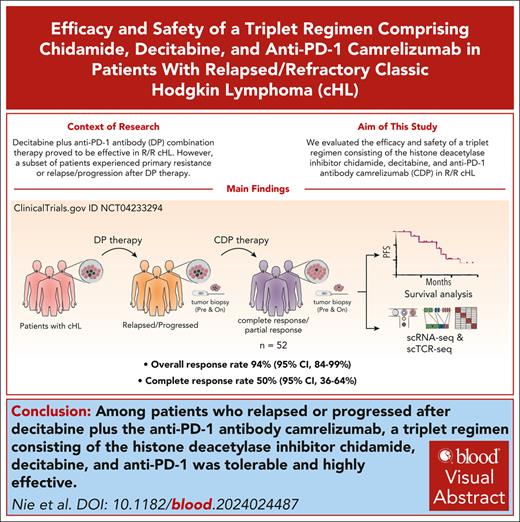
Key Points
Among patients relapsed or progressed after DP, CDP combination therapy is tolerable and highly effective.
CDP therapy can relieve immunosuppression, increase immunogenicity, and enhance the killing of heterogenous HRS cell populations.
Visual Abstract
DNA methyltransferase inhibitor decitabine plus anti–programmed cell death 1 (DP) therapy was effective in relapsed/refractory classic Hodgkin lymphoma (cHL). However, a subset of patients experienced primary resistance or relapse/progression after DP therapy. In this study, we evaluated the efficacy and safety of a triplet regimen consisting of the histone deacetylase inhibitor chidamide, decitabine, and anti–PD-1 camrelizumab (CDP) in 52 patients who previously received DP therapy. CDP treatment was well tolerated and resulted in an objective response rate of 94% (95% confidence interval [CI], 84-99), with 50% (95% CI, 36-64) of patients achieving complete response (CR). Notably, all patients who were recalcitrant to previous DP treatment exhibited therapeutic responses after CDP therapy, although their CR rate was lower than patients responsive to prior DP. Overall, the median progression-free survival was 29.4 months. Through single-cell RNA sequencing of pretreatment and on-treatment cHL tumor biopsy samples, we observed the heterogeneity of rare malignant Hodgkin Reed/Sternberg (HRS)–like cells. The classical CD30+ HRS-like cells interacted with abundant immunosuppressive IL21+CD4+ T helper cells, forming a positive feedback loop that supported their survival. While the CD30– HRS-like cell population showed potential resistance to anti–PD-1 immunotherapy. CDP treatment promoted the activation of diverse tumor-reactive CD8+ T cells and suppressed the proliferation of IL21+CD4+ T cells by inhibiting STAT1/3 signaling, thereby alleviating their immunosuppressive effects. These findings provide insights into the cHL microenvironment that contributes to anti–PD-1 resistance and highlight the therapeutic effectiveness of dual epi-immunotherapy in overcoming immunotherapy resistance. This trial was registered at www.clinicaltrials.gov as #NCT04233294.
Introduction
Programmed cell death 1 (PD-1) blockade therapy reinvigorates antitumor immunity and has been approved for patients with relapsed/refractory classic Hodgkin lymphoma (cHL) who have failed after autologous stem cell transplant (ASCT) or are ineligible for transplant. Most patients (65%-87%) with relapsed/refractory cHL obtained clinical responses, and 20% to 30% of patients acquired complete response (CR) and durable response.1-4 To further augment CR rate, various combination regimens involving anti–PD-1 have been explored. We previously reported that DNA methyltransferase inhibitor decitabine plus anti–PD-1 camrelizumab (DP) therapy resulted in high CR rate of 71% with a median progression-free survival (PFS) of 35 months among anti–PD-1 treatment–naïve patients with relapsed/refractory cHL.5 However, a subpopulation of patients with cHL exhibit primary resistance, and a proportion of patients develop acquired resistance after anti–PD-1 therapy.1-5 The underlying mechanisms of the effective action and resistance of PD-1 inhibitors in cHL remain largely unclear.
CHL represents a unique malignancy characterized by the rare cancerous Hodgkin Reed/Sternberg (HRS) cells interspersing among abundant immune cells. HRS cells, making up a tiny fraction (1%) of the tumor, create a tumor-supportive and immunosuppressive circumstance. Universal genetic alterations at chromosome 9p24.1 locus with hyperactivation of signaling via PD-L1, JAK/STAT, and NF-κB is detected in HRS cells,6 contributing to high responsiveness to anti–PD-1 treatment in cHL. Pathologically, HRS cells are primarily characterized by the expression of CD30 (TNFRSF8). Previous literature have reported that PD-L1+CD30+ HRS cells contacted with extensively infiltrated PD-1+CD4+ T cells in a specialized rosette-like cHL niche and identified a regulatory subset of LAG3+CD4+ T cells in cHL microenvironment.7,8 However, due to the scarcity of HRS cells in cHL tumors, exploring the intratumor heterogeneity of HRS cells and its association with cHL escape after immunotherapy remains challenging.
DNA demethylating agents could sensitize antitumor treatment in lymphoma and enhance the expansion and cytotoxicity of antigen-specific CD8+ T cells in mice tumor models.9-11 Although patients achieving CR with decitabine-plus-camrelizumab therapy often experienced durable responses even after treatment cessation,12 most patients who did not obtain CR eventually suffered disease progression.13 Histone deacetylase (HDAC) inhibitor chidamide was approved in China for the treatment of recurrent/refractory peripheral T-cell lymphoma. Although HDAC inhibitor monotherapy had shown poor clinical outcomes in relapsed/refractory cHL,14 vorinostat plus pembrolizumab was effective in patients with cHL refractory to prior PD-1 blockade.15 Given that targeting different epigenetic pathways can yield distinct antitumor effects and potentially overcome anti–PD-1 resistance, we conducted a trial using the combination regimen of chidamide, decitabine plus camrelizumab (CDP) in patients with cHL who relapsed/progressed after DP therapy (NCT04233294). Here, we reported the clinical outcome of CDP therapy and further investigated the antitumor mechanism using single-cell RNA sequencing (scRNA-seq) on paired cHL samples.
Methods
Study design
This study is a phase 2 clinical trial to test the safety and efficacy of chidamide, decitabine plus anti–PD-1 camrelizumab in patients with relapsed/refractory cHL who previously treated with DP therapy (NCT04233294). The study was approved by the ethics committee of Chinese People's Liberation Army General Hospital (S2020-026-01), in accordance with the ethical principles of Good Clinical Practice and the Declaration of Helsinki.
Patients
Eligible patients must have histologically confirmed relapsed/refractory cHL. Patients were aged ≥12 years, had measurable disease, Eastern Cooperative Oncology Group performance status of 0 or 1, and had adequate organ function. Patients must have at least 2 lines of antitumor therapy, and those who were transplant ineligible or refused transplant were allowed. All patients had received DP therapy, including those who had initially responded to DP but later experienced disease relapsed/progressed and those who were recalcitrant to DP with the best response of stable disease (SD)/progressive disease (PD). Biopsies from relapsing patients were histologically confirmed as cHL. Before study entry, the last therapy must be off at least 4 weeks. Key exclusion criteria included grade 3 or 4 immune-related adverse events within 3 months, prior ASCT within 100 days, active autoimmune disease or history of syndrome that requires systemic immunosuppressive medications, and any serious or unstable preexisting medical condition. All patients provided written informed consent before the study.
Treatment
To mitigate potential adverse events, low doses of chidamide and decitabine were administrated. Patients received chidamide orally (10 mg/d on days 1-4; 20 mg/d on days 8, 11, 15, and 18) and decitabine IV (10 mg/d on days 1-5) plus camrelizumab IV (200 mg on day 6), every 3 weeks. Treatment continued until disease progression, serious toxicity, withdrawal of consent, or treatment completion. Patients who achieved CR could discontinue CDP treatment after 1 year of persistent CR.
Efficacy assessments
Antitumor response was assessed using computed tomography (CT; magnetic resonance imaging or ultrasound) at baseline and every 2 cycles (6 weeks) thereafter, and positron emission tomography/CT was performed at baseline and every 4 cycles (12 weeks) thereafter, according to the Revised Response Criteria for Malignant Lymphoma (2014 Lugano classification). Tumor CR was confirmed by positron emission tomography/CT, with a Deauville score of ≤3 considered as metabolic CR. Patients achieving CR could discontinue treatment after 1 year of persistent CR.
Methods for scRNA-seq and bioinformatic analyses are included in supplemental Data, available on the Blood website.
Results
Patients
From 2 January 2020 to 31 August 2022, a total of 52 patients with cHL who relapsed/progressed after DP were treated with the triplet CDP combination therapy. The median age was 29 years (range, 13-54), and 50 patients (96%) had ≥3 lines of previous systemic therapy; the median number of prior therapies was 5 (range, 2-12). Twelve patients had received prior ASCT; the remaining 40 patients were considered transplant ineligible due to comorbidity (n = 4), intolerance (n = 8), or insensitivity (n = 27) to second-line chemotherapy or refusal of transplant because of high costs (n = 1). Five patients had received brentuximab vedotin (BV). Twenty-seven patients received ≥2 lines of anti–PD-1/anti–PD-L1–containing therapy previously. Before study entry, 44 patients (85%) received DP therapy as their last treatment, including 42 patients experiencing disease progression on active DP and 2 having relapsed cHL after DP discontinuation. The remaining 8 patients had received mechlorethamine hydrochloride, oncovin, doxorubicin hydrochloride liposome, and prednisone (MOAP) chemotherapy (n = 6), bendamustine plus anti–PD-1 or BV plus bendamustine before CDP therapy. The median cycles of previous DP was 12 (range, 4-44), resulting in an objective response rate of 77%, with 29% of patients achieving CR. The median PFS of prior DP therapy was 14.6 months (Table 1). Patients baseline characteristics and prior treatments were shown in supplemental Table 1.
Baseline characteristics
| Characteristicss . | Total (N = 52) . |
|---|---|
| Median age (range), y | 29 (13-54) |
| Sex, male | 34 (69%) |
| Disease stage at diagnosis | |
| II | 10 (19%) |
| III | 12 (23%) |
| IV | 30 (58%) |
| Extranodal disease at enrollment | 26 (50%) |
| Number of previous systemic therapy | |
| Median (range) | 5 (2-12) |
| ≥3 | 50 (96%) |
| Previous ASCT | 12 (23%) |
| Transplant ineligible | 40 (77%) |
| Previous BV | 5 (10%) |
| Last systemic therapy before enrollment | |
| DP therapy | 44 (85%) |
| Disease progression on active treatment | 42 (81%) |
| Disease relapse after DP discontinuation | 2 (4%) |
| Non-DP therapy | 8 (15%) |
| Previous DP therapy | |
| Median cycles (range) | 12 (4-44) |
| ORR (95% CI) | 77% (63-87) |
| CR rate (95% CI) | 29% (17-43) |
| Median PFS (mo) | 13.6 |
| Characteristicss . | Total (N = 52) . |
|---|---|
| Median age (range), y | 29 (13-54) |
| Sex, male | 34 (69%) |
| Disease stage at diagnosis | |
| II | 10 (19%) |
| III | 12 (23%) |
| IV | 30 (58%) |
| Extranodal disease at enrollment | 26 (50%) |
| Number of previous systemic therapy | |
| Median (range) | 5 (2-12) |
| ≥3 | 50 (96%) |
| Previous ASCT | 12 (23%) |
| Transplant ineligible | 40 (77%) |
| Previous BV | 5 (10%) |
| Last systemic therapy before enrollment | |
| DP therapy | 44 (85%) |
| Disease progression on active treatment | 42 (81%) |
| Disease relapse after DP discontinuation | 2 (4%) |
| Non-DP therapy | 8 (15%) |
| Previous DP therapy | |
| Median cycles (range) | 12 (4-44) |
| ORR (95% CI) | 77% (63-87) |
| CR rate (95% CI) | 29% (17-43) |
| Median PFS (mo) | 13.6 |
Efficacy of chidamide-decitabine-plus-camrelizumab therapy
Till 10 December 2023, of the 52 patients, the median number of CDP cycles was 20 (range, 4-44), with a median duration of CDP treatment of 21 months. Notably, 49 patients (94%; 95% confidence interval [CI], 84-99) achieved an objective response, of whom 26 patients (50%; 95% CI, 36-64) acquired CR after CDP therapy (Table 2). Detailed treatment and response information are listed in supplemental Table 2. Among patients who had received at least 5 prior lines, 90% of patients obtained an objective response. Comparable responses were observed for patients who had undergone ASCT and those ineligible for transplant. Patients benefited from CDP therapy regardless of sex, age, disease stage, and target lesions (supplemental Table 3). Notably, all 12 patients with SD/PD to previous DP therapy obtained therapeutic responses after CDP therapy, although they achieved a significantly lower CR rate than patients responsive to prior DP therapy (25% vs 58%; P = .048).
ORR and CR rate with CDP therapy
| . | Number . | ORR (95% CI) . | CR rate (95% CI) . |
|---|---|---|---|
| All patients | 52 | 94% (84-99) | 50% (36-64) |
| Patients with ≥5 lines of prior therapy | 30 | 90% (73-98) | 47% (28-66) |
| Patients with previous ASCT | 12 | 100% (74-100) | 58% (28-85) |
| Transplant-ineligible patients | 40 | 93% (80-98) | 48% (32-64) |
| Patients with previous DP therapy | |||
| CR/PR after DP therapy | 40 | 93% (80-98) | 58% (41-73) |
| SD/PD after DP therapy | 12 | 100% (74-100) | 25% (5-57) |
| . | Number . | ORR (95% CI) . | CR rate (95% CI) . |
|---|---|---|---|
| All patients | 52 | 94% (84-99) | 50% (36-64) |
| Patients with ≥5 lines of prior therapy | 30 | 90% (73-98) | 47% (28-66) |
| Patients with previous ASCT | 12 | 100% (74-100) | 58% (28-85) |
| Transplant-ineligible patients | 40 | 93% (80-98) | 48% (32-64) |
| Patients with previous DP therapy | |||
| CR/PR after DP therapy | 40 | 93% (80-98) | 58% (41-73) |
| SD/PD after DP therapy | 12 | 100% (74-100) | 25% (5-57) |
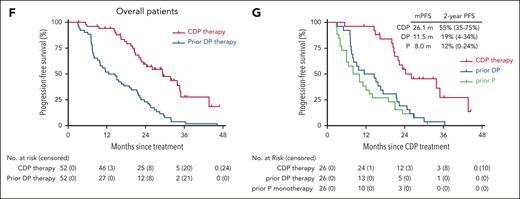
After a median follow-up of 32 months (range, 9-47 months), 12 patients in continuous CR over 1 year had completed and discontinued CDP treatment, 26 patients experienced disease progression during treatment, and 10 patients were still undergoing CDP therapy (supplemental Figure 1). No patients in remission underwent subsequent transplantation, and all patients survived (supplemental Figure 2A). The median time to response was 1.4 months (range, 1.4-11.2), and the median time to CR was 2.8 months (range, 2.8-14.9; Figure 1A; supplemental Figure 2B-C). The median duration of response was 33.2 months in CR patients vs 20.9 months in partial response (PR) patients (P = .016; Figure 1B). Overall, the median PFS was 29.4 months (95% CI, 20.7-38.0), and PFS rates at 1 year and 2 years were 94% (95% CI, 88-100) and 60% (95% CI, 45-74), respectively (Figure 1C). Patients who acquired CR and PR had longer PFS than nonresponders (median PFS in CR, 34.6 months; PR, 23.3 months; SD/PD, 5.8 months; Figure 1D). Among the 12 patients in CR who ceased CDP therapy and had no subsequent transplantation, 78% remained in remission at 1 year after treatment discontinuation, and the median relapse-free survival since treatment cessation was not obtained (supplemental Figure 2D).
PFS. (A) PFS for the 49 patients responsive to CDP therapy. Blue bar indicates CDP treatment, and its length shows the time from first dosing until disease progression, relapse, or discontinuation of CDP treatment. Orange bar indicates treatment cessation, and its length shows the time from cessation until disease relapse. The onset time of first response (CR or PR) is indicated as a red circle (CR) or a blue square (PR), respectively. (B) Duration of response (DOR) for patients with the best response of CR vs PR after CDP therapy. Shown are median DOR and 2-year DOR rates (95% CI); long-rank (Mantel-Cox) test. (C) Overall PFS for the entire cohort. (D) PFS for patients who acquired CR vs PR vs SD/PD after CDP therapy. Shown are median PFS (mPFS) and 2-year PFS rates (95% CI); long-rank (Mantel-Cox) test. (E) PFS for patients based on the best response to prior DP treatment (CR/PR vs SD/PD). Shown are mPFS (95% CI), long-rank (Mantel-Cox) test. (F) PFS for the overall patients achieved from CDP therapy vs prior DP therapy. (G) Twenty-six patients previously received anti–PD-1 monotherapy (P), DP therapy, and subsequently were treated with CDP therapy in this study. PFS curves of prior P monotherapy, prior DP therapy, and CDP therapy for the 26 patients are shown. mPFS and 2-year PFS rates are shown. P value <.05 is considered significant.
PFS. (A) PFS for the 49 patients responsive to CDP therapy. Blue bar indicates CDP treatment, and its length shows the time from first dosing until disease progression, relapse, or discontinuation of CDP treatment. Orange bar indicates treatment cessation, and its length shows the time from cessation until disease relapse. The onset time of first response (CR or PR) is indicated as a red circle (CR) or a blue square (PR), respectively. (B) Duration of response (DOR) for patients with the best response of CR vs PR after CDP therapy. Shown are median DOR and 2-year DOR rates (95% CI); long-rank (Mantel-Cox) test. (C) Overall PFS for the entire cohort. (D) PFS for patients who acquired CR vs PR vs SD/PD after CDP therapy. Shown are median PFS (mPFS) and 2-year PFS rates (95% CI); long-rank (Mantel-Cox) test. (E) PFS for patients based on the best response to prior DP treatment (CR/PR vs SD/PD). Shown are mPFS (95% CI), long-rank (Mantel-Cox) test. (F) PFS for the overall patients achieved from CDP therapy vs prior DP therapy. (G) Twenty-six patients previously received anti–PD-1 monotherapy (P), DP therapy, and subsequently were treated with CDP therapy in this study. PFS curves of prior P monotherapy, prior DP therapy, and CDP therapy for the 26 patients are shown. mPFS and 2-year PFS rates are shown. P value <.05 is considered significant.
There were no significant differences in PFS curves after CDP therapy among patients based on sex, age (≥29 vs <29 years), tumor stage (III/IV vs II), target lesions (lymph node only vs extranodal involvement), lines of prior therapies (≥5 vs <5), or between patients after ASCT and transplant-ineligible patients (supplemental Table 4). Although responders to prior DP tended to achieve longer PFS than DP nonresponders, statistical significance was not reached (P = .070; Figure 1E). Overall, PFS achieved from CDP therapy was longer than that from prior DP therapy (Figure 1F). In this study cohort, 26 patients were initially treated with anti–PD-1/anti–PD-L1 monotherapy and subsequently received DP treatment after disease progression. Among these patients, PFS from different immunotherapy regimens were compared, and CDP triplet therapy demonstrated potential therapeutic superiority (Figure 1G).
The most common treatment-related adverse events (TRAEs) with CDP therapy were leukocytopenia (73.1%), diarrhea (36.5%), and nausea (34.6%). The grade 3 TRAEs included leukocytopenia (25.0%), thrombocytopenia (1.9%), increased transaminase (1.9%), and pulmonary infection (1.9%), and 1 patient (1.9%) experienced grade 4 leukocytopenia (supplemental Table 5). Nine patients experienced treatment delays because of leukocytopenia (n = 1), pulmonary infection (n = 1) and COVID-19 infection (n = 7), whereas none discontinued treatment due to TRAEs.
scRNA-seq enables detailed characterization of HRS-like cells
To investigate the mechanism of epi-immunotherapy in cHL, we built a single-cell atlas of cHL (Figure 2A), which included 17 biopsy samples from 9 patients in this trial (core data set, Figure 2B) and an additional 7 tumors from patients in our other clinical studies, aiming to acquire more data for characterization of the rare HRS cells (supplemental Table 6). Furthermore, 3 tumors from patients UPN50 and UPN51, collected before and during CDP treatment, served as a validation set. After quality control, a total of 185 791 cells were obtained, most of which were immune cells (Figure 2C; supplemental Figure 3A-D; supplemental Table 7). To better characterize T cells and trace their clonal expansion, we performed single-cell T-cell receptor (TCR) sequencing. From 98 291 T cells with matched transcriptomic data and TCR data, we identified 82 684 clonotypes (supplemental Figure 3E-F).
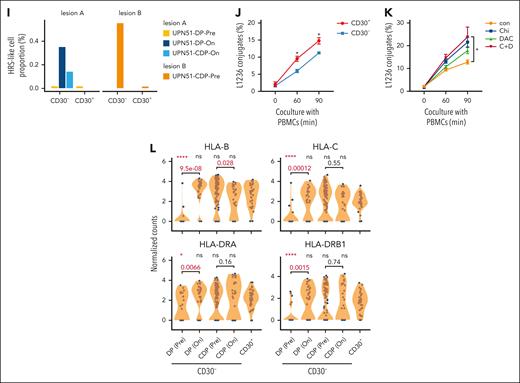
Dissection of cHL tumor microenvironment and the association of HRS-like cells with treatment response. (A) Schematic overview of the study. (B) Treatment regimens, response onset, and duration for tumors conducted scRNA-seq in this study. The color indicates DP or CDP therapy. The length of the bar shows PFS. The best clinical response for tumor biopsy is labeled. Patients with ongoing response are indicated by an arrow, and patients with progression or relapse are indicated by a cross. For UPN51, the biopsy sample before CDP therapy was a recurrent tumor, and the other 3 biopsy samples were collected at identical location. (C) Uniform manifold approximation and projection (UMAP) map of 185 791 cells in cHL. Colored by cell subtype. (D) Dot plots showing representative signature genes of cell subtypes. Color for z score scaled expression and size for detection rate. (E) Representative images of multiplex immunostaining results. The CD30 (red), DHRS2 (green), and PAX5 (pink) are used to highlight HRS-like cells. The CD30–DHRS2+PAX5dim+ cells are indicated by white arrows. At the time of sample collection, patient UPN33 was anti–PD-1 treatment naïve (P naive), and patient UPN19 had disease progression after prior anti–PD-1 therapy (P resist). (F) RNA velocities overlaid on principal component analysis showing the transition path of the HRS-like cells from CD30– cells to CD30+ cells. Streamlines show the RNA velocity field, and dots are colored by CD30 expression status. (G) Volcano plot showing differentially expressed genes between CD30+ and CD30– HRS-like cells. Red dots for significantly higher expression in the on-treatment samples; blue dots for significantly lower expression in the on-treatment samples. Representative genes are highlighted. (H) Box plots showing normalized counts of total HRS-like cells, CD30+ HRS-like cells, and CD30– HRS-like cells for paired pretreatment (Pre) and on-treatment (On) samples. Adjusted P values and fold change values by negative binomial-generalized linear models were also shown. (I) CD30– and CD30+ HRS cell proportions in a serial of tumors from the patient UPN51 who achieve PR after CDP treatment. (J) L1236-mCherry cells were stained with anti-CD30 antibody and incubated with peripheral blood mononuclear cells (PBMCs), which were stained with CellTrace eFluor dye, for 60 or 90 minutes at a ratio of 1:20. The percentages of conjugates formed by CD30+ or CD30– L1236 with PBMCs (gating from CD30+ or CD30– mCherry+ cells) were calculated. Results are pooled from 2 repeated experiments (n = 4 per group). Data are represented as mean ± standard error of the mean (SEM), by 2-tailed Student t test. (K) L1236-mCherry cells were pretreated with phosphate-buffered saline (PBS; con group), 100 nM chidamide (Chi group), 10 nM decitabine (DAC group), or 100 nM chidamide plus 10 nM decitabine (C + D group) for 3 days, followed by incubation with PBMCs-eFluor for 60 or 90 minutes at a ratio of 1:20. The percentages of conjugates formed by L1236 with PBMCs were calculated. Results are pooled from 2 repeated experiments (n = 4 per group). Data are represented as mean ± SEM, by 1-way analysis of variance (ANOVA). (L) Violin plots depicting the expression of major histocompatibility complex (MHC) class I/II genes in 5 groups. Comparisons were performed by the 2-sided Wilcoxon tests. NR, nonresponder; R, responder; scTCR-seq, single-cell TCR sequencing.
Dissection of cHL tumor microenvironment and the association of HRS-like cells with treatment response. (A) Schematic overview of the study. (B) Treatment regimens, response onset, and duration for tumors conducted scRNA-seq in this study. The color indicates DP or CDP therapy. The length of the bar shows PFS. The best clinical response for tumor biopsy is labeled. Patients with ongoing response are indicated by an arrow, and patients with progression or relapse are indicated by a cross. For UPN51, the biopsy sample before CDP therapy was a recurrent tumor, and the other 3 biopsy samples were collected at identical location. (C) Uniform manifold approximation and projection (UMAP) map of 185 791 cells in cHL. Colored by cell subtype. (D) Dot plots showing representative signature genes of cell subtypes. Color for z score scaled expression and size for detection rate. (E) Representative images of multiplex immunostaining results. The CD30 (red), DHRS2 (green), and PAX5 (pink) are used to highlight HRS-like cells. The CD30–DHRS2+PAX5dim+ cells are indicated by white arrows. At the time of sample collection, patient UPN33 was anti–PD-1 treatment naïve (P naive), and patient UPN19 had disease progression after prior anti–PD-1 therapy (P resist). (F) RNA velocities overlaid on principal component analysis showing the transition path of the HRS-like cells from CD30– cells to CD30+ cells. Streamlines show the RNA velocity field, and dots are colored by CD30 expression status. (G) Volcano plot showing differentially expressed genes between CD30+ and CD30– HRS-like cells. Red dots for significantly higher expression in the on-treatment samples; blue dots for significantly lower expression in the on-treatment samples. Representative genes are highlighted. (H) Box plots showing normalized counts of total HRS-like cells, CD30+ HRS-like cells, and CD30– HRS-like cells for paired pretreatment (Pre) and on-treatment (On) samples. Adjusted P values and fold change values by negative binomial-generalized linear models were also shown. (I) CD30– and CD30+ HRS cell proportions in a serial of tumors from the patient UPN51 who achieve PR after CDP treatment. (J) L1236-mCherry cells were stained with anti-CD30 antibody and incubated with peripheral blood mononuclear cells (PBMCs), which were stained with CellTrace eFluor dye, for 60 or 90 minutes at a ratio of 1:20. The percentages of conjugates formed by CD30+ or CD30– L1236 with PBMCs (gating from CD30+ or CD30– mCherry+ cells) were calculated. Results are pooled from 2 repeated experiments (n = 4 per group). Data are represented as mean ± standard error of the mean (SEM), by 2-tailed Student t test. (K) L1236-mCherry cells were pretreated with phosphate-buffered saline (PBS; con group), 100 nM chidamide (Chi group), 10 nM decitabine (DAC group), or 100 nM chidamide plus 10 nM decitabine (C + D group) for 3 days, followed by incubation with PBMCs-eFluor for 60 or 90 minutes at a ratio of 1:20. The percentages of conjugates formed by L1236 with PBMCs were calculated. Results are pooled from 2 repeated experiments (n = 4 per group). Data are represented as mean ± SEM, by 1-way analysis of variance (ANOVA). (L) Violin plots depicting the expression of major histocompatibility complex (MHC) class I/II genes in 5 groups. Comparisons were performed by the 2-sided Wilcoxon tests. NR, nonresponder; R, responder; scTCR-seq, single-cell TCR sequencing.
A small cell population was identified as HRS-like cells, which exhibited significant enrichment of previously established signature of purified HRS cells (gene set “Steidl 2012”16; supplemental Figure 4A), highly expressed the canonical marker TNFRSF8 (CD30)17 and a set of genes rarely detected in immunocytes of cHL microenvironment such as DHRS2 (Figure 2D). Interestingly, although CD30 was considered consistently expressed in HRS cells,18 we noticed a subpopulation of CD30– HRS-like cells. In these cells, other classical HRS markers PAX5, IRF4, and FUT4 were also downregulated, whereas the DHRS2 gene was specifically overexpressed in both CD30+ and CD30– HRS-like cells (supplemental Figure 4B-C). The presence of DHRS2+CD30– morphological HRS-like cells were verified by multiplex immunofluorescence staining in patients UPN1/19/26/33/40 and additional cHL samples as DHRS2+CD30–PAX5dim+ cells (Figure 2E; supplemental Figure 5). Both CD30+ and CD30– HRS-like cells enriched the gene set “Steidl 2012” (supplemental Figure 4D) and shared certain somatic mutations, including those occurring in NFKBIA and EEF1A1 (supplemental Figure 4E), hinting at a common clonal origin and HRS cell identity also for the CD30– population. Principal component analysis revealed a state continuum approximately aligned with PC1, with CD30+ and CD30– cells located at opposite ends, and the RNA velocities19 exhibited streamlines from CD30– to CD30+ cells (Figure 2F). CD30– cells exhibited higher differentiation potential than CD30+ cells based on relative expression orderings (REOs20; supplemental Figure 4F) but lower activities of certain pathways, including antigen processing and presentation and other pathways of immune system, with significantly downregulated genes such as CD274 (encoding PD-L1), ICAM1, and cytokine receptors IL21R and IL10RA (Figure 2G; supplemental Figure 4G-H; supplemental Table 8). Together, these results indicated the existence of both CD30+ and CD30– HRS-like cell populations.
CD30− HRS-like cells are insensitive to anti–PD-1 and respond to CDP therapy
We conducted treatment correlative analysis using 5 pairs of tumors responsive to CDP therapy and 3 pairs of tumors resistant to DP therapy to explore key factors in reversing anti–PD-1 resistance by CDP regimen. As expected, the abundance of total HRS-like cells significantly reduced after treatment in responsive tumors, whereas such reduction was not observed in tumors unresponsive to DP treatment (Figure 2H). Notably, CD30+ HRS-like cells were eliminated after treatment in DP nonresponders, whereas CD30– HRS-like cells only decreased in responders after CDP therapy, hinting that CD30– HRS-like cells may be less sensitive to anti–PD-1–based immunotherapy. We further validated this in patient UPN51, who was recalcitrant to DP and subsequently acquired PR after CDP therapy (supplemental Figure 6). Although the abundance of CD30+ HRS-like cells were too low to detect, the abundance of CD30– HRS-like cells in DP-resistant lesion A reduced after CDP treatment (Figure 2I). Furthermore, CD30– cells accounted for 97% of all HRS-like cells in lesion B (Figure 2I), which emerged after DP failure and achieved metabolic CR after CDP treatment. Together, the effectiveness of CDP regimen after DP failure may be partly related to the depletion or restriction of CD30– HRS-like cells.
We observed, after DP treatment, CD30– HRS-like cells had a transcriptional phenotype shift to CD30+ HRS-like cells but still differed from CD30+ HRS-like cells (supplemental Figure 7A). To evaluate the direct effects of epigenetic agents on HRS cells, we used L1236 HL cell line rather than L428 cells because almost all cells expressed CD30. Treatment with chidamide, decitabine, or their combination resulted in increased human leukocyte antigen (HLA) gene expression (supplemental Figure 7B). Although rosseting assay failed to be conducted in L1236 cells (supplemental Figure 7C-D), by in vitro conjugation assay, we observed that CD30– L1236 cells formed significantly fewer conjugates with peripheral blood mononuclear cells than CD30+ cells (Figure 2J). When L1236 cells were pretreated with chidamide plus decitabine, conjugation formation increased in both CD30+ and CD30– L1236 cells (Figure 2K; supplemental Figure 7E-F). The scRNA-seq data revealed that, despite tumors being unresponsive, HLA genes in CD30– HRS-like cells were significantly upregulated after DP treatment and remained at high levels even if disease progressed/relapsed (Figure 2L; supplemental Figure 7G). These results indicate that although epigenetic agents can increase immune recognition by upregulating HLA gene expression, this alone may be insufficient to eliminate tumors, and additional immuno-regulatory effects are required to potentiate the antitumor response.
Epi-immunotherapy activates antitumor response mediated by diverse tumor-reactive CD8+ T cells
After CDP treatment, we observed a significant increase in the abundance of CD8+ T cells rather than natural killer (NK) cells (Figure 3A). To identify cell subpopulations responsible for effective antitumor response, we performed reclustering on T cells/innate lymphoid cells (ILCs) (supplemental Figure 8; supplemental Table 9). Among 5 CD8+ T clusters highly expressed PDCD1, 1 was identified as CD8+ T follicular helper–like cells (CD8+ Tfh),21 and the other 4 displayed an exhaustion phenotype but also expressed cytotoxic genes (Figure 3B). Cytotoxicity was a prominent feature of certain PD-1low clusters, and multiple cytotoxic clusters exhibited enriched antigen specificities, as reflected by high clonality and proportion of clonal cells (Figure 3C).
Epi-immunotherapy promotes the antitumor response mediated by diverse CD8+ tumor-reactive T cells. (A) Box plots showing normalized counts of total CD8+ and NK cells for paired pretreatment (Pre) and on-treatment (On) samples. Adjusted P values and fold change values by negative binomial-generalized linear models were also shown. Box middle lines, median; box limits, upper and lower quartiles; box whiskers, 1.5× the interquartile range. (B) The expression pattern of representative genes related to exhaustion, costimulation, cytotoxicity, chemokine, adhesion, and transcription factor in CD8+ T-cell clusters. ∗adjusted P value < .01 and log2 fold change > 0.15; †adjusted P value < .01 and log2 fold change > 0; test by limma analysis. (C) Scatterplots showing CD8+ T-cell clusters’ clonality measured by Shannon entropy–based index, the proportion of clonal cells (clone size ≥ 5). Size for proliferation activity indicated by MKI67+ cell proportion. (D) Box plots comparing clonality between pretreatment (Pre) and on-treatment (On) samples. Tests by paired t tests. (E) Box plots comparing the fractions of expanded clones between treatment groups. Fraction is the number of expanded clones in a patient divided by the total number of clones in that patient. (F) Pie charts showing the cell state compositions of expanded/contracted clones in the 2 treatment groups. Toth indicates other T cells besides exhausted T cells (Tex). (G) Heat map showing TCR sharing strength between cluster HAVCR2+ Tex and other CD8+ clusters in the 2 treatment groups. Top clusters with the highest sharing strength with HAVCR2+ Tex are highlighted in bold. (H-I) Sankey plots showing the dominant-state dynamics of large clones. Cells of the same clone in a paired pretreatment and on-treatment sample are connected. Color for the dominant transcriptomic state. Clones dominated by HAVCR2+ Tex or CXCR5+ Tex are plotted. (H) clones in DP-treated patients, (I) clones in CDP-treated patients. (J) Heat map showing the abundance of clusters in pretreatment and on-treatment tumors from responders (R; treated by CDP) and nonresponders (NR; treated by DP). Color for the average of z score–scaled abundance. The abundance in responders is compared with nonresponders by negative binomial-generalized linear models for pretreatment and on-treatment samples separately. Only CD8+ T clusters exhibiting significant abundance differences (nominal P < .05) are shown. The orange bar next to the left side annotates clusters having significant abundance differences in the pretreatment samples, and the red bar next to the left side annotates other clusters. The clusters exhibited significant abundance change between pretreatment and on-treatment samples are highlighted in red (see supplemental Figure 8F). ∗∗adjusted P < .01; ∗adjusted P < .05; †nominal P < .05.
Epi-immunotherapy promotes the antitumor response mediated by diverse CD8+ tumor-reactive T cells. (A) Box plots showing normalized counts of total CD8+ and NK cells for paired pretreatment (Pre) and on-treatment (On) samples. Adjusted P values and fold change values by negative binomial-generalized linear models were also shown. Box middle lines, median; box limits, upper and lower quartiles; box whiskers, 1.5× the interquartile range. (B) The expression pattern of representative genes related to exhaustion, costimulation, cytotoxicity, chemokine, adhesion, and transcription factor in CD8+ T-cell clusters. ∗adjusted P value < .01 and log2 fold change > 0.15; †adjusted P value < .01 and log2 fold change > 0; test by limma analysis. (C) Scatterplots showing CD8+ T-cell clusters’ clonality measured by Shannon entropy–based index, the proportion of clonal cells (clone size ≥ 5). Size for proliferation activity indicated by MKI67+ cell proportion. (D) Box plots comparing clonality between pretreatment (Pre) and on-treatment (On) samples. Tests by paired t tests. (E) Box plots comparing the fractions of expanded clones between treatment groups. Fraction is the number of expanded clones in a patient divided by the total number of clones in that patient. (F) Pie charts showing the cell state compositions of expanded/contracted clones in the 2 treatment groups. Toth indicates other T cells besides exhausted T cells (Tex). (G) Heat map showing TCR sharing strength between cluster HAVCR2+ Tex and other CD8+ clusters in the 2 treatment groups. Top clusters with the highest sharing strength with HAVCR2+ Tex are highlighted in bold. (H-I) Sankey plots showing the dominant-state dynamics of large clones. Cells of the same clone in a paired pretreatment and on-treatment sample are connected. Color for the dominant transcriptomic state. Clones dominated by HAVCR2+ Tex or CXCR5+ Tex are plotted. (H) clones in DP-treated patients, (I) clones in CDP-treated patients. (J) Heat map showing the abundance of clusters in pretreatment and on-treatment tumors from responders (R; treated by CDP) and nonresponders (NR; treated by DP). Color for the average of z score–scaled abundance. The abundance in responders is compared with nonresponders by negative binomial-generalized linear models for pretreatment and on-treatment samples separately. Only CD8+ T clusters exhibiting significant abundance differences (nominal P < .05) are shown. The orange bar next to the left side annotates clusters having significant abundance differences in the pretreatment samples, and the red bar next to the left side annotates other clusters. The clusters exhibited significant abundance change between pretreatment and on-treatment samples are highlighted in red (see supplemental Figure 8F). ∗∗adjusted P < .01; ∗adjusted P < .05; †nominal P < .05.
We next examined clonotype dynamics. Firstly, the clonality of overall CD8+ T cells significantly increased after CDP treatment (Figure 3D). Secondly, we identified individual expanded or contracted clones during treatment (supplemental Figure 9A; supplemental Table 10). More expanded CD8+ T cell clones were detected in CDP-responsive tumors (Figure 3E), in which the contracted clones contained more exhausted T (Tex) cells, than in DP-unresponsive tumors (40% vs 15%; Figure 3F), suggesting that CDP treatment alleviated T-cell exhaustion. Thirdly, we analyzed the transcriptional state shifts of presumable tumor-reactive T cells (pTRTs) during treatments. The HAVCR2+ Tex exhibited high cytotoxicity and clonality, accounting for 75.17% of Tex cells, indicating their presumed tumor reactivity.22 Using HAVCR2+ Tex as a “bait” and Shannon entropy based TCR-sharing calculation,23 the enrichment of pTRTs in clusters was quantified. After both treatments, CXCR5+ progenitor exhausted T cells, which possessed high expansion potential and pivotal roles during anti–PD-1 immunotherapy,22 showed enhanced TCR sharing with HAVCR2+ Tex (Figure 3G). Additionally, the cluster distributions of pTRTs were more diverse, because multiple nonexhausted cytotoxic clusters (early effector T cells [Teff], CD160+ Teff, and KLRG1+ Teff) exhibited high TCR sharing with HAVCR2+ Tex after treatment (Figure 3G). The changes in dominant transcriptional states of individual large clones directly demonstrated the transition of exhausted clones to nonexhausted effector states during treatment (Figure 3H-I). Differentiation trajectories inference revealed that HAVCR2+ Tex was one ending branch, whereas clusters strongly sharing TCR with HAVCR2+ Tex occupied transitional positions (supplemental Figure 9B-C). Taken together, epi-immunotherapy, particularly the CDP regimen, enhances the clonal expansion of tumor-reactive CD8+ T cells and sustains them in pre-exhaustion effector states.
Finally, we investigated the associations of CD8+ T clusters with clinical response and mainly observed 2 classes. The first class contained clusters already exhibiting differences in the pretreatment samples (Figure 3J; supplemental Figure 9D), providing a basis for predictive biomarkers. The second class consisted of clusters only showing differences in the on-treatment samples, indicative of the effect of CDP treatment. Indeed, most clusters in the second class increased dramatically in abundance after CDP treatment, including highly cytotoxic ones such as KLRG1+ Teff, CD160+ Teff, ZNF683+ effector memory T (Tem), and CX3CR1+ recently activated effector memory (Temra) (Figure 3J; supplemental Figure 9E). CD8+ central memory T cells also increased in abundance only after effective CDP treatment (supplemental Figure 9E). Notably, before CDP treatment, HAVCR2+ Tex was abundant in tumors, suggesting that prior treatments had activated tumor-reactive T cells, but other factors likely impeded effective antitumor immune response.
Immunosuppressive IL21+ Th cells interact with malignant HRS cells
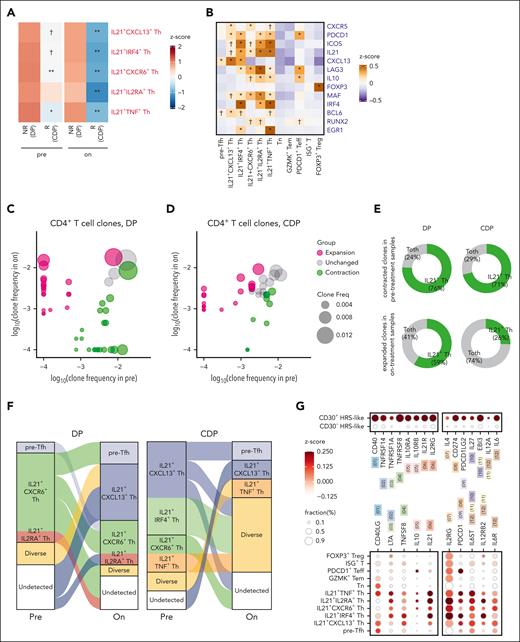
Among CD4+ T cells, 5 interleukin (IL) 21+ T helper (Th) clusters significantly decreased in abundance after CDP treatment in responders (supplemental Figure 10A). In addition, these clusters tended to be more abundant in nonresponsive patients than in responsive patients in both pretreatment and on-treatment tumors (Figure 4A). In the validation data set, the proportions of IL21+ Th decreased in DP-resistant lesions after CDP treatment, whereas the new lesion contained few IL21+ Th cells (supplemental Figure 10B).
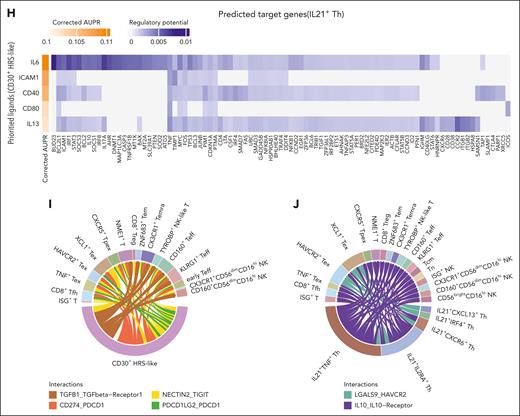
Epi-immunotherapy inhibits tumor-promoting and immunosuppressive IL21+ Th cells. (A) Heat map showing the abundance of clusters in pretreatment and on-treatment tumors from responders (R; treated by CDP) and nonresponders (NR; treated by DP). Color for the average of z score–scaled abundance. The abundance in responders is compared with nonresponders by negative binomial-generalized linear models for pretreatment and on-treatment samples separately. Only CD4+ T cells exhibiting significant abundance differences (nominal P < .05) are shown. The clusters exhibited significant abundance change between pretreatment and on-treatment samples are highlighted in red (see supplemental Figure 10A). ∗∗adjusted P < .01; ∗adjusted P < .05; †nominal P < .05. (B) The expression pattern of representative signature genes in CD4+ T-cell clusters. ∗adjusted P < .01; and log2 fold change > 0.15; †adjusted P < .01; and log2 fold change > 0; test by limma analysis. (C-D) Scatterplots illustrating TCR clone frequencies in pretreatment and on-treatment samples from patients treated by DP (C) and CDP (D). Only large clones (with sizes ≥5 either in pretreatment or on-treatment samples) are shown. Color for categories of T-cell expansion/contraction; size for clone frequencies in patients. (E) Pie charts showing the cell state compositions of expanded/contracted clones in treatment group DP and CDP. Toth indicates other T cells besides IL21+ Th. (F) Sankey plots showing the dominant-state dynamics of large clones. Cells of the same clone in a paired pretreatment and on-treatment sample are connected. Color for the dominant transcriptomic state. Clones dominated by IL21+ Th cluster are plotted. (G) Dot plots showing the expression of ligand/receptor genes. The left panel is for receptor genes on HRS-like cells, and cognate ligand genes on IL21+ Th cells; the right panel is for receptor genes on IL21+ Th cells and cognate ligand genes on HRS-like cells. Paired ligand-receptors are labeled in the same color and number. (H) Heat map showing top-ranked ligands predicted to have high regulatory potentials on IL21+ Th cells by the NicheNet analysis. Genes were ranked by corrected AUPR. (I-J) Chord diagrams illustrating representative interactions of high specificity between CD30+ HRS-like cells (I) or IL21+ Th cells (J) and CD8+ T cells/NK cells. Width of the chord for interaction strength. AUPR, area under the precision-recall curve.
Epi-immunotherapy inhibits tumor-promoting and immunosuppressive IL21+ Th cells. (A) Heat map showing the abundance of clusters in pretreatment and on-treatment tumors from responders (R; treated by CDP) and nonresponders (NR; treated by DP). Color for the average of z score–scaled abundance. The abundance in responders is compared with nonresponders by negative binomial-generalized linear models for pretreatment and on-treatment samples separately. Only CD4+ T cells exhibiting significant abundance differences (nominal P < .05) are shown. The clusters exhibited significant abundance change between pretreatment and on-treatment samples are highlighted in red (see supplemental Figure 10A). ∗∗adjusted P < .01; ∗adjusted P < .05; †nominal P < .05. (B) The expression pattern of representative signature genes in CD4+ T-cell clusters. ∗adjusted P < .01; and log2 fold change > 0.15; †adjusted P < .01; and log2 fold change > 0; test by limma analysis. (C-D) Scatterplots illustrating TCR clone frequencies in pretreatment and on-treatment samples from patients treated by DP (C) and CDP (D). Only large clones (with sizes ≥5 either in pretreatment or on-treatment samples) are shown. Color for categories of T-cell expansion/contraction; size for clone frequencies in patients. (E) Pie charts showing the cell state compositions of expanded/contracted clones in treatment group DP and CDP. Toth indicates other T cells besides IL21+ Th. (F) Sankey plots showing the dominant-state dynamics of large clones. Cells of the same clone in a paired pretreatment and on-treatment sample are connected. Color for the dominant transcriptomic state. Clones dominated by IL21+ Th cluster are plotted. (G) Dot plots showing the expression of ligand/receptor genes. The left panel is for receptor genes on HRS-like cells, and cognate ligand genes on IL21+ Th cells; the right panel is for receptor genes on IL21+ Th cells and cognate ligand genes on HRS-like cells. Paired ligand-receptors are labeled in the same color and number. (H) Heat map showing top-ranked ligands predicted to have high regulatory potentials on IL21+ Th cells by the NicheNet analysis. Genes were ranked by corrected AUPR. (I-J) Chord diagrams illustrating representative interactions of high specificity between CD30+ HRS-like cells (I) or IL21+ Th cells (J) and CD8+ T cells/NK cells. Width of the chord for interaction strength. AUPR, area under the precision-recall curve.
These IL21+ Th clusters exhibited high IL21 expression and medium-to-high levels of CXCR5, PDCD1, and ICOS, indicative of a Tfh-like phenotype (Figure 4B). Their molecular phenotypes suggested potential immunosuppressive functions, as characterized by high levels of CXCL13, LAG3, and IL10.7,24 By differentiation trajectory inference, IL21+ Th cells formed a branch with pre-Tfh as a precursor state (supplemental Figure 10C). Furthermore, IL21+ Th cells were among the CD4+ clusters with the highest clonality (supplemental Figure 10D).
Examining the clonotype dynamics, no significant changes in the clonality of overall CD4+ T cells were observed in either DP or CDP group (supplemental Figure 10E). However, we identified individual expanded or contracted clones (Figure 4C-D). Both treatments led to the contraction of IL21+ Th cell–enriched clones, but DP nonresponsive tumors exhibited expanded clones with a higher enrichment of IL21+ Th cells after treatment (Figure 4E). Notably, multiple novel expanded clones contributed to the IL21+ Th pool in DP group, whereas in CDP group, majority of IL21+ Th dominated clonotypes transited to the precursor state or exhibited diverse states (Figure 4F). Therefore, CDP treatment inhibits the expansion of IL21+ Th cells and traps those antigen-stimulated cells in less differentiated states.
Next, we inferred the cell-cell interaction network among cell populations.25 CD30+ HRS-like cells displayed more interactions with other cells, including IL21+ Th cells (supplemental Figure 11A), than CD30– HRS-like cells. Besides PD-1/PD-L1 interaction, multiple interactions were observed between CD30+ HRS-like cells and IL21+ Th cells (supplemental Table 11). CD30+ HRS-like cells secreted cytokines (IL6, IL4, and IL27), and IL21+ Th cells expressed the corresponding receptors (Figure 4G). NicheNet analysis26 indicated that IL6 and CD40 were top-ranked ligands with high regulatory potentials on IL21+ Th, suggesting the potential influence of CD30+ HRS-like cells on IL21+ Th cells (Figure 4H). Meanwhile, IL21+ Th cells expressed several ligands that could promote the survival and proliferation of HRS cells, including TNFSF8 (CD30 ligand), CD40LG,27LTA,28IL10, and IL21,29 with CD30+ HRS-like cells expressing the corresponding receptors (Figure 4G). cHL tumors exhibited significantly higher IL10 pathway activity than other lymphomas or lymph node samples30 (supplemental Figure 11B), and CD30+ HRS-like cells had higher IL10 signaling activation in cHL TME (supplemental Figure 11C). This suggested that IL10 secreted by IL21+ Th cells may also regulate CD30+ HRS-like cells. Additionally, CD30+ HRS cells expressed TGFB1, CD274, and NECTIN2, potentially suppressing CD8+ T/NK cell function (Figure 4I). IL21+ Th cells could also negatively regulate CD8+ T/NK cells via IL10/IL10 receptor and LGALS9/HAVCR231 (Figure 4J; supplemental Figure 11D-E; supplemental Table 12). In summary, IL21+ Th cells and CD30+ HRS-like cells interact closely, forming a positive feedback loop in which CD30+ HRS-like cells induce an immunosuppressive phenotype in IL21+ Th cells, which in turn supports the survival of CD30+ HRS-like cells.
Epi-immunotherapy reprograms IL21+ Th cells
To further unravel the mechanism underlying CDP therapy, we focused on IL21+ Th cells and HRS cells, both of which were suppressed after treatment. Given that chidamide selectively inhibits HDAC1/2/3/10,32,33 we used the average expression of HDAC1 core complex (HDAC1, HDAC2, RBBP4, and RBBP7) as an indicator of potential chidamide sensitivity. Among CD4+ T cells, IL21+ Th clusters exhibited the highest HDAC scores (Figure 5A). In the 3 DP-treated samples, IL21+ Th clusters in CDP responsive patients (UPN30 and UPN51) had higher HDAC scores than the nonresponsive patient UPN50 (Figure 5B).
CDP triplet therapy reprograms IL21+Th cells. (A) Dot plots showing the ordering of CD4+ T cell clusters by mean expression of HDAC core. Color for z-scaled mean expression; size for percentage of cells in which HDAC core is detected. (B) Box plots showing HDAC core expression levels by IL21+ Th clusters in the DP on-treatment samples. IL21+CXCL13+ Th cells and IL21+IRF4+ Th in UPN30 and UPN51 exhibited higher HDAC scores than UPN50. (C) Volcano plots showing differentially expressed genes between pretreatment and on-treatment samples in IL21+ Th cells from CDP-treated patients. Red dots for significantly higher expression in the on-treatment samples; blue dots for significantly lower expression in the on-treatment samples. Representative genes are highlighted. (D) GSEA plots for 2 pathways with significantly decreased expression in IL21+ Th cells from the on-treatment samples treated by CDP. (E) Bar plots showing the regulon specificity scores of the top 10 transcription factors of IL21+ Th cells. (F) Box plots showing the activities of STAT1 regulon across CD4+ T cell clusters. (G) Genes in IL6/IL21 signaling pathways. Solid lines indicate inferred regulation by STAT1, dashed lines indicate no direct regulation relationship inferred. The differential expression analysis result comparing IL21+ Th cells between on-treatment and pretreatment samples treated by CDP is also shown. Color for log fold change; size for significance. The genes with significant expression difference are highlighted with orange borders. (H) Box plots showing the average expression levels of HDAC core genes in CD30− and CD30+ HRS-like cells. Two-sided Wilcoxon test is applied. (I) Stacked bar plots illustrating relative communication probabilities of ligands and receptors in pretreatment and on-treatment tumors from CDP-treated patients. Interactions from CD8+ T/NK cells to CD30− HRS-like cells with remarkable difference between treatment time points and high communication probabilities in any 1 time point are shown. (J) Network plots showing cell-cell communication probabilities in on-treatment tumors from CDP-treated patients. Dot for cluster, line width for strength of interaction between FASL on CD8+ T/NK cells and FAS on CD30− HRS-like cells. Note, FASL-FAS interactions from CD8+ T/NK cells to CD30− HRS-like in pretreatment tumors were not detected. FASL, FAS ligand; GSEA, Gene Set Enrichment Analysis.
CDP triplet therapy reprograms IL21+Th cells. (A) Dot plots showing the ordering of CD4+ T cell clusters by mean expression of HDAC core. Color for z-scaled mean expression; size for percentage of cells in which HDAC core is detected. (B) Box plots showing HDAC core expression levels by IL21+ Th clusters in the DP on-treatment samples. IL21+CXCL13+ Th cells and IL21+IRF4+ Th in UPN30 and UPN51 exhibited higher HDAC scores than UPN50. (C) Volcano plots showing differentially expressed genes between pretreatment and on-treatment samples in IL21+ Th cells from CDP-treated patients. Red dots for significantly higher expression in the on-treatment samples; blue dots for significantly lower expression in the on-treatment samples. Representative genes are highlighted. (D) GSEA plots for 2 pathways with significantly decreased expression in IL21+ Th cells from the on-treatment samples treated by CDP. (E) Bar plots showing the regulon specificity scores of the top 10 transcription factors of IL21+ Th cells. (F) Box plots showing the activities of STAT1 regulon across CD4+ T cell clusters. (G) Genes in IL6/IL21 signaling pathways. Solid lines indicate inferred regulation by STAT1, dashed lines indicate no direct regulation relationship inferred. The differential expression analysis result comparing IL21+ Th cells between on-treatment and pretreatment samples treated by CDP is also shown. Color for log fold change; size for significance. The genes with significant expression difference are highlighted with orange borders. (H) Box plots showing the average expression levels of HDAC core genes in CD30− and CD30+ HRS-like cells. Two-sided Wilcoxon test is applied. (I) Stacked bar plots illustrating relative communication probabilities of ligands and receptors in pretreatment and on-treatment tumors from CDP-treated patients. Interactions from CD8+ T/NK cells to CD30− HRS-like cells with remarkable difference between treatment time points and high communication probabilities in any 1 time point are shown. (J) Network plots showing cell-cell communication probabilities in on-treatment tumors from CDP-treated patients. Dot for cluster, line width for strength of interaction between FASL on CD8+ T/NK cells and FAS on CD30− HRS-like cells. Note, FASL-FAS interactions from CD8+ T/NK cells to CD30− HRS-like in pretreatment tumors were not detected. FASL, FAS ligand; GSEA, Gene Set Enrichment Analysis.
We speculated, IL21+ Th cells could be directly targeted by CDP therapy. We observed significant changes in gene expression and pathway activity in IL21+ Th cells after CDP treatment, including decreased activity in mitotic cell cycle (supplemental Table 13). On-treatment IL21+ Th cells showed significant lower expression of genes such as IKZF3, IL21, IL6ST, STAT1, and STAT3 and decreased activity in IL6 and IL21 signaling. Key transcription factors regulating IL21 expression, including STAT1/3 and MAF were notably decreased (Figure 5C-D). The transcriptional regulatory network constructed by SCENIC34 suggested that IKZF3 and STAT1 were the most relevant transcription factors for IL21+ Th cells (Figure 5E-F; supplemental Table 14). Additionally, most genes in IL6 and IL21 pathways were targets of STAT1 (Figure 5G).
CD30– HRS-like cells exhibited lower HDAC scores (Figure 5H), implying a possible indirect effect of CDP treatment on these cells. Interactions from CD8+ T/NK cells to CD30− HRS-like cells were enhanced during treatment (supplemental Figure 11F). These subpopulations expressed several pairing ligand and receptor genes, such as FASL/FAS, TNF/TNFRSF1B, and LGALS9/CD44 (Figure 5I). Interestingly, FASL/FAS interaction between CD8+ T/NK cells and CD30– HRS cells emerged by CDP treatment, indicating an activated cytotoxic response (Figure 5J).
In cHL microenvironment, CD30+ HRS tumor cells secreted various cytokines including IL6, interacted with IL21+ Th subset, and received prosurvival signals from immunosuppressive IL21+ Th cells, facilitating immune evasion. CDP therapy altered the phenotype and proliferation of IL21+ Th cells by targeting STAT1/3, leading to decreased IL21 expression and IL6/IL21 signaling, which abrogated the supportive effects on CD30+ HRS cells. Moreover, CDP therapy upregulated major histocompatibility complex (MHC) molecules in CD30– HRS cells and activated diverse tumor-reactive CD8+ T cells. In summary, CDP epi-immunotherapy alleviated immunosuppression, increased immunogenicity, and enhanced the cytotoxic response against heterogenous HRS cell populations.
Discussion
Although achieving high responsiveness, a proportion of patients with relapsed/refractory cHL experienced PD after anti–PD-1 monotherapy or DP therapy. Here, we reported the therapeutic response of CDP triplet regimen among patients with cHL previously treated with DP. In patients with cHL refractory to prior anti–PD-1 (n = 18), HDAC inhibitor vorinostat plus pembrolizumab resulted in ORR and CR rates of 56% and 11%, respectively, with a median PFS of 5.8 months.15 As previously reported, ORR and CR rates were 45% and 18%, respectively, in anti–PD-1–refractory patients (n = 22) treated with decitabine plus camrelizumab, with a median PFS of 14.6 months.13 In this study, among patients with SD/PD after previous DP therapy, CDP regimen achieved a higher response (ORR, 100%; CR rate, 25%) and longer survival (median PFS, 22.3 months). These data suggest that combining 2 epigenetic agents bring more effectiveness in reversing resistance to anti–PD-1 immunotherapy.
The scarcity of HRS cells has always hindered the study of cHL. Leveraging scRNA-seq and paired biopsies, we built a high-resolution transcriptome atlas of relapsed/refractory cHL and identified the CD30− HRS-like subpopulation, which showed poor tumorigenicity and few interactions with immunocytes in tumor microenvironment. Moreover, the CD30– HRS-like cells were associated with treatment resistance. HRS cells were generally flow-sorted by intermediate-to-high CD30 expression, so CD30– HRS cells were not explicitly mentioned in previous literature. Although we did not examine the abundance of CD30– HRS in anti–PD-1–naïve patients, quite a few DHRS2+CD30– HRS cells were detected in anti–PD-1–resistant patients (scRNA-seq and multiplex immunofluorescence data). Additionally, recent reports have documented decreased CD30 expression in relapsed cHL after anti-CD30 therapies.35,36 Similarly, reduced CD30 level was reported in cancerous cells of anaplastic large cell lymphoma and CD30+ lymphoid neoplasm after BV therapy.37,38 It is worthwhile to explore whether cHL tumors with negative or low CD30 expression arise from immune escape and expansion of the initial few CD30– HRS cells. Regardless, targeting CD30–/low HRS cells could be a promising clinical strategy for patients after failure of immune checkpoint blockade, anti-CD30 chimeric antigen receptor T-cell therapy, or BV treatment. Among the 5 patients who had prior BV in our study, a median PFS of 34.9 months was achieved after CDP therapy, providing a potential therapeutic option for patients after BV-based therapy.
Our single-cell atlas also revealed the widespread remodeling of tumor-infiltrated lymphocytes after epi-immunotherapy and suggested a feedforward loop between the immunosuppressive IL21+ Th cells and CD30+ HRS-like cells, which might drive the pathogenesis and treatment resistance. In physiological conditions, Tfh cells and germinal center B cells had reciprocal interactions.39 Our results suggested the probable coevolution of IL21+ Tfh-like cells and B-cell–originated HRS cells. Interestingly, CDP treatment decreased the expression of transcription factors STAT1/3, IKZF3, and signal transducer IL6ST and suppressed IL6/IL21 signaling in IL21+ Th cells, disrupting the regulatory cycle and reshaping cHL tumor microenvironment.
Admittedly, our research is inadequate by its small sample size that performed single-cell sequencing and lack of data from patients responsive to DP therapy, leaving the detailed antitumor advantages of the CDP triplet regimen compared with DP treatment in responders undefined. Additionally, direct evidence of chidamide’s effects on molecular phenotypic changes of key cells in cHL through epigenetic modifications (such as H3K27 acetylation) requires future studies. Despite the observed efficacy of CDP treatment in patients after prior DP, it remains uncertain whether patients with cHL who progressed/relapsed after anti–PD-1 monotherapy or even anti–PD-1–naïve patients would derive greater clinical benefits from CDP therapy vs either DP or chidamide-plus-anti-PD-1 regimen. A randomized clinical trial comparing DP with CDP in anti–PD-1–resistant patients with cHL is currently underway (NCT04514081).
Acknowledgments
The authors thank the patients involved in this trial and all the members of the Department of Bio-therapeutic for discussion and support. The authors also thank Zemin Zhang for support in data analysis.
This work was supported by the National Natural Science Foundation of China (82370228 to J.N.; 31991171 and 81830002 to W.H.; and 82022057 to J.N.) and the National Key R&D Program of China (2022YFC2505000 and 2022YFC2505002 to Analytical Biosciences Limited).
Authorship
Contribution: J.N. and W.H. contributed to the conception, design, and execution of the study; Chunmeng Wang, Y.L., Y.C., Y.P., and Q.Y. contributed to the acquisition of clinical data; J.N. and Chunmeng W. contributed to the analysis of clinical data; L.Z., Chengcheng Wang, Y.H., and X.H contributed to the analysis of scRNA-seq data; J.N. and L.Z. contributed to the writing of the manuscript; J.N., L.Z., X.H., and W.H. supervised the work; and all authors contributed to review and revision of the manuscript.
Conflict-of-interest disclosure: L.Z., Chengcheng Wang, Y.H., and X.H reported being current employees at Analytical Biosciences Limited. The remaining authors declare no competing financial interests.
Correspondence: Liangtao Zheng, Analytical Biosciences Limited, Beijing, China; email: tao2013@gmail.com; Xueda Hu, Analytical Biosciences Limited, Beijing, China; email: huxueda@abiosciences.com; Jing Nie, Chinese PLA General Hospital, 28 Fuxing Rd, HaiDian District, Beijing 100853, China; email: nnjj2002@163.com; and Weidong Han, Chinese PLA General Hospital, 28 Fuxing Rd, HaiDian District, Beijing 100853, China; email: hanwdrsw@163.com.
References
Author notes
J.N., C.W., and L.Z. contributed equally to this study.
The sequencing data can be accessed from the Genome Sequence Archive database (accession number PRJCA019905).
Complete deidentified patient data (including study protocol) and additional information is available from the corresponding author upon reasonable request, Weidong Han (hanwdrsw@163.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal