In this issue of Blood, Strong et al1 demonstrate the compatibility of mRNA lipid nanoparticle (LNP) transfection to genetically engineer platelet components meant for transfusion. Using previously developed LNPs, the authors show their applicability to platelet transfusion products, demonstrating no changes in platelet function or lifespan after transfection. The work highlights the potential for new therapeutic strategies involving the genetic manipulation of donor platelets for improved clinical outcomes (see figure). In addition, development of these tools is laying the groundwork for enhanced tools for studying the fundamentals of platelet biology.
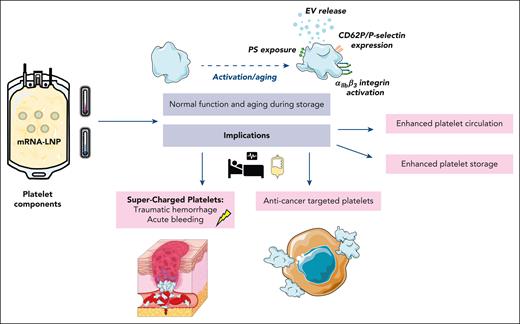
Possible clinical implications for use of lipid nanoparticles for genetic engineering of platelets compatible with blood bank storage conditions. EV, extracellular vesicles; PS, phosphatidylserine. Illustration prepared using Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 4.0 Unported License (https://creativecommons.org/licenses/by/4.0).
Possible clinical implications for use of lipid nanoparticles for genetic engineering of platelets compatible with blood bank storage conditions. EV, extracellular vesicles; PS, phosphatidylserine. Illustration prepared using Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 4.0 Unported License (https://creativecommons.org/licenses/by/4.0).
Anucleate platelets were originally thought to lack the capability for both transcription and translation, with early reports suggesting that, basally, platelets do not synthesize proteins. Consistent with a lack of translation, platelets generally lose protein as they age within the circulation, highlighting the limited capacities of these unique cells.2 However, the presence of translational machinery, including polyribosome complexes and regulatory elements for translation initiation, indicate that platelets do indeed have the ability to synthesize new proteins.3 Recent work has demonstrated that, after stimulation, platelets use residual splicing machinery inherited from progenitor megakaryocytes to produce mature mRNA for subsequent translation.3 Although the mechanisms and consequences of protein synthesis in platelets have yet to be fully established, several groups have reported activation-dependent translation for a variety of proteins, including cyclooxygenase-1, plasminogen activator-1, interleukin-1β, and tissue factor, highlighting the importance of protein synthesis for their hemostatic function.3
Given the inherent nature of platelets to release their intracellular cargo, they have been explored as drug-delivery systems. However, to date, engineering donor-derived platelets to provide better clinical outcomes has not been studied extensively. Clinically, platelet transfusion may be required for a myriad of reasons such as acute traumatic, surgical, or obstetric hemorrhage, or in those with a low platelet count due to disease or chemotherapy.
Platelet products are manufactured using different methods, such as (1) direct donation by 1 donor through apheresis, (2) buffy coat–derived pooled platelets from whole blood donation, or (3) the platelet-rich plasma method. Platelet components are conventionally stored at 21°C to 24°C (room temperature), under slight agitation for 5 to 7 days in 100% plasma or 30% to 40% plasma combined with 70% platelet additive solution. To prevent bacterial growth, along with the detrimental changes that occur during platelet storage at room temperature, alternative platelet-storage methods, such as cold storage or cryopreservation, are used and are being further assessed in clinical trials.4
Previous work on engineering platelets has focused on introducing alterations at the megakaryocyte level, thereby facilitating the production of modified platelets. Strong and colleagues robustly demonstrate the ability to viably transfect platelets stored under conventional blood bank storage conditions in platelet additive solutions and in plasma, and thus the implications of their work may be extensive. The ability to genetically engineer platelets may enhance their circulatory capabilities posttransfusion, leading to improved outcomes. For example, by targeting enzymes that specifically preserve carbohydrates on the platelet surface, such as St3gal4 sialyl-transferase, the platelets’ half-life in the circulation would increase in vivo because removal of sialic acid is an important “eat-me” signal.5
During storage, platelets slowly become dysfunctional, eventually undergoing cell death pathways, and therefore have a limited shelf life. Using these genetic manipulation tools, it may be possible to modify the expression of Bcl-2 family proteins other than Bax/Bak,6 and alter the mechanisms involved in cell death pathways, potentially enhancing the shelf life of transfusion products. Platelet apoptosis during cold storage is also linked to platelet signaling by the adhesion receptor for von Willebrand factor, glycoprotein Ibα, scaffolding 14-3-3ζ, release of bioreactive lipid arachidonic acid, and downstream RhoA GTPAse activity.7,8 Targeting these mechanisms, proteins, or enzymes involved may lead to platelets being preserved better and longer, preventing platelet wastage.
In addition to increasing storage longevity, tailoring platelet function to a patient’s needs could be highly advantageous. For patients who are actively bleeding, “ultra”-functional platelets could be manufactured with increased prohemostatic or procoagulant capabilities. For example, enhancing phosphatidylserine exposure on the platelet surface by targeting TMEM16 scramblase may facilitate the release of more phosphatidylserine-positive extracellular vesicle release during storage, which may support a more rapid return to hemostasis in actively bleeding patients.
Oncology patients are recipients of the most platelet transfusions. Patients with cancer are also prone to thrombosis, because platelets become hyperactive and can activate tumor cells. It is well established that there is crosstalk between platelet and tumor cells, with direct interactions occurring between CLEC-2 and podoplanin on tumor cells.9 Genetic modification of CLEC-2 in platelet transfusion components may reduce subsequent platelet-tumor cell interactions, potentially limiting metastasis and reducing the incidence of thrombotic events in these patients. These tools also offer the potential to genetically modify platelet components to express anticancer agents, which could be transferred to tumor cells through direct interactions and the exchange of cargo.
This elegant study by Strong et al provides the first evidence that using mRNA-loaded LNPs to enhance protein expression is viable in platelet components used in blood banks globally. Importantly, the authors also show that these transfected platelets age and retain their function in a manner similar to that of nontransfected platelets. Animal studies should be performed to ensure that the engineered platelets are safe and functional for cessation of bleeding in vivo. Assessing platelet half-life and clearance after transfusion in animal models, and eventually in human clinical trials, will provide more answers. In addition, platelets are important immune cells capable of releasing a plethora of immunomodulatory cytokines, such as platelet-derived growth factor, soluble CD40 ligand, and high-mobility group box 1, during platelet storage.10 Testing the immune potential of the newly engineered platelets will be crucial in the prevention of severe transfusion-related diseases.
In conclusion, Strong et al describe an exciting technological advancement in producing genetically engineered platelets compatible with platelet transfusion components. Building on this proof-of-concept work, future studies focused on genetic engineering with physiologically relevant proteins will be paramount. Such work will pave the way for the use of genetic engineering in transfusion products and open further avenues for platelet cell therapies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal