In this issue of Blood, Oluwole et al address the important question of the incidence of stroke in adults as well as in children with sickle cell disease (SCD), using a California database formed from hospital discharge and emergency department information.1 The cumulative incidence of the cerebrovascular events (CVEs) (ischemic and hemorrhagic stroke and transient ischemic attack) appears to have increased in California during the decade from 2010 to 2019 across all age groups, including children and adolescents.
This finding is disappointing given the availability since 1998 of transcranial Doppler (TCD) screening and regular transfusion for abnormal TCD velocity >200 cm/s for children with homozygous SCD. This program was initially associated with a reduction in childhood strokes in SCD in the same state.2 Moreover, the Affordable Care Act expanded health care access since 2010, and in 2014, the guideline suggesting hydroxyurea use for life from 9 months of age was published; universal implementation3 and adherence to the prescribed dose are important. Despite these advances, the problem of CVE is not yet solved for individuals with SCD.
Comparison of the current study to the prehydroxyurea Cooperative Study of Sickle Cell Disease4 is difficult because of differences in age groups and in definition of CVEs, in part related to improvements in neuroimaging. While for young adults the incidence may be lower now, for adults aged 31 to 50 years there is little evidence of improvement. Unfortunately, data on compliance with, and effectiveness of, screening and treatment at the provider and patient level are not available in Oluwole et al’s article. This makes interpretation of recurrence data particularly difficult.
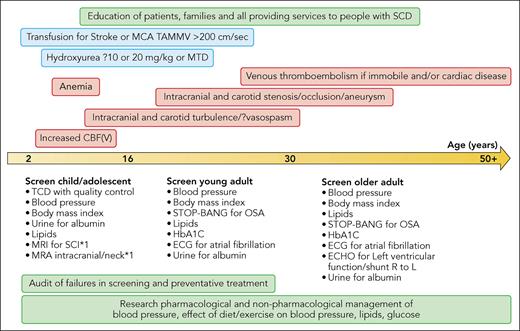
TCD measures velocity, which may be increased secondary to either vessel narrowing if there is intracranial vasculopathy, which may reduce cerebral blood flow (CBF) focally, or to global increase in CBF, which reduces the cerebrovascular reserve (CVR), that is, the ability to further increase CBF for increased metabolic demand when required. Abnormal velocities predict stroke secondary to vasculopathy or reduced CVR. TCD screening may not prevent stroke because the childhood population is not comprehensively screened, because of time and travel costs for separate appointments, or if the study is suboptimal, or because the mechanism is not high intracranial vessel velocity.5 Suboptimal studies may occur because sonographers undertake TCD screening in SCD relatively rarely, and the technique is operator dependent.6 Technical problems leading to suboptimal studies include inability to detect and track all vessels or to recognize artefact, as well as reporting of peak systolic rather than the time-averaged mean of the maximum velocity (TAMMV) on which the efficacy data were based.2,6 Whereas nonimaging TCD is typically used to interrogate the entire length of the middle and anterior cerebral arteries without angle correction, imaging TCD studies, with or without angle correction, may report only on the maximum at 1 or 2 points in each vessel, appropriately detecting high CBF but potentially missing short segments of vasculopathy. TCD cannot diagnose risk factors for hemorrhagic stroke, for example, aneurysms, or vasculopathy in the extracranial carotid or vertebral arteries; low velocities for age or asymmetry or inadequate studies may be a clue to the latter and should be followed up with neck vessel imaging. It is essential that the local standard operating protocol ensures that it is TAMMV that is measured,2,6 that the correct threshold for conditional and abnormal TAMMV is applied for imaging TCD depending on whether or not angle correction is used, and that training is adequate and is reinforced regularly via peer review.6 There should also be a pathway for alternative imaging for diagnosis of intracranial and extracranial vasculopathy, for example, magnetic resonance angiography or computed tomography angiography. Education of families and providers, with audits of screening and treatment failure at population and individual level, is needed now more than ever (see figure).
Timeline illustrating known risk factors for CVEs in SCD from childhood onward. Red boxes indicate risk factors; blue boxes indicate evidence-based management strategies; black text outside boxes indicates suggested screening; green boxes indicate population-based strategies for education to improve compliance with screening and evidence-based management strategies, closure of the audit loop if these strategies fail and stroke occurs, and research priorities to reduce stroke risk long term. MCA, middle cerebral artery; CBF(V), cerebral blood flow (velocity); MRI, magnetic resonance imaging; SCI, silent cerebral infarction; MRA, magnetic resonance angiography; STOP-BANG, online questionnaire Snoring? Tired? Observed? Pressure? Body Mass Index >35 kg/m2? Age? Neck circumference? Gender = male?; OSA, obstructive sleep apnea; HbA1C, hemoglobin A1C (glycated hemoglobin); ECG, electrocardiogram; ECHO, echocardiography; L, left; R, right. Professional illustration by Patrick Lane, ScEYEnce Studios.
Timeline illustrating known risk factors for CVEs in SCD from childhood onward. Red boxes indicate risk factors; blue boxes indicate evidence-based management strategies; black text outside boxes indicates suggested screening; green boxes indicate population-based strategies for education to improve compliance with screening and evidence-based management strategies, closure of the audit loop if these strategies fail and stroke occurs, and research priorities to reduce stroke risk long term. MCA, middle cerebral artery; CBF(V), cerebral blood flow (velocity); MRI, magnetic resonance imaging; SCI, silent cerebral infarction; MRA, magnetic resonance angiography; STOP-BANG, online questionnaire Snoring? Tired? Observed? Pressure? Body Mass Index >35 kg/m2? Age? Neck circumference? Gender = male?; OSA, obstructive sleep apnea; HbA1C, hemoglobin A1C (glycated hemoglobin); ECG, electrocardiogram; ECHO, echocardiography; L, left; R, right. Professional illustration by Patrick Lane, ScEYEnce Studios.
SCD complications including acute chest syndrome, renal failure, and liver failure were risk factors for intracranial hemorrhage in Oluwole et al’s data. Despite the limitations of the data set resulting in reporting of minimum prevalences, 2 common modifiable risk factors in the general population, hypertension and hyperlipidemia, were associated with all CVEs. Hypertension has long been known to predict stroke in SCD4 and is associated with extension of silent cerebral infarction in adults,7 but there is debate about whether the range of normal is lower or higher than in the general population, which means that the threshold for increased stroke risk remains unclear.8 Monitoring 24-hour ambulatory blood pressure and regular clinic measurement with plotting of individual trajectories to predict worsening hypertension are likely to be important in preventing renal and cardiac, as well as cerebral, complications in patients with SCD. In addition, there are few studies on the effect of pharmacological treatment for hypertension, and nonpharmacological strategies employed in the general population, such as reducing salt intake, preventing an increase in body mass index, or relaxation techniques including breathing exercises, have received little attention. In Oluwole et al’s article, the diagnosis of hyperlipidemia does not appear to have been made until the age of 30 to 60 years. Again, in the SCD population, there is little published data on pharmacological or nonpharmacological methods of improving lipid profile and whether such interventions reduce stroke risk.
Other risk factors not explored in the current study, including atrial arrythmia,9 obstructive sleep apnea, diabetes, and venous thromboembolism, need to be considered. Most of the stroke syndromes characterized in the general population, including extracranial and intracranial stenosis, dissection and occlusion, embolic stroke, posterior reversible encephalopathy syndrome, and sinovenous thrombosis, have been reported in SCD.5 The problem could be due to accelerated ageing,10 with chronic damage of the cerebral vasculature in older patients where there has been turbulence related to the increased global CBF in the developing brain (see figure). Analysis of available longitudinal data, for example, on those with abnormal TCD in childhood or with serial measurement of blood pressure, is likely to be informative. Future research into reduction of CVE in SCD might compare pharmacological and nonpharmacological methods of improving the blood pressure, lipid, and glucose profiles of people with SCD (see figure).
Conflict-of-interest disclosure: F.J.K. has received funding from Global Blood Therapeutics, a wholly owned subsidiary pf Pfizer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal