In this issue of Blood, Maurer et al1 identify predictors of response at 1 year to the chimeric antigen receptor T-cell (CAR-T) therapy, axicabtagene ciloleucel (axi-cel), by undertaking multimethodology, multi–time-point analysis of 3 patient cohorts with large B-cell lymphoma (LBCL). Axi-cel is 1 of 3 Food and Drug Administration–approved CAR-T therapies for LBCL and has shown real-world outcomes that match clinical trial data.2 Yet responses to CAR-T therapy are not uniformly durable.3 Identifying the factors that predict durable responses from multiomic observations will lead to improved patient selection, as well as the formulation of next-generation cellular therapies.
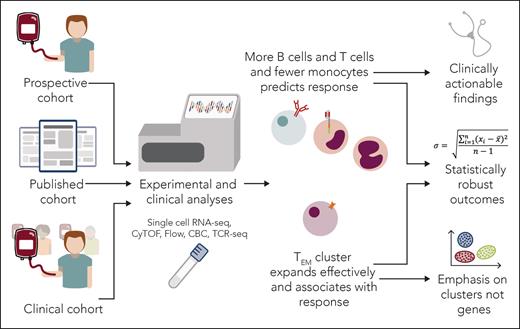
In the article, Maurer et al analyzed 3 different patient cohorts at various time points with diverse methods: complete blood counts (CBCs), flow cytometry, single-cell RNA sequencing (scRNA-seq), T-cell receptor sequencing (TCR-seq), and cytometry by time of flight (see figure). The diverse, concatenated data and multiple analytic platforms contribute to the statistical robustness of the work. The article also demonstrates that parsing the effects of measurable biological variables from clinical covariates when predicting response to CAR-T therapy may require even larger confirmatory studies as well as other technologies. Nonetheless, as far as we are aware, this is the largest published multiomic dataset of patients with LBCL receiving CAR-T therapy, and thus presents intriguing findings.
Maurer et al have undertaken a manifold analysis of multiple patient cohorts at multiple times receiving CAR-T therapy. The conclusions provide further insight into this vital therapy and chart a path forward for clinical application and further studies. CyTOF, cytometry by time of flight; TEM, effector memory T cell. Professional illustration by Somersault18:24.
Maurer et al have undertaken a manifold analysis of multiple patient cohorts at multiple times receiving CAR-T therapy. The conclusions provide further insight into this vital therapy and chart a path forward for clinical application and further studies. CyTOF, cytometry by time of flight; TEM, effector memory T cell. Professional illustration by Somersault18:24.
The authors show there was no clear transcriptomic association with response when analyzing postinfusion circulating peripheral blood mononuclear cells by scRNA-seq. With over 308 000 cells analyzed, this may not be due to lack of statistical power, but may imply that when comparing responders with nonresponders, timing of analysis may influence detection of response in that noncompetent cells in nonresponders may have died by the time of sampling. Future studies of postinfusion predictors of response may also require different technologies and statistical approaches that account for the tumor microenvironment, the effects of lymphodepletion, and the transcriptional noise inherent in CAR-T expansion.
In addressing preinfusion predictors, the authors find that the prelymphodepletion ratio of absolute lymphocytes to absolute monocytes (ALC:AMC) and circulating B-cell numbers was associated with response and that these 2 factors appear to operate independently. Importantly, this finding was validated in a large cohort of CBC from 99 patients from the pivotal ZUMA-1 trial of axi-cel in LBCL. This is an intriguing finding and raises questions about underlying mechanisms and potential to co-opt these factors in future iterations of CAR-T therapy. However, confounding clinical factors, in particular time from last therapy, are difficult to control for. The authors do report that the association with response holds when stratifying prelymphodepletion B-cell numbers by time from therapy, though the patient numbers in the stratified groups were small. Likewise, the association of baseline ALC:AMC with response may also be confounded by time from last therapy. However, if these 2 variables do operate independently of time from therapy, there may be scope in the future for appropriate patient selection on the basis of these readily available laboratory parameters.
Concentrating on the role of T-cell subsets at baseline, Maurer et al show that frequency of prelymphodepletion host CD8+ T cells predicts response. Supporting this, a growing number of recent preclinical and clinical studies intimate a role for the host immune system in assisting CAR-T therapy.4,5 Furthermore, there were more non-CAR-T peripheral blood CD8+ T cells in nonresponders following infusion, implying that CAR− cells in responders may migrate rapidly to tumors following CAR-T infusion. However, further studies on the role of time from last therapy on baseline CD8+ T-cell count and controlling for the effect of lympholytic doses of steroids for cytokine release syndrome on the T-cell compartment of responders will be required.
Turning to the infusion product (IP), the authors use high-dimensional scRNA-seq and TCR-seq to elucidate predictors of durable response and identify expanding clones from the IP following expansion. Akin to the findings in the postinfusion samples, there were no genes of note associated with response within the IP, pointing the way toward future studies that will account for patient and tumor factors, as well as statistical noise in transcriptional output in highly activated manufactured CAR-T products.
When looking at cell clusters, not genes, in the IP, the authors showed that CD8+ T cells and, in particular, non-CAR CD8+ T cells were associated with durable response. They further show that the expanding clones of response-associated T cells from the IP were numerically of a more effector memory (EM) phenotype. Ostensibly, this contradicts other studies in which less differentiated central memory (CM) and naïve CAR-Ts are posited as the most desired IP phenotype to achieve responses, and genes associated with differentiated effector cells tend to be associated with poor responses.6,7 Likewise, in clinical and preclinical studies, premanufactured products derived from CM or naïve cells tend to be associated with enhanced responses and persistence, respectively.8,9 However, the contrast between Maurer et al and other studies may be a consequence of the peculiarities of T-cell clone counting, timing of analysis, and manufacturing. If competent EM cells in responders expand more in manufacturing and postinfusion, they are more likely to be detected.10 The corollary would be fewer competent EM cells in nonresponders, as these cells have failed to persist, resulting in a subsequent relative overrepresentation of CM cells. Furthermore, accounting for clonal tracking using more sensitive barcoding technologies in the apheresis product may reveal that CM and naïve cells are indeed the precursor to the competent EM fraction in responders. Regardless, the finding across multiple RNA-seq studies that responses are associated with groups of cells and not individual genes indicates that the quality of the population may be more important than isolated genetic predictors.
The real-world applicability of the findings from Maurer et al is patent. The article charts avenues to creating next-generation CAR-T products that preserve baseline immunity, such as by avoiding or altering lymphodepletion. However, the study also highlights the complexities of large-scale data analysis, calling for future studies that consider stable markers of cell phenotype, patient factors, and the tumor microenvironment.
Conflict-of-interest disclosure: S.F. has filed and received license fees on patents for optimizing CAR-T function, has received research laboratory grants from Bristol Myers Squibb (BMS), and has consulted ad hoc for Prescient Therapeutics. C.J.T. received research funding from Juno Therapeutics/BMS, Nektar Therapeutics, and NanoString; serves on Scientific Advisory Boards for Caribou Biosciences, T-CURX, Myeloid Therapeutics, Arsenal Bio, CARGO Therapeutics, Celgene/BMS Cell Therapy, Differentia Bio, eGlint, and Advesya; is a Data Safety and Monitoring Board member for Kyverna; has served in ad hoc advisory/consulting roles for Prescient Therapeutics, Century Therapeutics, IGM Biosciences, AbbVie, Boxer Capital, and Novartis; holds stock options for Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, Arsenal Bio, CARGO Therapeutics, and eGlint; and has the right to receive payment from Fred Hutch Cancer Center as an inventor on patents related to CAR-T therapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal