∗∗P < .001; Wilcoxon rank-sum test. (F) Barplot showing the fate bias of single HSC/MPPs computed by FateID. ns, not significant; ∗∗P < .01; ∗∗∗P < .001; 2-sided Fisher exact test. (G) UMAP visualization of 19 706 CMP cells from fl/fl and Δ/Δ mice categorized into 8 clusters. (H) UMAP plot showing the cell subset distribution frequencies in the fl/fl and Δ/Δ CMP cell populations. (I) Transcriptome cluster frequency changes in Atf4-depleted CMP cells relative to the control. ∗P < .05; ∗∗∗P < .001; χ2 test. (J) Cell cycle distributions of Ery1 and Ery2 in fl/fl and Δ/Δ LK+ cells. (K) Violin plots showing the expression of the G1/S phase transition gene set in Ery1 and Ery2 cells from fl/fl and Δ/Δ group. (L) Violin plots showing the expression level of the DNA replication gene set in Ery1 and Ery2 cells from fl/fl and Δ/Δ groups. ns, not significant; ∗∗∗P < .001; Wilcoxon rank-sum test. (M) Gene set enrichment analysis (GSEA) of DNA replication in Ery1 cells from the fl/fl and Δ/Δ groups. (N) Expression levels of representative genes related to DNA replication in Ery1 subsets within fl/fl and Δ/Δ LK+ cell populations. (O). Violin plots showing the expression of the replication stress gene set in Ery1 and Ery2 cells from fl/fl and Δ/Δ groups. (P) GSEA of replication stress in Ery1 cells from the fl/fl and Δ/Δ groups. (Q) GSEA of DNA damage in Ery1 cells from fl/fl and Δ/Δ groups. (R) Violin plots showing the expression of the DNA repair gene set in Ery1 and Ery2 cells from the fl/fl and Δ/Δ groups. (S) Violin plots showing the expression of the apoptosis gene set in Ery1 and Ery2 from the fl/fl and Δ/Δ groups. (T) GSEA of apoptosis in Ery1 cells from the fl/fl and Δ/Δ groups. ns, not significant; ∗P < .05; ∗∗∗P < .001; Wilcoxon rank-sum test.
CMPs lie upstream of MEPs and their lineage output is highly heterogeneous.29 We then performed scRNA-seq of flow-cytometry–sorted CMP cells from Δ/Δ and fl/fl mice. Clustering analysis generated a map with 8 transcriptional subpopulations (Figure 4G; supplemental Figure 5G-I; supplemental Table 2). In the Δ/Δ group, the size of the erythroid groups was significantly reduced (Figure 4H-I). These results suggest that Aft4 deletion biased the lineage output of CMPs and impaired erythroid differentiation.
ATF4 deletion forces early erythroid progenitors into S phase
To elucidate the mechanism of impaired erythropoiesis in Δ/Δ mice, we examined transcriptomically detected Ery1 and Ery2 cells at a steady state. Cell cycle analysis revealed a higher proportion of Ery1 cells in the Δ/Δ group in the S and G2/M phases (Figure 4J). Similarly, the expression of genes associated with G1/S transition was significantly increased in Ery1 cells from Δ/Δ mice (Figure 4K; supplemental Figure 6A). Conversely, in accordance with the changes observed in flow-defined MEP cells, fewer Ery2 cells from the Δ/Δ group were in the S and G2/M phases (Figures 3H-I and 4J). DNA replication was hyperactivated in Ery1 cells from Δ/Δ mice (Figure 4L-N), which induced the Ery1 cells response to replication stress (Figure 4O-P; supplemental Figure 6B). Furthermore, the DNA damage response and DNA repair pathways were induced in Atf4-depleted Ery1 cells (Figure 4Q-R; supplemental Figure 6C-D). Consequently, apoptosis-related gene (eg, Bax) expression was elevated (Figure 4S-T; supplemental Figure 6E).
We explored the existence of this phenomenon in CMPs by cell cycle analysis of erythroid clusters in Δ/Δ and fl/fl mice. A greater number of E cells from the Δ/Δ group were in S phase, as evidenced by the increased expression of G1/S transition-related genes (supplemental Figure 6F-G). Consistent with the changes in Ery1 cells, Atf4-deleted E cells displayed higher levels of DNA replication and subsequent replication stress, which induced DNA damage, DNA repair, and apoptosis (supplemental Figure 6H-L). These transcriptomic changes were validated by computational screening for surface markers overrepresented in the E clusters and isolating E clusters with an immunophenotype of Lin–cKit+Sca1–CD34+CD16/32lowCD55+CD63–CD41– from the BM (supplemental Figure 6M-O) for in vitro experiments (supplemental Figure 6P-R). These results suggest that the absence of ATF4 forced erythroid progenitors into the S phase, leading to replication stress and activation of both the DNA damage response and apoptosis.
ATF4 directs the transcriptional program of erythropoiesis
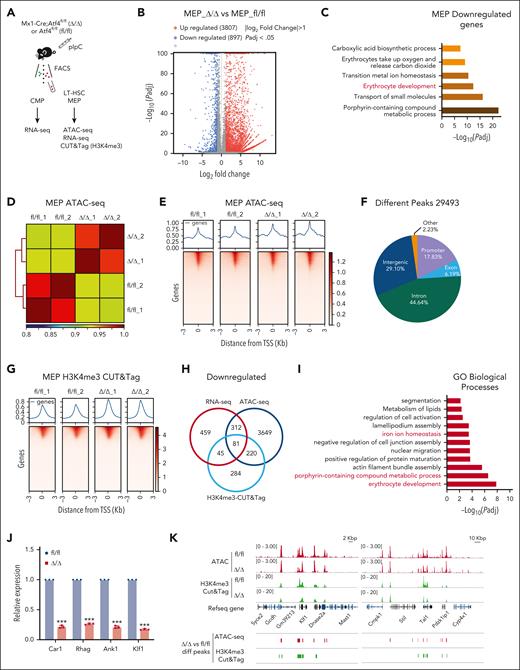
To investigate the molecular mechanism by which Atf4 deficiency causes severe impairment of erythropoiesis, we sorted MEPs from Δ/Δ and fl/fl mice 4 weeks after Atf4 deletion for RNA sequencing (RNA-seq; Figure 5A). In total, we identified 4704 differentially expressed genes between the Δ/Δ and fl/fl groups, of which 897 were downregulated (Figure 5B). Gene ontology (GO) analysis of downregulated genes revealed impaired erythrocyte development in Δ/Δ mice (Figure 5C), which was consistent with the colony formation assay results (Figure 3J-K). In addition, we performed assay for transposase-accessible chromatin with sequencing (ATAC-seq) with MEP cells to explore changes in chromatin accessibility after Atf4 deletion (Figure 5A,D-E). We identified 29 493 different peaks of accessibility between the Δ/Δ and fl/fl mouse groups,17.83% of which were localized to the promoter (Figure 5F).
ATF4 governs the transcriptional program of erythropoiesis. (A) Experimental workflow showing the strategy used to sort the indicated cell types from the BM of fl/fl and Δ/Δ mice (n = 8-10 mice) for bulk RNA-seq, ATAC-seq, and CUT&Tag (H3K4me3). (B) Plots showing the differentially expressed genes (DEGs) identified from bulk RNA-seq analysis of fl/fl vs Δ/Δ MEP cells. (C) GO term analysis of genes identified as downregulated by bulk RNA-seq in Atf4-depleted MEP cells (vs the control group). (D) Heat map showing replication of samples of ATAC-seq from fl/fl and Δ/Δ BM MEP cell samples. (E) ATAC-seq profile and heat map showing the degree of chromatin accessibility around transcription start site (TSS) ± 3 Kbp of fl/fl and Δ/Δ MEP cells. (F) Venn diagram of ATAC-seq data showing the 29 493 different peak distributions of Atf4-deficient MEP cells. (G) H3K4me3 CUT&Tag profile and heat map showing TSS ± 3 kb. (H) Venn diagram showing the overlap between downregulated and transcriptionally repressed genes identified by ATAC-seq, H3K4me3 CUT&Tag analysis, and RNA-seq after Atf4 deletion. (I) GO term analysis of overlapping downregulated genes; only the top 11 GO terms are listed. (J) qRT-PCR analysis of the indicated genes in MEP cells sorted from the fl/fl and Δ/Δ mice (n = 3 samples). (K) Integrative genomics viewer (IGV) software was used to visually present the important genomic regions of Klf1 and Tal1 using findings from ATAC-seq and H3K4me3 CUT&Tag (shown as peaks). Data represent the mean ± SD. ∗∗∗P < .001; unpaired 2-tailed Student t test.
ATF4 governs the transcriptional program of erythropoiesis. (A) Experimental workflow showing the strategy used to sort the indicated cell types from the BM of fl/fl and Δ/Δ mice (n = 8-10 mice) for bulk RNA-seq, ATAC-seq, and CUT&Tag (H3K4me3). (B) Plots showing the differentially expressed genes (DEGs) identified from bulk RNA-seq analysis of fl/fl vs Δ/Δ MEP cells. (C) GO term analysis of genes identified as downregulated by bulk RNA-seq in Atf4-depleted MEP cells (vs the control group). (D) Heat map showing replication of samples of ATAC-seq from fl/fl and Δ/Δ BM MEP cell samples. (E) ATAC-seq profile and heat map showing the degree of chromatin accessibility around transcription start site (TSS) ± 3 Kbp of fl/fl and Δ/Δ MEP cells. (F) Venn diagram of ATAC-seq data showing the 29 493 different peak distributions of Atf4-deficient MEP cells. (G) H3K4me3 CUT&Tag profile and heat map showing TSS ± 3 kb. (H) Venn diagram showing the overlap between downregulated and transcriptionally repressed genes identified by ATAC-seq, H3K4me3 CUT&Tag analysis, and RNA-seq after Atf4 deletion. (I) GO term analysis of overlapping downregulated genes; only the top 11 GO terms are listed. (J) qRT-PCR analysis of the indicated genes in MEP cells sorted from the fl/fl and Δ/Δ mice (n = 3 samples). (K) Integrative genomics viewer (IGV) software was used to visually present the important genomic regions of Klf1 and Tal1 using findings from ATAC-seq and H3K4me3 CUT&Tag (shown as peaks). Data represent the mean ± SD. ∗∗∗P < .001; unpaired 2-tailed Student t test.
We also conducted cleavage under targets and tagmentation (CUT&Tag) assays using MEP cells and an antibody against trimethylation of histone H3 lysine 4 (H3K4me3) histone modification (Figure 5G). Integrative analyses of the RNA-seq, ATAC-seq, and H3K4me3 CUT&Tag data sets yielded 81 genes that were downregulated (transcriptionally repressed) in Δ/Δ vs fl/fl mice (Figure 5H; supplemental Table 3). GO analysis of these genes revealed that several terms related to erythropoiesis were highly diminished in Atf4-deficient MEPs (Figure 5I). qRT-PCR analysis further confirmed the decreased expression of erythrocyte differentiation-related genes (Figure 5J). Visualization of ATAC-seq and H3K4me3 CUT&Tag data showed that the peaks of erythroid master regulators Klf1 and Tal1 were decreased in Atf4-depleted MEPs (Figure 5K).
Interestingly, the top-ranked downregulated gene sets from the bulk RNA-seq data of Atf4-depleted CMPs and LT-HSCs were implicated in erythrocyte development (supplemental Figure 7A-D). GO analysis of the ATAC-seq and H3K4me3 CUT&Tag data sets showed that 56 overlapping genes were downregulated in Atf4-deficient LT-HSCs, which were enriched in pathways associated with the negative regulation of cell proliferation (supplemental Figure 7E-I). These results suggest that the absence of ATF4 impairs erythroid differentiation of HSCs.
ATF4 activates the transcription of Rps19bp1 to regulate ribosome biogenesis
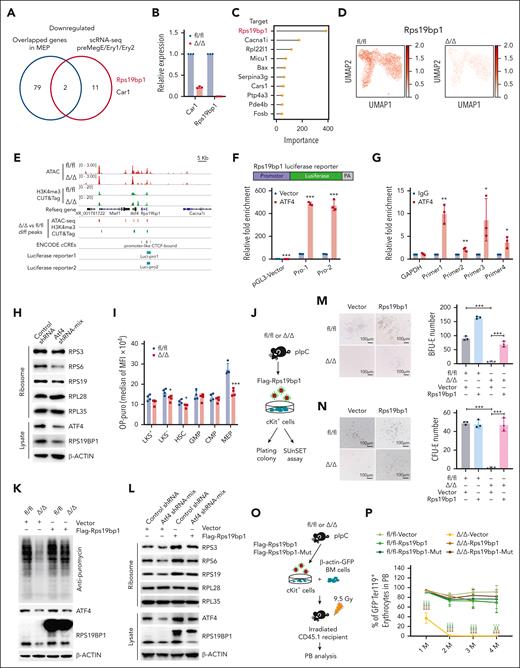
In a subsequent integrative analysis of the genes that were identified as downregulated in MEPs by RNA-seq, ATAC-seq, and H3K4me3 CUT&Tag, and genes that were identified as downregulated in erythroid progenitors (ie, PreMegE, Ery1, and Ery2) by scRNA-seq, we identified 2 genes, Car1 and Rps19bp1 (Figure 6A). Intriguingly, Rps19bp1 was among the overlapping downregulated genes identified in the RNA-seq analysis of LT-HSCs and HSCs/MPPs (supplemental Figure 8A). qRT-PCR analysis showed a marked reduction in Rps19bp1 expression in Atf4-deleted MEPs compared with controls (Figure 6B). Analysis of scRNA-seq data using python based single-cell regulatory network inference and clustering (pySCENIC) predicted that Rps19bp1 is a target gene of Atf4 (Figure 6C). scRNA-seq data of CMPs further showed lower Rps19bp1 expression in Atf4-deleted HSPCs, especially in Atf4–deleted erythroid progenitors (Figure 6D; supplemental Figure 8B).
ATF4 regulates ribosome biogenesis by directly activating the transcription of Rps19bp1. (A) Venn diagram depicting the overlapping downregulated/transcriptionally repressed genes identified from 4 different data sets (ie, ATAC-seq, H3K4me3 CUT&Tag, RNA-seq, and scRNA-seq) after Atf4 deletion. (B) qRT-PCR analysis of the indicated genes in MEP cells sorted from the fl/fl and Δ/Δ mice (n = 3 samples). (C) Lollipop plot displaying the top 10 predicted ATF4-targeting genes within the CMP population using pySCENIC. (D) Expression of Rps19bp1 projected onto the UMAP plot of fl/fl and Δ/Δ CMP cells, based on scRNA-seq data. Color intensity indicates the expression levels. (E) IGV software was used to visually present the important genomic regions of Rps19bp1; ATAC-seq and H3K4me3 CUT&Tag peaks, predicted cis-regulated elements, and luciferase reporter clone regions are shown. (F) Schematic diagrams of the pGL3-Rps19bp1-promoter-luciferase reporter constructs (Pro-1 contains the CTCF-bound region; Pro-2 does not contain the CTCF-bound region); relative luciferase activity was determined by sequential normalization to Renilla luciferase and pGL3-vector activity (n = 3 samples). (G) qChIP of ATF4 with primers covering the promoters of Rps19bp1 (n = 3 samples). (H) Ribosomes were separated from MEL cells transduced with control or Atf4 shRNA mix (shRNA-1 and shRNA-2) and then analyzed by western blotting using antibodies targeting the indicated proteins. (I) Protein synthesis in HSPCs based on OP-Puro incorporation in vivo (n = 4 mice in 3 independent experiments). (J) Experimental workflow showing how cKit+ cells were sorted from the BM of fl/fl and Δ/Δ mice and then transduced with vector-TD or Flag-Rps19bp1-TD for the plating colony and SUnSET assays. (K) TD+ cKit+ cells of the fl/fl and Δ/Δ mice were treated with puromycin and then analyzed by western blotting using antibodies against the indicated proteins. (L) Ribosomes were separated from MEL cells transduced with control or Atf4 shRNA mix and vector-TD or Flag-Rps19bp1-TD then analyzed by western blotting using antibodies against the indicated proteins. (M-N) BFU-E (M) or CFU-E (N) colony assays of 2 × 104 transduced TD+ cKit+ cells from fl/fl or Δ/Δ mice cultured in MethoCult SF M3436 or M3334 methylcellulose-based medium with EPO cytokine for 10 days (M) or 48 hours (N), respectively (n = 3 wells in 3 independent experiments). Representative images (left) and colony numbers (right) are shown. (O) Experimental workflow of the transplantation assay in which 105 cKit+ cells (sorted from the BM of the fl/fl or Δ/Δ mice and transduced with vector-TD or Flag-Rps19bp1-TD, Flag-Rps19bp1-Mut-TD) and 106 BM cells from donor mice (β-actin-GFP) were infected into irradiated CD45.1 recipient mice. (P) Percentage of GFP–Ter119+ erythrocytes in PB erythrocytes of recipient mice at the indicated time points after BM transplantation (n = 3-5 mice). Data represent the mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test for Figure 6B,F-G,I; 1-way analysis of variance followed by an unpaired 2-tailed Student t test for figure 6M-N,P. EPO, erythropoietin; OP-Puro, O-propargyl-puromycin; qChIP, quantitative chromatin immunoprecipitation; shRNA, short hairpin RNA; SUnSET, surface sensing of translation.
ATF4 regulates ribosome biogenesis by directly activating the transcription of Rps19bp1. (A) Venn diagram depicting the overlapping downregulated/transcriptionally repressed genes identified from 4 different data sets (ie, ATAC-seq, H3K4me3 CUT&Tag, RNA-seq, and scRNA-seq) after Atf4 deletion. (B) qRT-PCR analysis of the indicated genes in MEP cells sorted from the fl/fl and Δ/Δ mice (n = 3 samples). (C) Lollipop plot displaying the top 10 predicted ATF4-targeting genes within the CMP population using pySCENIC. (D) Expression of Rps19bp1 projected onto the UMAP plot of fl/fl and Δ/Δ CMP cells, based on scRNA-seq data. Color intensity indicates the expression levels. (E) IGV software was used to visually present the important genomic regions of Rps19bp1; ATAC-seq and H3K4me3 CUT&Tag peaks, predicted cis-regulated elements, and luciferase reporter clone regions are shown. (F) Schematic diagrams of the pGL3-Rps19bp1-promoter-luciferase reporter constructs (Pro-1 contains the CTCF-bound region; Pro-2 does not contain the CTCF-bound region); relative luciferase activity was determined by sequential normalization to Renilla luciferase and pGL3-vector activity (n = 3 samples). (G) qChIP of ATF4 with primers covering the promoters of Rps19bp1 (n = 3 samples). (H) Ribosomes were separated from MEL cells transduced with control or Atf4 shRNA mix (shRNA-1 and shRNA-2) and then analyzed by western blotting using antibodies targeting the indicated proteins. (I) Protein synthesis in HSPCs based on OP-Puro incorporation in vivo (n = 4 mice in 3 independent experiments). (J) Experimental workflow showing how cKit+ cells were sorted from the BM of fl/fl and Δ/Δ mice and then transduced with vector-TD or Flag-Rps19bp1-TD for the plating colony and SUnSET assays. (K) TD+ cKit+ cells of the fl/fl and Δ/Δ mice were treated with puromycin and then analyzed by western blotting using antibodies against the indicated proteins. (L) Ribosomes were separated from MEL cells transduced with control or Atf4 shRNA mix and vector-TD or Flag-Rps19bp1-TD then analyzed by western blotting using antibodies against the indicated proteins. (M-N) BFU-E (M) or CFU-E (N) colony assays of 2 × 104 transduced TD+ cKit+ cells from fl/fl or Δ/Δ mice cultured in MethoCult SF M3436 or M3334 methylcellulose-based medium with EPO cytokine for 10 days (M) or 48 hours (N), respectively (n = 3 wells in 3 independent experiments). Representative images (left) and colony numbers (right) are shown. (O) Experimental workflow of the transplantation assay in which 105 cKit+ cells (sorted from the BM of the fl/fl or Δ/Δ mice and transduced with vector-TD or Flag-Rps19bp1-TD, Flag-Rps19bp1-Mut-TD) and 106 BM cells from donor mice (β-actin-GFP) were infected into irradiated CD45.1 recipient mice. (P) Percentage of GFP–Ter119+ erythrocytes in PB erythrocytes of recipient mice at the indicated time points after BM transplantation (n = 3-5 mice). Data represent the mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test for Figure 6B,F-G,I; 1-way analysis of variance followed by an unpaired 2-tailed Student t test for figure 6M-N,P. EPO, erythropoietin; OP-Puro, O-propargyl-puromycin; qChIP, quantitative chromatin immunoprecipitation; shRNA, short hairpin RNA; SUnSET, surface sensing of translation.
Luciferase reporter and quantitative chromatin immunoprecipitation assays confirmed that ATF4 regulates Rps19bp1 transcription (Figure 6E-F; supplemental Figure 8C) and binds directly to its promoter region (Figure 6G). These data indicate that ATF4 is a transcriptional activator of Rps19bp1.
Because Rps19bp1 has been reported to interact with RPS19 to function in ribosome biogenesis,30 we measured the relative ribosomal protein abundance in ribosomes extracted from Atf4-knockdown and WT murine erythroleukemia (MEL) cells. Atf4 downregulation decreased the abundance of 40S proteins (Figure 6H). We then administered O-propargyl-puromycin to BM HSPCs to assess their translation rates. The MEPs from Δ/Δ mice exhibited markedly lower levels of fluorescence than those from fl/fl mice (Figure 6I). Moreover, surface sensing of translation assays confirmed a reduction in the global protein synthesis rate of cKit+ cells from Δ/Δ mice compared with the same cells from fl/fl mice (Figure 6J-K). Transfection of Flag-Rps19bp1 plasmids into Atf4-deficient MEL cells or primary cKit+ cells from Δ/Δ mice showed that Rps19bp1 overexpression reversed the decrease in 40 S protein levels (Figure 6L). These results indicate that ATF4 participates in ribosome biogenesis and protein synthesis by regulating Rps19bp1 transcription.
ATF4 deletion induces Rps19bp1 downregulation and ultimately perturbs erythropoiesis
To investigate the impact of Rps19bp1 on erythroid differentiation in Atf4-depleted HSPCs, we transfected Flag-Rps19bp1 plasmids into primary cKit+ cells from Δ/Δ and fl/fl mice and performed in vitro CFU-E and BFU-E colony formation assays (Figure 6J). Rps19bp1 overexpression reversed the decrease in CFU-E and BFU-E colony numbers caused by Atf4 deletion (Figure 6M-N; supplemental Figure 8D). We then transplanted cKit+ cells from Δ/Δ or fl/fl mice transfected with Flag-Rps19bp1-TD, Flag-Rps19bp1-Mut-TD plasmid (introducing an enzyme activity mutation by deleting amino acids 64-73 homologous to human amino acids 62-71),31 or vector plasmids, together with fresh BM cells from β-actin–GFP transgenic reporter mice, into CD45.1 recipients (Figure 6O). The defective erythropoiesis phenotype of Atf4-depleted cells was effectively rescued by overexpression of Rps19bp1 or the Rps19bp1 mutant (Figure 6P; supplemental Figure 8F-G). Interestingly, Rps19bp1 overexpression also significantly improved HSC repopulating ability (supplemental Figure 8H-I). In addition, Rps19bp1 knockdown impaired the repopulation ability and erythrocyte output of cKit+ cells (supplemental Figure 8J-N). These findings imply that ATF4 promotes erythroid differentiation and HSC function by boosting Rps19bp1 transcription, which in turn increases ribosome assembly.
ATF4 depletion accelerates BM hematopoietic failure in response to 5-FU–induced stress
To better understand the consequences of decreased ribosome levels on translation after Atf4 deletion, we sorted CMP cells from Δ/Δ and fl/fl mice 4 weeks after Atf4 deletion and conducted ribosome profiling32 (Figure 7A). We identified 249 significantly differentially translated proteins between the Atf4-depleted and control groups (Figure 7B; supplemental Table 4). Gene set enrichment analysis further demonstrated that ribosome biogenesis-related protein expression was significantly lower in Atf4-depleted than in WT CMP cells, despite no change in their messenger RNA levels (Figure 7C-D). This observation suggests that perturbation of ribosome homeostasis has a reciprocal impact on the translation of ribosome-related proteins, potentially exacerbating protein synthesis impairment. The expression of gene sets linked to the PreCFU-E signature was also lower in Atf4-depleted than in WT CMPs, suggesting that the reduction in ribosome biogenesis significantly affected the translation efficiency of erythroid-related pathway components, thereby impeding erythroid lineage commitment (Figure 7E). Additionally, MEPs exhibited the highest levels of translational activity, whereas those of CMPs and GMPs were considerably lower, demonstrating a preferential requirement for ribosome biogenesis in early EPCs (Figure 7F). Polysome profiling of FACS–sorted BM cKit+ cells from pIpC–induced Δ/Δ and fl/fl mice also showed a reduction in the relative abundance of the 40S subunit, assembled 80S ribosomes, and polysomes in Δ/Δ cKit+ cells (Figure 7G).
5-FU stress accelerates Atf4-deletion–induced BM hematopoietic failure. (A) Experimental workflow for sorting of CMP cells from the BM of fl/fl and Δ/Δ mice for ribosome profiling (Ribo-seq). (B) Plots showing the DEGs identified by Ribo-seq analysis in CMP cells from fl/fl vs Δ/Δ mice. (C) GSEA plot of ribosome biogenesis based on Ribo-seq data from CMP cells in the fl/fl and Δ/Δ groups. (D) GSEA plot of ribosome biogenesis based on RNA-seq data from CMP cells of the fl/fl and Δ/Δ groups. (E) GSEA plot of PreCFU-E based on Ribo-seq data from CMP cells of the fl/fl and Δ/Δ groups (left). Relative changes in translation efficiency for selected transcripts from the PreCFU-E gene set are shown in blue, and relative changes in messenger RNA expression are shown in red (right). (F) Sorted cell populations from fl/fl and Δ/Δ mice were treated with puromycin and then analyzed by western blotting using antibodies against the indicated proteins. (G) Representative polysome profiles from cKit+ cells of fl/fl and Δ/Δ mice. The absorption profile of a linear sucrose gradient at 254 nm is depicted, with sedimentation and major ribosomal peaks indicated. (H) Survival curves of fl/fl and Δ/Δ mice treated with a single dose of 5-FU. (I) BM cell numbers in fl/fl and Δ/Δ mice 10 days after 5-FU treatment (n = 3-4 mice). (J) Number of LT-HSCs in the BM of fl/fl and Δ/Δ mice 10 days after 5-FU administration (n = 3 mice). (K) Routine blood parameters of fl/fl and Δ/Δ mice at the indicated time points after 5-FU treatment (n = 4-9 mice). (L) cKit+ cells from the BM of fl/fl and Δ/Δ mice were given 5-FU and then 10 days later treated with puromycin and analyzed by western blotting using antibodies against the indicated proteins. Data represent the mean ± SD. ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test.
5-FU stress accelerates Atf4-deletion–induced BM hematopoietic failure. (A) Experimental workflow for sorting of CMP cells from the BM of fl/fl and Δ/Δ mice for ribosome profiling (Ribo-seq). (B) Plots showing the DEGs identified by Ribo-seq analysis in CMP cells from fl/fl vs Δ/Δ mice. (C) GSEA plot of ribosome biogenesis based on Ribo-seq data from CMP cells in the fl/fl and Δ/Δ groups. (D) GSEA plot of ribosome biogenesis based on RNA-seq data from CMP cells of the fl/fl and Δ/Δ groups. (E) GSEA plot of PreCFU-E based on Ribo-seq data from CMP cells of the fl/fl and Δ/Δ groups (left). Relative changes in translation efficiency for selected transcripts from the PreCFU-E gene set are shown in blue, and relative changes in messenger RNA expression are shown in red (right). (F) Sorted cell populations from fl/fl and Δ/Δ mice were treated with puromycin and then analyzed by western blotting using antibodies against the indicated proteins. (G) Representative polysome profiles from cKit+ cells of fl/fl and Δ/Δ mice. The absorption profile of a linear sucrose gradient at 254 nm is depicted, with sedimentation and major ribosomal peaks indicated. (H) Survival curves of fl/fl and Δ/Δ mice treated with a single dose of 5-FU. (I) BM cell numbers in fl/fl and Δ/Δ mice 10 days after 5-FU treatment (n = 3-4 mice). (J) Number of LT-HSCs in the BM of fl/fl and Δ/Δ mice 10 days after 5-FU administration (n = 3 mice). (K) Routine blood parameters of fl/fl and Δ/Δ mice at the indicated time points after 5-FU treatment (n = 4-9 mice). (L) cKit+ cells from the BM of fl/fl and Δ/Δ mice were given 5-FU and then 10 days later treated with puromycin and analyzed by western blotting using antibodies against the indicated proteins. Data represent the mean ± SD. ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test.
Higher levels of protein synthesis are required for HSPC regeneration after 5-FU exposure. Therefore, to further investigate the effect of ATF4 depletion on BM hematopoiesis under conditions of 5-FU stress, we treated Δ/Δ and fl/fl mice with a single dose of 5-FU (150 mg/kg). The survival rate of Δ/Δ mice was strikingly reduced compared with fl/fl mice (Figure 7H). Moreover, BM cellularity and LT-HSC numbers were substantially lower in the Δ/Δ mice (Figure 7I-J). We also observed a drastic reduction in the number of RBCs, white blood cells, and platelets in Δ/Δ mice on day 10 after treatment (Figure 7K). Further analysis of cKit+ cells isolated on day 10 after treatment using surface sensing of translation assay showed a more pronounced reduction in protein synthesis levels in Δ/Δ mice, even after treatment with 5-FU, compared with fl/fl mice (Figure 7L). These results reveal that the absence of ATF4 under stress conditions hinders hematopoietic lineage development, as it relies on robust ribosome biogenesis and ultimately results in BM failure.
Discussion
Previous studies have indicated that erythroid progenitors have unique cell cycle signatures, with shorter cell cycle duration and faster DNA synthesis rate during the commitment from self-renewal to differentiation.33,34 Our data showed that MEPs exhibited much higher protein synthesis rates than did GMPs or CMPs, suggesting that they are especially sensitive to ribosome biogenesis defects. This may explain the severe perturbation of the erythroid lineage in Atf4-depleted mice. Our findings highlight the crucial importance of protein synthesis regulation during erythroid lineage commitment.
RPS19BP1, which is associated with 40S ribosomal subunits,30 has been reported to bind directly to SIRT1. This interaction enhances the ability of SIRT1 to deacetylate multiple TFs or cofactors, including p53, HIF-1α, and NF-κB, to regulate the transcription of their target genes, which impact multiple biological processes such as cell differentiation, apoptosis, autophagy, and metabolism.35 However, the observation that overexpression of a catalytically inactive form of RPS19BP1 in Atf4-deleted cKit+ cells rescued the erythroid differentiation blockade caused by ATF4 knockout indicates that the ATF4-RPS19BP1 signaling axis might not regulate erythroid differentiation via the SIRT1 pathway.
Although most Atf4 germ line knockout mice (Atf4–/–) died neonatally,21 only a few survivors exhibited phenotypic expansion of most HSPC subsets (including HSCs, MPPs, CMPs, and GMPs), myeloid differentiation skewing, defective colony-forming ability, and impaired self-renewal activity of HSCs,19 which is consistent with the phenotype observed in our study using Mx1-Cre;Atf4fl/fl mice. Furthermore, Atf4–/– and Scl-Cre-ERT;Atf4fl/fl mice displayed normal erythropoiesis in the steady state.19 In contrast, Mx1-Cre;Atf4fl/fl mice exhibited severe anemia characterized by reduced MEPs, RBCs, and lower hemoglobin levels than control mice. Under conditions of iron deficiency stress, Atf4–/– mice displayed microcytic hypochromic anemia with inhibited erythroid differentiation starting from basophilic erythroblasts or an earlier stage, resulting in reduced erythroblasts and reticulocytes in the spleen and decreased Ter119+ cells in the BM.23 Therefore, using different mouse models, we expanded our understanding of the role of ATF4 in hematopoiesis.
In summary, by using niche or hematopoietic cell-specific conditional knockout mouse models, we demonstrated that unlike in the fetal liver, ATF4 governs adult HSC function and erythropoiesis in a cell-intrinsic manner. We revealed a novel role of ATF4 in erythropoiesis, which links it to RPS19BP1, ribosome biogenesis, and protein translation. Our findings highlight the crucial importance of protein synthesis regulation during erythroid lineage commitment, a discovery that has extended implications for understanding and treating ribosomopathy–associated erythroid failure.
Acknowledgments
The authors are grateful to the laboratory members for their assistance with experiments.
This work was supported by grants from the Ministry of Science and Technology of the People's Republic of China (2021YFA1100900, 2020YFE0203000, and 2022YFA1103500), the National Natural Science Foundation of China (92368202, 82270120, 82222003, 92268117, and 82161138028), the Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00016), the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (2021-I2M-1–019 and 2021-I2M-1–040), and the CAMS Fundamental Research Funds for Central Research Institutes (3332021093).
Authorship
Contribution: Z. Zheng, S.Y., F.G., C.T., and Z. Zhang designed the study; Q.G., G.S., P.J., N.W., X.Z., J.K., Y.W., Y.H., M.Y., and S.L. helped with mouse experiments; T.L. helped with bioinformatics analysis; and P.Q., P.Z., H.C., and T.C. proposed the study, designed the experiments, interpreted the results, wrote the paper, and oversaw the research project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hui Cheng, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Center for Stem Cell Medicine, 288 Nanjing Rd, Tianjin 300020, China; email: chenghui@ihcams.ac.cn; Ping Zhu, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Center for Stem Cell Medicine, 288 Nanjing Rd, Tianjin 300020, China; email: zhuping@ihcams.ac.cn; Pengxu Qian, School of Medicine, Zhejiang University, Room 209, Zijingang Campus, No 866 Yuhangtang Rd, Hangzhou 310030, China; email: axu@zju.edu.cn; and Tao Cheng, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Center for Stem Cell Medicine, 288 Nanjing Rd, Tianjin 300020, China; email: chengtao@ihcams.ac.cn.
References
Author notes
Z. Zheng, S.Y., F.G., C.T., and Z. Zhang contributed equally to this study.
RNA sequencing and single-cell RNA sequencing data have been deposited in the Gene Expression Omnibus database (accession codes GSE233677 and GSE235798, respectively). Assay for transposase-accessible chromatin with sequencing and H3K4me3 CUT&Tag data have been deposited in the Sequence Read Archive (accession codes PRJNA992183 and PRJNA992885, respectively).
All other data are available upon reasonable request from the corresponding authors, Tao Cheng (chengtao@ihcams.ac.cn), Hui Cheng (chenghui@ihcams.ac.cn), Ping Zhu (zhuping@ihcams.ac.cn), and Pengxu Qian (axu@zju.edu.cn). The computational code used in this study can be obtained from the corresponding author, Ping Zhu (zhuping@ihcams.ac.cn).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Mx1-Cre;Atf4fl/fl mice experience severe macrocytosis. (A) Representative images of femurs and PB smears. (B) Routine blood parameters of fl/fl and Δ/Δ mice (n = 4-5 mice). (C) Survival curves of fl/fl and Δ/Δ mice treated with 25 μg/g pIpC delivered on alternate days for a total of 3 doses. (D) Percentages of terminal erythroid cell populations in BM (n = 3-4 mice). (E) Representative flow cytometry dot plots of CD44 expression vs forward scatter (FSC), after gating on TER119+ cells (left) and quantification of terminally differentiated erythroid subsets in fl/fl and Δ/Δ mice (right) (n = 4-5 mice). (F) Quantitative results from BFU-E colony formation assays performed using 3 × 103 cKit+ BM cells from fl/fl and Δ/Δ mice cultured in MethoCult SF M3436 methylcellulose-based medium for 10 to 14 days (n = 4 mice). (G) Quantitative results (left) from CFU-E colony formation assays performed using 3 × 103 cKit+ BM cells from fl/fl and Δ/Δ mice cultured in MethoCult SF M3334 methylcellulose-based medium for 48 hours and representative images from 3 independent experiments (right). Scale bar, 25 μm (n = 4 mice). (H) Representative flow cytometry dot plots (bromodeoxyuridine [BrdU]/Hoechst) of MEP cells (left) and a graph showing the percentage of BrdU+ MEP cells (right) (n = 3-4 mice). (I) Percentages of MEP cells in different cell cycle phases (n = 3-4 mice). (J) Quantitative results (left) from CFU-E colony formation assays performed using 500 fresh BM MEP sorted from fl/fl and Δ/Δ mice and cultured in MethoCult SF M3334 methylcellulose-based medium for 48 hours (right) (n = 3 mice). (K) Quantitative results (left) from BFU-E colony formation assays performed using 1000 fresh BM MEP cells sorted from fl/fl and Δ/Δ mice and cultured in MethoCult SF M3436 methylcellulose-based medium for 2 weeks (right), (n = 3 mice). Data represent the mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; unpaired 2-tailed Student t test. HGB, hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/7/10.1182_blood.2023021901/2/m_blood_bld-2023-021901-gr3.jpeg?Expires=1769103615&Signature=LvZYzqtI9399Vsgw4voOni5IvzbBcurVI7nyl0ht5z8hGKtHhNxO-vVD4YpgSiAsRJTMvZ~IhMaaoMq2BXm0oh4HuZJ1jkhCKfnmF5QogQlPKUxwr6xkwYTJl6-ZDwTK7R9QuK1NIX~v8fObRDcfS5L1IN0UnERmjGaNDD7d0uBXXfo7VgxImQGG~UAIitaZ4C15E07mEjKe9rjPRGCCYU85k-3sf-qOAY3VXpAMRFp-jTlZuXHVJgRLzxA0VqYfA1OpTRrrNkLJ7gXZ~ZfVrOlJQMDNzTlWoS~hy9kNc4sZPdQq1A5IK6aZ5vWBax2A0fuPR8D8yWbMx7jVOMIybA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal