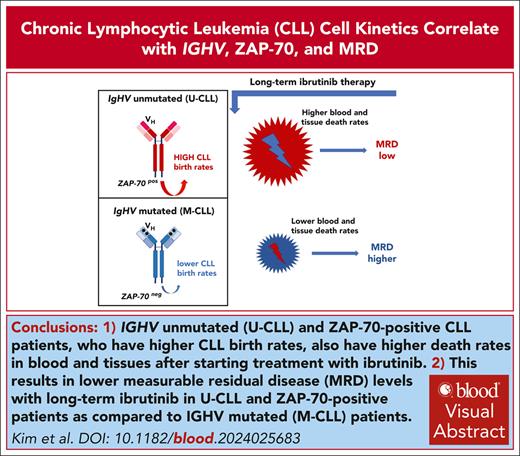
Visual Abstract
Deuterated (“heavy”) water labeling in patients with chronic lymphocytic leukemia (CLL) demonstrates that IGHV unmutated and ZAP-70+ patients have higher blood and tissue CLL death rates on ibrutinib therapy, resulting in lower measurable residual disease levels with long-term ibrutinib treatment. This trial was registered at www.clinicaltrials.gov as #NCT01752426.
TO THE EDITOR:
Deuterated (“heavy”) water labeling directly in patients with chronic lymphocytic leukemia (CLL) demonstrated that ibrutinib treatment blocks proliferation and accelerates CLL cell death.1 Here, we conducted long-term analyses of patients treated with ibrutinib in this clinical trial to determine whether in vivo CLL cell kinetics correlate with disease characteristics and depth of remission. Bruton tyrosine kinase inhibitors (BTKis) are a standard therapy for patients with CLL. Ibrutinib is the first in class BTKi that demonstrated significantly improved progression-free survival (PFS) as compared with chemotherapy, CD20 antibodies, or combinations thereof in a series of randomized trials in untreated patients and patients with relapsed or refractory CLL.2 Importantly, ibrutinib treatment can overcome the negative prognostic impact of CLL risk factors, such as the mutational status of the immunoglobulin heavy-chain variable (IGHV) genes.3IGHV sequencing distinguishes mutated CLL (M-CLL) with ≥2% deviation from the IGHV germ line sequence from unmutated CLL (U-CLL) with <2% deviation.4,5 Patients with M-CLL present with more indolent disease, whereas patients with U-CLL typically have more aggressive disease features. This clinical difference has been linked to greater B-cell receptor (BCR) signaling responsiveness in U-CLL,6 with higher surface IgM and more robust signaling after IgM stimulation in U-CLL,7 resulting in enhanced CLL cell viability.8,9 Expression of the ζ-chain-associated protein kinase 70 (ZAP-70) in CLL correlates with U-CLL, with more aggressive disease presentation, and appears to have stronger predictive value than IGHV mutation status in discordant cases.10 ZAP-70, which normally regulates T-lymphocyte signaling, enhances constitutive and ligand-induced BCR signaling in CLL cells.11,12
The patients enrolled in this study drank deuterated water during a 4-week labeling phase, followed by 6 to 12 weeks of a resting phase. Then continuous long-term ibrutinib monotherapy was started. Peripheral blood (PB) measurable residual disease (MRD) was quantified by flow cytometry with a sensitivity of 10–4.13 Immunohistochemical staining for ZAP-70 protein was performed as described.14 CLL cell birth and death rates were quantified as described.1,15 Briefly, quantification of 2H enrichment in the DNA of CLL cells was done in serial samples by gas chromatography/pyrolysis/isotope ratio-mass spectrometry, as the basis for calculating CLL cell birth and death rates. Tissue CLL cell death rates were calculated based on kinetic changes in tissue volumes (spleen, lymph nodes).1,16 The Kaplan-Meier method was used to estimate survival, including overall survival and PFS. The Wilcoxon rank sum test was used to compare 2 groups of continuous variables. The Spearman rank test was used to test the correlation between 2 groups. Groups with high or low CLL cell death rates were defined as being above or below the median.
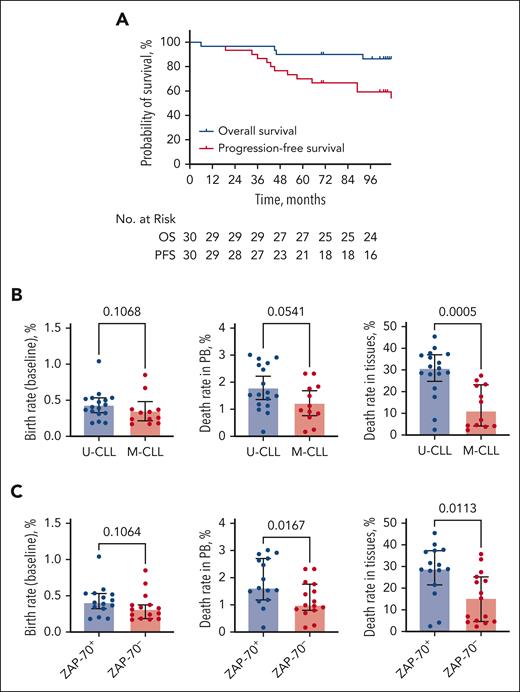
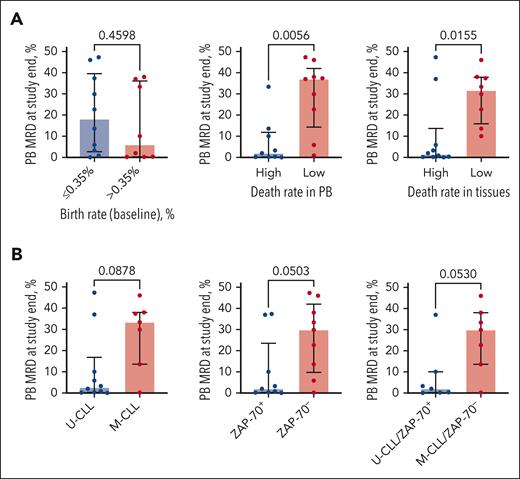
Out of 30 previously untreated patients with CLL enrolled in the trial, 22 patients (73.3%) completed 5 years of ibrutinib treatment on study and subsequently continued treatment with commercial ibrutinib. After a median follow-up of 107 months (range, 6-111), 21 patients discontinued treatment, and 9 patients still received ibrutinib. Five-year overall survival and PFS rates were 90% (95% confidence interval, 79.88%-100%) and 70% (95% confidence interval, 55.38%-88.48%, Figure 1A), respectively. Five patients (17%) achieved a complete remission, 24 (80%) achieved a partial remission, and 1 patient (3%) had stable disease, resulting in an overall response rate of 97%. The detailed patient characteristics, reasons for ibrutinib discontinuation, remission rates at different time points, treatment outcome in each individual patient, and correlations between PB and tissue death rates can be found in the supplemental Material, available on the Blood website. CLL cell birth rates were slightly higher in patients with U-CLL and ZAP-70+, without reaching significance, and were comparable to the measured birth rates in a larger study where this difference (ie, higher birth rates in U-CLL) was statistically significant.17 CLL cell death rates in PB also tended to be higher in patients with U-CLL vs patients with M-CLL (1.79% ± 0.2% vs 1.22% ± 0.21%, mean ± standard error of the mean, P = .054) (Figure 1B). Tissue CLL cell death rates were significantly higher in patients with U-CLL compared with patients with M-CLL (28.88% ± 2.75% vs 13.16% ± 2.82%, P = .0005) (Figure 1B). A similar pattern was observed for ZAP-70+ vs ZAP-70– patients (Figure 1C). PB MRD levels were tested in 18 patients after 5 years of ibrutinib therapy. We found that all patients had detectable MRD (median: 11.8%, range, 0.06%-47.30%). CLL cell death rates in PB and tissues inversely correlated with the level of PB MRD after 5 years (r = −0.5872, P = .0104 and r = −0.4407, P = .0672) (Figure 2A). Specifically, MRD levels were significantly lower in patients with high CLL cell death rates in the PB (7.03% ± 3.68% vs 29.46% ± 5.53%, P = .0056) and in the tissues (9.73% ± 5.49% vs 28.89% ± 4.42%, P = .0155) as compared with patients with lower CLL cell death rates (Figure 2A). MRD levels also were lower in patients with U-CLL and ZAP-70+ CLL (10.71% ± 5.39% vs 28.36% ± 6.02%, P = .0878 and 10.15% ± 5.22% vs 26.34% ± 5.65%, P = .0503) (Figure 2B).
Survival on ibrutinib therapy and CLL cell kinetics during the first months of treatment. (A) Overall survival (OS) and PFS of patients enrolled in the study. (B) CLL cell birth rates prior to the start of therapy (left) and CLL cell death rates in PB (middle) and tissues (right), measured at the beginning of ibrutinib therapy. Depicted are comparisons between patients with unmutated vs mutated IGHV (U-CLL vs M-CLL). Median with interquartile range. (C) The same parameters were compared between patients with ZAP-70+ and ZAP-70– CLL.
Survival on ibrutinib therapy and CLL cell kinetics during the first months of treatment. (A) Overall survival (OS) and PFS of patients enrolled in the study. (B) CLL cell birth rates prior to the start of therapy (left) and CLL cell death rates in PB (middle) and tissues (right), measured at the beginning of ibrutinib therapy. Depicted are comparisons between patients with unmutated vs mutated IGHV (U-CLL vs M-CLL). Median with interquartile range. (C) The same parameters were compared between patients with ZAP-70+ and ZAP-70– CLL.
Measures of CLL cell kinetics early in the course of treatment with ibrutinib correlate with PB MRD. (A) PB MRD after 54 to 60 cycles of ibrutinib in patients with high or low CLL cell birth rate (left), and CLL cell death rate in PB (middle) or tissues (right) after ibrutinib treatment initiation. Median with interquartile range. (B) PB MRD after 54 to 60 cycles of ibrutinib in patients stratified by IGHV mutational status and ZAP-70 expression (positive vs negative). Median with interquartile range.
Measures of CLL cell kinetics early in the course of treatment with ibrutinib correlate with PB MRD. (A) PB MRD after 54 to 60 cycles of ibrutinib in patients with high or low CLL cell birth rate (left), and CLL cell death rate in PB (middle) or tissues (right) after ibrutinib treatment initiation. Median with interquartile range. (B) PB MRD after 54 to 60 cycles of ibrutinib in patients stratified by IGHV mutational status and ZAP-70 expression (positive vs negative). Median with interquartile range.
These findings, together with prior CLL kinetic studies,17 demonstrate that patients with U-CLL have more proliferative disease, and higher cell death rates are observed when patients with U-CLL are treated with the BTKi ibrutinib, pointing toward a high importance of BCR- and BTK-mediated signaling for the growth and survival of U-CLL clones. The finding that this translates into better disease clearance, resulting in lower MRD in U-CLL, is novel and helps explaining the high efficacy of BTKi therapy in U-CLL. Whether the depth of remission or MRD is clinically relevant in patients with CLL treated with BTKi therapy remains controversial. Woyach et al18 noted that low-risk patients with M-CLL with del(13q) often have slow disease clearance with prolonged lymphocytosis, without having an inferior PFS. In frontline CLL therapy, however, the combination of acalabrutinib with obinutuzumab resulted in deeper remissions and longer PFS than acalabrutinib monotherapy.19
To our knowledge this is the first study reporting about deeper remissions and greater responsiveness of patients with U-CLL and ZAP-70+ disease to BTKi monotherapy, resulting in higher blood and tissue cell death rates (Figure 1) and lower PB MRD levels with long-term BTKi therapy (Figure 2). Interestingly, opposite findings were seen after frontline fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy, where patients with U-CLL had fewer complete remissions and higher MRD levels than patients with M-CLL.20 Frontline treatment with venetoclax plus obinutuzumab (CLL14 trial) did not result in significantly different MRD levels in U-CLL vs M-CLL at the end of therapy.21 In contrast, and consistent with the data presented here, the CAPTIVATE trial investigators reported that patients with U-CLL achieve deeper remissions after treatment with ibrutinib plus venetoclax.22 Similarly, the GLOW and FLAIR trials also reported about deeper remissions in patients with U-CLL treated with ibrutinib plus venetoclax,23,24 suggesting that the ibrutinib component of this treatment favors achievement of deeper remissions. Whether similar findings will be seen when second-generation BTKis are used alone or in combination with venetoclax remains to be seen.
Collectively, these findings indicate that the CLL cells from patients with unmutated IGHV and ZAP-70+ are highly dependent on BTK and BCR signaling for their survival and growth. Consequently, blockade of BTK and BCR signaling with ibrutinib in U-CLL/ZAP-70+ patients induces deeper remissions when ibrutinib is used alone or in combination with venetoclax. However, with time-limited therapy, patients with U-CLL also likely will relapse sooner,23 despite achieving deeper remissions, which can be attributed to the U-CLL-inherent higher proliferative drive of the leukemia cells.
This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board, and informed consent was obtained from patients in accordance with institutional guidelines and the Declaration of Helsinki. The study was registered at clinicaltrials.gov (NCT01752426), was conducted in accordance with the Declaration of Helsinki, and was sponsored by Pharmacyclics, an AbbVie Company.
Acknowledgments
The authors thank the patients who participated in this trial and their families and the research nurses and study coordinators at MD Anderson who helped to conduct this study. The investigators and their research teams collected the data. The investigators confirmed the accuracy of the data and compiled these for analyses.
The study was supported by a Cancer Center Support Grant (National Institutes of Health, National Cancer Institute grant P30 CA016672), MD Anderson’s Moon Shots Program in CLL, and Pharmacyclics, an AbbVie company.
Authorship
Contribution: E.K., S.-S.C., M.S., and D.W. analyzed and summarized the data; H.H. and X.H. performed statistical analysis; A.F., N.J., and W.G.W. provided patient care and reviewed the manuscript; N.C. and J.A.B. designed the research and wrote the paper with E.K.; and all authors had full access to the data and were involved in data interpretation, as well as the revisions and final approval of the manuscript and vouched for the accuracy of the reported data, confirmed adherence to the protocol, and made the decision to submit the data for publication.
Conflict-of-interest disclosure: J.A.B. reports research funding from Pharmacyclics, BeiGene, and AbbVie and honoraria from Janssen. A.F. reports research funding from AstraZeneca and BeiGene. W.G.W. reports research funding from AbbVie and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Jan A. Burger, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Unit 428, PO Box 301402, Houston, TX 77230-1402; email: jaburger@mdanderson.org.
References
Author notes
Primary results were presented in part at the 64th Annual Meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
Data are available on request from the corresponding author, Jan A. Burger (jaburger@mdanderson.org).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal