Visual Abstract
Large granular lymphocytic leukemia (LGLL) is a rare lymphoproliferative chronic disorder characterized by expansion of either T or natural killer (NK) cytotoxic cells. In contrast to Epstein-Barr virus–induced aggressive NK-LGLL, chronic T-LGLL and NK-LGLL are indolent diseases affecting older patients with a median age of 66.5 years. LGLL is frequently associated with autoimmune disorders, most frequently rheumatoid arthritis. An auto-/alloantigen is tentatively implicated in disease initiation. Large granular lymphocyte expansion is then triggered by proinflammatory cytokines such as interleukin-15, macrophage inflammatory protein 1 (MIP-1), and RANTES (regulated upon activation, normal T cell expressed, and secreted). This proinflammatory environment contributes to deregulation of proliferative and apoptotic pathways. After the initial description of the JAK-STAT pathway signaling activation in the majority of patients, recurrent STAT3 gain-of-function mutations have been reported. The JAK-STAT pathway plays a key role in LGL pathogenesis by promoting survival, proliferation, and cytotoxicity. Several recent advances have been made toward understanding the molecular landscapes of T- and NK-LGLL, identifying multiple recurrent mutations affecting the epigenome, such as TET2 or KMT2D, and cross talk with the immune microenvironment, such as CCL22. Despite an indolent course, published series suggest that the majority of patients eventually need treatment. However, it is noteworthy that many patients may have a long-term observation period without ever requiring therapy. Treatments rely upon immunosuppressive drugs, namely cyclophosphamide, methotrexate, and cyclosporine. Recent advances have led to the development of targeted approaches, including JAK-STAT inhibitors, cytokine targeting, and hypomethylating agents, opening new developments in a still-incurable disease.
Introduction
Large granular lymphocytic leukemia (LGLL) is a rare hematological malignancy characterized by clonal expansion of cytotoxic T cells leading to diverse clinical and biological manifestations. LGLL accounts for 2% to 5% of chronic lymphoproliferative disorders, with an estimated incidence of 0.2 to 0.72 cases per million population per year.1 LGLL can be divided into 2 entities according to phenotype, namely T-cell LGLL (T-LGLL) and natural killer (NK) cell LGLL (NK-LGLL), accounting for 85% and 15% of cases, respectively, and sharing a common pathophysiology and presentation.2 With an estimated 10-year overall survival (OS) of >70% in T-LGLL and 65% in NK-LGLL, LGLL is an indolent disease.2-4 However, approximately half of patients eventually need treatment because of symptomatic cytopenias or associated autoimmune manifestations. Infections represent the main cause of death.
The clonal nature of the disease was proved in 1985, and the description of the T-cell and NK cell entities was made in 1993.5,6 The discovery of JAK-STAT pathway deregulation and the later identification of recurrent STAT3 mutations in both T-LGLL and NK-LGLL represented a key step in the understanding of the pathophysiology and unified the 2 entities.7 T-LGLL and aggressive NK-LGLL were included in the World Health Organization (WHO) classification in 2001 in the “Mature T- and NK-cell neoplasms” group. Until recently, NK-LGL proliferation was considered a “provisional entity” because of difficulties demonstrating clonality. therefore, the term “chronic lymphoproliferative disorder of natural killer cells” was used, opposed to the aggressive NK leukemia. Thanks to the identification of recurrent mutations in clonal NK lymphoproliferations, the last 2022 WHO classification included both T- and NK-LGLL.8 By contrast, the 2022 International Consensus Classification kept the T-LGL and chronic lymphoproliferative disorder of natural killer cells denominations.9
This review covers pathogenesis of the disease, clinicobiological features, diagnostic workflows, and treatment recommendations, including future directions.
Pathophysiology
After initial expansion, thought to be due to a chronic antigen stimulation, persistence of monoclonal large granular lymphocytes (LGLs) is the consequence of resistance to the Fas-induced apoptosis, a proinflammatory cytokine environment, activation of the JAK-STAT pathway, and epigenetic alterations (Figure 1).
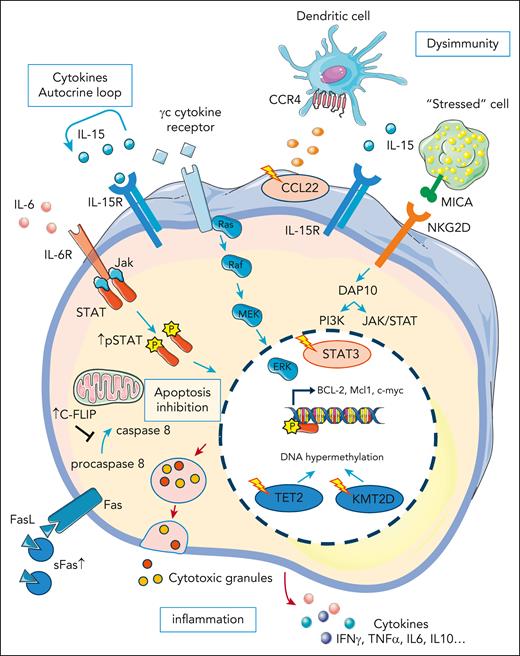
LGLL pathogenesis: polyclonal LGL expansion is thought to be initiated by a viral/autoantigen. LGL expansion is then sustained by inflammatory cytokines that can also contribute to the frequently associated autoimmune disorders. LGL expansion and clonal selection can also be favored by the occurrence of specific mutations including STAT3 and CCL22, inducing a resistance to apoptosis, increased proliferative capacities, and dysregulation of the cross talk with the immune microenvironment, respectively. Even in the absence of STAT3 mutation, the JAK-STAT pathway is activated in the majority of patients with LGLL. Epigenetic modifications have also been described with TET2 mutations, possibly representing an early event, observed in ∼30% of cases. Production of inflammatory cytokines and release of the cytotoxic granules by the leukemic LGLs in the infiltrated tissues lead to a spectrum of clinical and biological manifestations including cytopenia, fatigue, and autoimmune diseases. CCL22, C-C motif chemokine ligand 22; FasL: Fas ligand; IP10 (CXCL10), interferon-gamma–induced protein 10; PDGF, platelet-derived growth factor; STAT3, signal transducer and activator of transcription 3; TET2, ten-eleven translocation-2; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand.
LGLL pathogenesis: polyclonal LGL expansion is thought to be initiated by a viral/autoantigen. LGL expansion is then sustained by inflammatory cytokines that can also contribute to the frequently associated autoimmune disorders. LGL expansion and clonal selection can also be favored by the occurrence of specific mutations including STAT3 and CCL22, inducing a resistance to apoptosis, increased proliferative capacities, and dysregulation of the cross talk with the immune microenvironment, respectively. Even in the absence of STAT3 mutation, the JAK-STAT pathway is activated in the majority of patients with LGLL. Epigenetic modifications have also been described with TET2 mutations, possibly representing an early event, observed in ∼30% of cases. Production of inflammatory cytokines and release of the cytotoxic granules by the leukemic LGLs in the infiltrated tissues lead to a spectrum of clinical and biological manifestations including cytopenia, fatigue, and autoimmune diseases. CCL22, C-C motif chemokine ligand 22; FasL: Fas ligand; IP10 (CXCL10), interferon-gamma–induced protein 10; PDGF, platelet-derived growth factor; STAT3, signal transducer and activator of transcription 3; TET2, ten-eleven translocation-2; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand.
Initiating event
LGL clones commonly display an effector memory phenotype, suggesting the involvement of chronic stimulation by an auto-/alloantigen in leukemogenesis, further supported by peculiar associations with autoimmune disorders and documented interactions between LGLs and dendritic cells.10 Several viral pathogens, including human T-lymphotropic virus 1/2 and Epstein-Barr virus, have been suspected despite no documented evidence of direct viral DNA insertion.11-13 Interestingly, hematological responses to antiviral therapies have been observed in patients with LGLL who are infected (hepatitis C virus, hepatitis B virus, and HIV).14,15 Importantly, no common T-cell receptor alpha (TRA) or T-cell receptor beta (TRB) loci clonotype shared by different patients was identified in single-cell analyses coupled with T-cell receptor (TCR) profiling.16,17 However, in most patients, LGL clones shared a TCR target with their normal counterpart. Although the triggering event may likely be a result of different antigens, it would appear that the mechanisms sustaining pathogenesis are common and may be independent of the inciting antigen.
The role of inflammatory cytokines in LGL expansion
In LGLL, sustained LGL expansion is favored by several cytokines, including interleukin-15 (IL-15), IL-18, interferon-γ–induced protein 10 (CXCL10), and platelet-derived growth factor.18-21 Deregulation of cytokine production leads to a proinflammatory microenvironment, contributing to LGL pathophysiology (Figure 2).22 LGL clones secrete high levels of cytokines and their cognate receptors forming an autocrine loop activating survival and proliferations pathways.20,23,24 IL-15 plays a crucial role in the genesis and homeostasis of NK- or T-LGLs: mice lacking the IL-15α receptor display an NK-cell and CD8pos memory T-cell defect.25 IL-15 is overexpressed by LGL leukemic clones and in vitro culture of LGLs isolated from patients are IL-15 dependent.26 In addition, transgenic mice overexpressing IL-15 develop either T- or NK-LGLL.27 Finally, IL-15 chronic in vitro exposure leads to a leukemic transformation due to IL-15–induced centrosome aberration and DNA hypermethylation. IL-6 could be implicated in LGLL pathogenesis because high levels of IL-6, produced, in part, by nonleukemic clones, were observed in some patients with LGLL.28,29
Main deregulated pathways in LGLL: deregulation of apoptosis pathways is a key element in LGLL pathogenesis. LGL clones are resistant to the Fas-mediated apoptosis. Secreted soluble Fas (sFas) act as a decoy of FasL preventing the formation of the Fas-mediated death-inducing signaling complex (DISC). Apoptosis cascade is also inhibited by an increased level of an inhibitory protein named cellular FADD-like IL-1–converting enzyme inhibitory protein (c-FLIP). Ras and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathways are also activated in LGLL and contribute to the apoptosis inhibition. The JAK-STAT pathway is constitutively activated in LGLL and leads to the transcription of B-cell lymphoma 2 (BCL-2) and myeloid cell leukemia 1 (Mcl-1) antiapoptotic proteins expression. Indeed, the JAK-STAT pathway is activated by several mechanisms. Gain-of-function STAT3 mutations are observed in 30% to 60% of patients and have recently been shown to trigger the expansion of cytotoxic LGL cells expressing high level of natural-killer group 2, member D (NKG2D). The JAK-STAT pathway is also activated downstream to the cytokines’ receptors. Increased secretion of inflammatory cytokines creates an autocrine loop. Mutations affecting genes implicated in epigenetic mechanisms such as ten-eleven translocation-2 (TET2) and lysine methyltransferase 2D (KMT2D) have been recently described. Gain-of-function CCL22 mutation induce a defect in its CCR4 receptor internalization, leading to an increase adhesion of myeloid and dendritic cells. These immune cells can stimulate LGL cells survival and proliferation in part by IL-15 secretion. Inflammatory cytokines secretion and cytotoxic granules release lead to the clinical symptoms and biological features observed in LGLL.
Main deregulated pathways in LGLL: deregulation of apoptosis pathways is a key element in LGLL pathogenesis. LGL clones are resistant to the Fas-mediated apoptosis. Secreted soluble Fas (sFas) act as a decoy of FasL preventing the formation of the Fas-mediated death-inducing signaling complex (DISC). Apoptosis cascade is also inhibited by an increased level of an inhibitory protein named cellular FADD-like IL-1–converting enzyme inhibitory protein (c-FLIP). Ras and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathways are also activated in LGLL and contribute to the apoptosis inhibition. The JAK-STAT pathway is constitutively activated in LGLL and leads to the transcription of B-cell lymphoma 2 (BCL-2) and myeloid cell leukemia 1 (Mcl-1) antiapoptotic proteins expression. Indeed, the JAK-STAT pathway is activated by several mechanisms. Gain-of-function STAT3 mutations are observed in 30% to 60% of patients and have recently been shown to trigger the expansion of cytotoxic LGL cells expressing high level of natural-killer group 2, member D (NKG2D). The JAK-STAT pathway is also activated downstream to the cytokines’ receptors. Increased secretion of inflammatory cytokines creates an autocrine loop. Mutations affecting genes implicated in epigenetic mechanisms such as ten-eleven translocation-2 (TET2) and lysine methyltransferase 2D (KMT2D) have been recently described. Gain-of-function CCL22 mutation induce a defect in its CCR4 receptor internalization, leading to an increase adhesion of myeloid and dendritic cells. These immune cells can stimulate LGL cells survival and proliferation in part by IL-15 secretion. Inflammatory cytokines secretion and cytotoxic granules release lead to the clinical symptoms and biological features observed in LGLL.
Resistance to Fas-induced apoptosis
Physiologically, pathogen clearing is followed by activation of the Fas-mediated activation-induced cell death. LGL clones are characterized by resistance to Fas-induced apoptosis, almost always without evidence of FAS gene mutation as observed in autoimmune lymphoproliferative syndrome.30-32 FAS-L overexpression in LGL clones leads to secretion of soluble Fas acting as a decoy-receptor inhibiting Fas-dependent apoptosis.31 Moreover, in LGLL, increased expression of the inhibiting C-FLIP molecule, downstream to the Fas-receptor, leads to decreased caspase 8 cleavage and ultimately inhibits the death-inducing signaling complex.33
The genetic landscape of LGLL
Since the discovery of constitutive activation of STAT3 in 2001 as the hallmark of LGLL, several mutations affecting key T-cell cellular functions, such as cytokine secretion, proliferation, apoptosis, and epigenetics, have been reported.7,34,35 As observed in the vast majority of hematological malignancies, LGLL is characterized by a clonal heterogeneity with the cooccurrence of different mutations (Figure 3).32
Mutational landscape of LGLL. (A) Clinicomutational correlation. STAT3, STAT5B, and TET2 mutations have been associated with specific clinicobiological features. ∗Compared with wild-type STAT3. ∗∗CCL22 and STAT3 mutations are mutually exclusive. (B) Mutational landscape of LGLL (N = 120 patients). Courtesy of Cédric Pastoret, Hematology Department, Rennes University Hospital. AITL, angioimmunoblastic T-cell lymphoma; CMML, chronic myelomonocytic leukemia; MDS, myelodysplastic syndrome; Ref, references.
Mutational landscape of LGLL. (A) Clinicomutational correlation. STAT3, STAT5B, and TET2 mutations have been associated with specific clinicobiological features. ∗Compared with wild-type STAT3. ∗∗CCL22 and STAT3 mutations are mutually exclusive. (B) Mutational landscape of LGLL (N = 120 patients). Courtesy of Cédric Pastoret, Hematology Department, Rennes University Hospital. AITL, angioimmunoblastic T-cell lymphoma; CMML, chronic myelomonocytic leukemia; MDS, myelodysplastic syndrome; Ref, references.
A key role of JAK-STAT signaling
The JAK/STAT pathway plays a major role in the regulation of T cells. The role of STAT3 in LGLL was identified in 2001, with high levels of phosphorylated STAT3.35 STAT3 inhibition using an antisense oligonucleotide restored Fas-dependent apoptosis. STAT3 gain-of-function mutations were later identified in T- and NK-LGLL, unifying both entities.7,36STAT3 mutations are identified in up to 60% of T-LGLL and 30% of NK-LGLL.37,38STAT3 mutations are classically missense mutations found in the region encoding for the Src homology 2 domain with the D661Y and Y640F mutations accounting for 80% of all mutations found.36 These mutations induced constitutive dimerization and phosphorylation of STAT3, leading to increased transcription of antiapoptotic genes (BCLXL and FLIP), cell cycle genes (CDKN1D and CMYC), and proinflammatory cytokine coding genes (IL-6, IL-12, IL-17, IL-21, IL10, and interferon-γ). Multiple STAT3 mutations can be found in patients with LGLL, supporting the existence of subclonal evolution as observed in acute leukemia.39STAT3 gain-of-function mutation leads to a more cytotoxic profile, higher blood LGL level, and deeper cytopenia. It is associated with an hypermethylated status, a higher expression of FAS ligand (FASL), and an increased reactive oxygen species level.16,29,40 Interestingly, STAT3 gain-of-function mutations are described in several autoimmune diseases associated with a CD8pos T-cell expansion.41 In a mouse model, STAT3 gain-of-function induced expansion of NKG2DposCD8posCD62LnegCD44pos T-cells and overexpression of cell cycle and killer cell genes (GZMA and GZMB) through natural killer group 2 member D (NKG2D)/IL2-IL15 receptor interactions.42 Although current opinion states autoimmune disease and LGLL can both arise from a common proinflammatory context, this study demonstrated that the LGL clone itself may play a crucial role in autoimmunity development. In addition to STAT3, STAT5B mutations have been reported in patients with LGLL, particularly in Tγδ-LGLL (6%) or in the rare indolent form of CD4pos Tαβ-LGLL (45%).40,43-46 Mutations of other genes implicated in the JAK-STAT pathway, such as JAK1, JAK3, IL6R, PTPRT, and GNAS have also been described.32,47 The JAK/STAT pathway can also be activated by nonmutational mechanisms, such as cytokine modulation and epigenetic inactivation of JAK-STAT pathway inhibitors, including microRNAs (miRs) modifications. miR-181a and miR-146b have been associated with STAT3 activation in patients without mutations.48,49 Finally, deregulation of other signaling pathways (ie, mitogen-activated protein kinases, phosphoinositide-3-kinase–protein kinase B/Akt, or NF-κB) is reported in LGLL inducing prosurvival signals.50-52 Accordingly, NRAS, KRAS, PI3KCD, and TNFAIP3 mutations complete the genomic landscape of LGLL.
Mutations affecting epigenetic pathways
Epigenetic modifications are recent developments in understanding LGLL pathophysiology. DNA-methylation alteration seems to be a crucial event in NK-LGLL development, as suggested by the high frequency of TET2 (ten-eleven translocation 2) mutations in this LGLL subtype. Initially described in few cases of LGLL, we recently reported that TET2 mutations are present in 28% to 34% of patients with NK-LGLL.38,53 However, TET2 mutations are also reported in clonal hematopoiesis of indeterminate potential, affecting the myeloid compartment but not normal T cells.54TET2 mutations have been initially described in myeloid disorders, aplastic anemia, and also in various T-cell malignancies, including angio-immunoblastic lymphoma.55,56 Most recently, clonal hematopoiesis was reported in some patients with LGLL with neutropenia, with TET2 mutation seen in a small percentage.57 In myelodysplastic syndromes, TET2 mutations are associated with hypermethylation of genes implicated in NK cell function including killer immunoglobulin-like receptors (KIR), perforin, and TNFA.58 In NK-LGLL, TET2 mutations were associated with a methylation pattern distinct from normal NK cells, including the hypermethylation of PTPRD (a negative regulator of STAT3) and the JAK inhibitor SOCS3, all contributing to the activation of the JAK-STAT pathway.38,53,59 We explored the clonal hierarchy of genomic alterations in NK-LGLL. In some cases, TET2 mutations can occur early and be shared between NK cell and myeloid compartments. In this case, TET2 mutations can explain the link between NK-LGLL and the development of other TET2-derived malignancies, such as myelodysplastic syndrome. TET2 may support the selection of NK cell clones, which can secondarily acquire STAT3 mutations leading to the cytotoxic phenotype, and ultimately define a symptomatic NK-LGLL. This hypothesis is supported by the cooccurrence of TET2 and STAT3 or CCL2 in a third of TET2-mutated cases. TET2 mutations can also be restricted to NK cell clones and support STAT3 activation through epigenetic silencing of JAK-STAT regulators. TET2 mutations seems to be a crucial event in NK-LGLL development, presenting relevant clinical features, such as more thrombopenia and frequent associations with other malignancies. In T-LGLL, mutations of the histone monomethyl-transferase KMT2D are detected in up to 20% of cases.32 The exact role of KMT2D in LGLL pathogenesis remains unclear, but chromatin accessibility profiling can discriminate LGLL from other mature T-cell malignancies.60 Clearly, epigenetic modifications constitute a new field in LGLL pathophysiology, which should be elucidated with single cell analyses and functional studies, detailed later.
Role of the immune microenvironment
Mutations affecting the CCL22 encoding gene are observed in 27% of NK-LGLL.61CCL22 mutations were enriched at highly conserved residues and mutually exclusive of STAT3 mutations. They consist of gain-of-function alterations, inducing a CCR4 internalization defect in targeted cells, leading to increased cellular chemotaxis and dysregulated cross talk with the hematopoietic microenvironment. For example, myeloid cells and dendritic cells produce higher IL-15 levels in vitro with mutated CCL22, which may favor NK cell proliferation. Similarly, a recent study reported that patients with STAT3 mutation display skewed Th17/T regulatory T-cells ratios and abnormal distributions of monocyte populations with increases in intermediate and nonclassical monocytes.62 CCL5, a cytokine produced by LGL clones induced high-level of IL-6 release by monocytes. Further studies are needed to gain mechanistic insight and to test potential therapeutic intervention targeting this dysregulated cross talk.
Clinicobiological features
With a median age of 66.5 years, LGLL usually affects older people with similar incidence in men and women. Symptomatology is dominated by neutropenia-related infections, which represent the highest risk of mortality. The main clinicobiological features reported in the literature are summarized in Table 1.
Clinical and biologic presentation: data from the largest retrospective series
| Parameters . | Loughran et al (1993)115 . | Semenzato et al (1997)80 . | Bareau et al (2010)2 . | Sanikommu et al (2017)70 . | Zhu et al (2020)116 . | Dong et al (2021)71 . |
|---|---|---|---|---|---|---|
| Number of patients | 129 | 162 | 229 | 204 | 108 | 319 |
| T-LGLL (%) | 100 | 100 | 88 | 90 | 100 | 93 |
| NK-LGLL (%) | NA (aggressive form) | 0 | 12 | 10 | 0 | 8 |
| Median age, y (min-max) | 57 (15-88) | 59 | 59 (12-87) | 63 (54-72) | 51 (21-78) | 65 (17-90) |
| Sex ratio (M/F) | 0.8 | 0.8 | 0.8 | 0.9 | 0.9 | 0.9 |
| Biological features | ||||||
| LGL count (×109/L) | NA | NA | <1 × 109/L, 37% >4 × 109/L, 15% | 1.7 (0.8-3.3) | 2.07 (0.3-45.5) | 0.9 (0.5-2.3) |
| Median neutrophil count (min-max) | NA | NA | NA | 1.56 (0.8-2.6) | 1.36 (0.04-9.6) | NA |
| Neutropenia (%) (ANC <1.5 × 109/L) | 84 | NA | 59 | 46 | 57 | 41 |
| Severe neutropenia (ANC <0.5 × 109/L) (%) | 48 | 37 | 24 | 17 | 10 | 17 |
| Median Hb level (min-max) | NA | NA | NA | 11.7 (10-13) | 7.5 (3.1-15.6) | NA |
| Anemia (%) | 49 | 26 | 24 | 40 | 59 | 41 |
| Transfusion dependency (%) | NA | NA | 6 | 22 | 58 | 19 |
| Thrombocytopenia (%) | 19 | 9 | 17 | 30 | 8 | 26 |
| Antinuclear antibodies | 38 | 38 | 17 | NA | 45 | 22 |
| Rheumatoid factor (%) | 57 | 43 | 14 | NA | 10 | 39 |
| STAT3 mutation (%) | NA | NA | NA | 36 | 28 | 40∗ |
| Clinical manifestations | ||||||
| Treatment requirement | 73 | 33 | 44 | 58 | 97 | 57 |
| B symptoms (%) | NA | 26 | 7 | NA | 0 | 63 |
| Hepatomegaly (%) | 23 | 32 | 10 | NA | 6 | NA |
| Splenomegaly (%) | 50 | 44 | 24 | 24 | 41 | 28 |
| Lymphadenopathies (%) | 1 | 12 | 6 | NA | 7 | NA |
| Recurrent infections (%) | 39 | 61 | 22 | NA | 12 | NA |
| Autoimmune manifestations (%) | 28 | NA | 32 | 25 | 23 | 26 |
| RA (%) | 28 | NA | 17 | 15 | 2.7 | 12 |
| Other hematological malignancies (%) | NA | NA | 10 | 19 | 0 | 19 |
| Solid tumor (%) | NA | NA | 4 | 17 | 0 | 21 |
| LGL-related death (%) | 36 | NA | 7 | 5 | 3 | NA |
| Parameters . | Loughran et al (1993)115 . | Semenzato et al (1997)80 . | Bareau et al (2010)2 . | Sanikommu et al (2017)70 . | Zhu et al (2020)116 . | Dong et al (2021)71 . |
|---|---|---|---|---|---|---|
| Number of patients | 129 | 162 | 229 | 204 | 108 | 319 |
| T-LGLL (%) | 100 | 100 | 88 | 90 | 100 | 93 |
| NK-LGLL (%) | NA (aggressive form) | 0 | 12 | 10 | 0 | 8 |
| Median age, y (min-max) | 57 (15-88) | 59 | 59 (12-87) | 63 (54-72) | 51 (21-78) | 65 (17-90) |
| Sex ratio (M/F) | 0.8 | 0.8 | 0.8 | 0.9 | 0.9 | 0.9 |
| Biological features | ||||||
| LGL count (×109/L) | NA | NA | <1 × 109/L, 37% >4 × 109/L, 15% | 1.7 (0.8-3.3) | 2.07 (0.3-45.5) | 0.9 (0.5-2.3) |
| Median neutrophil count (min-max) | NA | NA | NA | 1.56 (0.8-2.6) | 1.36 (0.04-9.6) | NA |
| Neutropenia (%) (ANC <1.5 × 109/L) | 84 | NA | 59 | 46 | 57 | 41 |
| Severe neutropenia (ANC <0.5 × 109/L) (%) | 48 | 37 | 24 | 17 | 10 | 17 |
| Median Hb level (min-max) | NA | NA | NA | 11.7 (10-13) | 7.5 (3.1-15.6) | NA |
| Anemia (%) | 49 | 26 | 24 | 40 | 59 | 41 |
| Transfusion dependency (%) | NA | NA | 6 | 22 | 58 | 19 |
| Thrombocytopenia (%) | 19 | 9 | 17 | 30 | 8 | 26 |
| Antinuclear antibodies | 38 | 38 | 17 | NA | 45 | 22 |
| Rheumatoid factor (%) | 57 | 43 | 14 | NA | 10 | 39 |
| STAT3 mutation (%) | NA | NA | NA | 36 | 28 | 40∗ |
| Clinical manifestations | ||||||
| Treatment requirement | 73 | 33 | 44 | 58 | 97 | 57 |
| B symptoms (%) | NA | 26 | 7 | NA | 0 | 63 |
| Hepatomegaly (%) | 23 | 32 | 10 | NA | 6 | NA |
| Splenomegaly (%) | 50 | 44 | 24 | 24 | 41 | 28 |
| Lymphadenopathies (%) | 1 | 12 | 6 | NA | 7 | NA |
| Recurrent infections (%) | 39 | 61 | 22 | NA | 12 | NA |
| Autoimmune manifestations (%) | 28 | NA | 32 | 25 | 23 | 26 |
| RA (%) | 28 | NA | 17 | 15 | 2.7 | 12 |
| Other hematological malignancies (%) | NA | NA | 10 | 19 | 0 | 19 |
| Solid tumor (%) | NA | NA | 4 | 17 | 0 | 21 |
| LGL-related death (%) | 36 | NA | 7 | 5 | 3 | NA |
ANC, absolute neutrophils count; F, female; Hb, hemoglobin; M, male; max, maximum; min, minimum; NA, not available.
Performed only on 25 patients.
Clinical manifestations
Clinical manifestations vary largely among the population of patients with LGLL. Neutropenia-related infections affect 15% to 39% of patients and involve the skin, oropharynx, perirectal area, and the lungs. Life-threatening bloodstream infections may occur. Opportunistic bacterial, fungal, and viral infections are uncommon. Some patients have prolonged neutropenia without any infection. Splenomegaly is seen in 20% to 50% of cases whereas hepatomegaly and lymphadenopathy are less common. Fatigue and B symptoms are observed in 20% to 30% of cases. Tissue infiltrations of the skin, nerves, or kidney have been described sporadically.63-65 As opposed with indolent LGLL, aggressive NK LGLL is characterized by systematic bone marrow and organ involvement, marked deterioration of the general status, and an extremely poor OS.66 In this context, the diagnosis of hepato-splenic T-cell lymphoma (generally CD4negCD8negCD56pos) must be also ruled out.67
LGLL is at the interface between hematology and autoimmunity, with autoimmune disorders reported in ∼25% to 32% of cases.68,69 Several autoimmune disorders have been described, mostly in case reports and small cohorts. Rheumatoid arthritis (RA) represents the main autoimmune disorder associated with LGLL, affecting 10% to 15% of patients and is usually diagnosed before or concomitantly to the LGLL. Sjogren syndrome, rhizomelic pseudopolyarthritis, and unclassified inflammatory arthritis have also been described.2,70-72 Psoriasis incidence seems to be preferentially observed in patients carrying the CCL22 mutation.61 Pulmonary artery hypertension has also been described.73 Vasculitis such as cryoglobulinemia, cutaneous leukocytoclastic angiitis, and antineutrophil cytoplasmic antibody–negative microscopic polyangiitis have been reported. The regression of vasculitis-related manifestations after the treatment of LGLL highlights the close relationship between the 2 conditions.74 Occasionally, systemic lupus erythematosus and inclusion body myositis have been reported.
Solid cancers and hematological malignancies are found in 5% to 21% of patients with LGLL.2,71 Monoclonal gammopathy of undetermined significance, multiple myeloma, and B-cell lymphomas have been reported.75 Overrepresentation of cancers in patients with LGLL is difficult to ascertain because of its high incidence above the age of 60 years.
STAT3 mutations are associated with a more symptomatic phenotype, with a higher incidence of neutropenia, associated autoimmune disorders including RA, a more frequent treatment requirement, and a reduced OS.76,77 More specifically, STAT3 mutation D661Y is correlated with the presence of macrocytic anemia.18
Biological features
Neutropenia is observed in 70% to 85% of cases, with a higher incidence in T-LGLL compared with its NK counterpart.2 Fas-mediated apoptosis has been implicated in the pathogenesis of neutropenia. Fas-ligand, produced in excess by LGL clones, binds to the neutrophil Fas-receptors, inducing apoptosis.78 Moreover, autoantibodies are found in 20% to 40% of patients and can form immune complexes binding to the Fc receptors of neutrophils. Anemia is usually moderate and <10% to 20% of patients are transfusion dependent. Conversely, anemia is the most frequent cytopenia in Asia, affecting 29% to 42% of patients with LGLL mainly due to pure red cell aplasia (PRCA). Hemolytic anemia and immune thrombocytopenia have occasionally been reported.79 Thrombocytopenia, usually moderate, is observed in 19% of patients. Bone marrow involvement, observed in 70% to 90% of cases, is unlikely to directly contribute to cytopenias because infiltration is subtle in the majority of patients. Lymphocytosis is inconstant and LGL threshold was lowered to 0.5 × 109/L even if the 2 × 109/L threshold is still retained as a diagnostic criterion in the last 2022 International Consensus Classification and WHO classifications.8,9,80 Indeed, authentic LGLLs are described with a circulating LGL count between 0.5 × 109/L and 2 × 109/L.
Serum electrophoresis frequently shows hypergammaglobulinemia. Antinuclear antibodies are seen in 20%. Increased β2 microglobulin and soluble Fas ligand are seen in 65% and 95%, respectively.
Tγδ LGLL variant represents only 5% of all T-LGLLs. Even if Tγδ and Tαβ entities share clinical and biological features including STAT3/5B mutations, the frequencies of cytopenia is higher in Tγδ subtypes and survival lower than that of Tαβ LGLL.4,81,82 Recent whole-exome sequencing data found shared somatic mutations and putative drivers between Tαβ-LGL and Tγδ-LGLL.32
The CD4pos T-LGLL: a rare entity with a specific presentation and outcome
Alongside with the largely predominant CD3posCD8pos T-LGLL phenotype, a rare CD3posCD4posCD8neg entity has been reported.83 Compared with classical CD8pos T-LGLL, patients with a CD4pos T-LGLL seem to have a more indolent evolution with less cytopenia and autoimmune manifestations. A higher incidence of associated neoplasia such as monoclonal B-cell lymphocytosis and plasma cell disorders has been reported.83,84 CD4pos T-LGLLs are characterized by a high frequency of STAT5B mutation.43 In the rare CD4pos T-LGLL, the diagnosis of prolymphocytic leukemia, sharing similarities in term of phenotype and molecular profile, should be ruled out.45,83
Diagnosis
In a compatible clinicobiological context, the aim of diagnostic procedures is to distinguish between reactive LGL expansion and LGLL. Reactive polyclonal LGL expansion can be seen in various situations, such as viral infections (cytomegalovirus, Epstein-Barr virus, HIV, etc), after splenectomy, and organ or stem cell transplantation.85,86 A polyclonal LGL expansion has also been described in association with hematological malignancies and autoimmune conditions.87
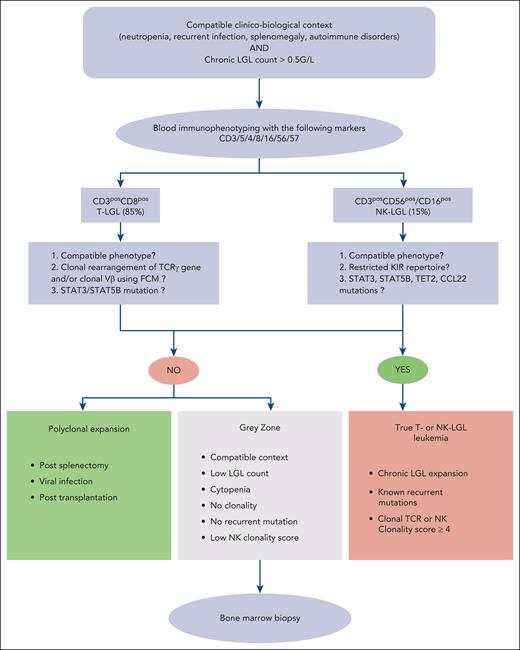
Therefore, diagnosis is based on 2 mandatory criteria: (1) the identification of an elevated number (>0.5 × 109/L) circulating LGL cells based on the complete blood cell count and/or compatible phenotypic pattern, and (2) a proof of clonality obtained by flow cytometry and/or molecular biology. The diagnosis workflow is presented in Figure 4.
Diagnosis algorithm of LGLL. ∗NK clonality score based on 4 parameters: NK cell count of >11 × 109/L: 2 points; KIR restricted phenotype: 2 points; CD94 or NKG2Ahi: 1 point; STAT3, STAT5B, TET2, or TNFAIP3 mutation: 2 points.38
Diagnosis algorithm of LGLL. ∗NK clonality score based on 4 parameters: NK cell count of >11 × 109/L: 2 points; KIR restricted phenotype: 2 points; CD94 or NKG2Ahi: 1 point; STAT3, STAT5B, TET2, or TNFAIP3 mutation: 2 points.38
Cytology
LGLs are easily identified on a blood smear. These large cells (15-20 μm) are characterized by abundant cytoplasm containing azurophilic granules and a reniform or round nucleus with mature chromatin. In a normal setting, LGLs represent 10% to 15% of mononuclear cells, that is, 0.21 × 109/L to 0.29 × 109/L. Leukemic and normal LGLs are morphologically indistinguishable, justifying phenotypic analyses when LGLL is suspected. Caution is needed in the case of normal lymphocyte count. Blood analyses are usually sufficient to ascertain the diagnosis, and bone marrow exploration is only recommended in atypical presentations or in case of associated confounding conditions such as PRCA or myelodysplastic syndrome. This exploration is particularly useful in patients with low (<1 × 109/L) LGL count to confirm the diagnosis.
Bone marrow involvement, observed in the vast majority of patients, is frequently subtle without any correlation between the degree of LGL marrow infiltration and the severity of cytopenia. Histological analysis classically shows intrasinusoidal linear clusters of LGLs.88 LGLs are in most cases CD8pos, granzyme B–positive, and TiA1pos.
Clonality assessment
LGLL has a CD3pos T phenotype in 85% of diagnoses whereas its CD3negCD56pos/CD16pos NK counterpart represents 15% of cases. In most cases, leukemic T-LGLs display a terminal effector memory T-cell phenotype (TCRαβposCD45posD8posCD57posCD16posCD62Lneg) and an aberrant dim expression of CD5.89 TCRγδ LGLLs with a CD4negCD8neg phenotype are seen in ∼15% of cases.81 The proof of clonality is a key step in the diagnosis of T-LGLL. TCR Vβ analysis by flow cytometry can be used as a surrogate marker of TCR clonality with commercially available antibodies covering ∼70% of the Vβ repertoire. However, clonal rearrangement of TCRγ gene by polymerase chain reaction using primers targeting conserved region of the variable diversity joining segments remains the gold standard.90,91 In TCRαβpos LGLL, flow cytometry–based analysis of the constant region 1 of the TCRβ chain has been recently developed with high sensitivity and good correlation with the TCR sequencing.92,93 However, this procedure is neither widely available and its high sensitivity could lead to the identification of T-cell clones in patients not meeting LGL-diagnostic criteria or corresponding to the T-cell clone of undetermined significant entity.94 Similarly, clonality can be assessed by deep sequencing of the TCR repertoire in highly specialized laboratories.44
Normal NK cells can be divided into 2 main phenotypes based on the expression of CD16 and CD56. Indeed, CD56lowCD16high NK cells are mainly characterized by their cytotoxic properties whereas CD56highCD16low NK cells are cytokine-producing cells.95,96 In NK-LGLL, leukemic cells usually display the following phenotype: CD2possCD3negCD3εposTCRαβnegCD4negCD8posCD16highCD56low. However, phenotypic analysis does not allow the easy distinction between normal and leukemic NK cells. Compared with T-LGLL, proof of clonality is far more difficult to obtain in chronic NK-LGLL because NK T cells do not express TCR. A restricted KIR repertoire has been used as a surrogate marker of clonality.97,98 Similarly to T-LGLL, recurrent mutations have been recently described in the NK entity with STAT3 , TET2, and CCL22 mutations observed in ∼30% each.38,53,61 In order to distinguish authentic NK-LGLL from reactive NK cell proliferation, we recently proposed a NK clonality score combining KIR repertoire analysis by flow cytometry and molecular analysis.38
How we treat LGLL
LGLL remains an indolent but incurable disease, with a reported 10-year OS >70% in the largest retrospective studies.2,3,70,71 Treatment indication and strategy do not differ between T- and NK-LGLL. First-line treatment still relies on immunosuppressive drugs (Figure 5). Recent advances in our understanding of pathogenesis have opened the way to the development of new therapeutic approaches targeting the IL-15 signaling and JAK-STAT pathways, and epigenetic deregulation.
When do we treat?
Consistent with the indolent evolution, a watch-and-wait strategy can be proposed in nearly half of the cases but only 20% will not need treatment in their lifetime. Severe neutropenia (neutrophils of <0.5 × 109/L), neutropenia-related infections, transfusion dependency, and symptomatic anemia represent the main indications. Autoimmune disorders represent an indication of treatment in 12% of cases in our experience.2 Evaluation of the treatment response is mostly based on the blood parameters and transfusion dependency. The role for minimal residual disease detection using flow cytometry or molecular approaches remains to be determined.
First-line treatment
Single-agent cyclophosphamide, methotrexate, and cyclosporine are the 3 recommended drugs in first line. No consensus exists regarding the treatment sequence.2,3,70,99,100 Overall response rates (ORRs) reported with the 3 available drugs range from 38% to 75%, depending on the retrospective or prospective nature of the studies.2,3,37,70,99,101,102 Only 2 prospective studies have been conducted in naïve patients. The Eastern Cooperative Oncology Group study reported an ORR of 38% after methotrexate.101 A total of 160 patients have been enrolled in the prospective randomized study comparing cyclophosphamide and methotrexate in France (ClinicalTrials.gov identifier: NCT01976182).37 Results of the interim analysis performed on the first 96 patients showed an ORR to the first line of 55% at 4 months with only 16% complete remission and a high rate of relapse occurring in 67% of the cohort. Methotrexate (10 mg/m2 per week) and cyclosporine A (3 mg/kg per day) are generally given until progression whereas cyclophosphamide (100mg per day) should not be given for >1 year because of the mutagenic risk. Treatment response is assessed after a minimum of 4 months. Cyclophosphamide or cyclosporine may be more adapted for patients with anemia, especially PRCA. Methotrexate is appropriate in patients with RA because of its activity in both conditions. In the absence of response to 1 drug, a switch is recommended. Cyclophosphamide and methotrexate are generally considered as the best first-line option. Cyclosporine A is usually considered for patients with LGLL with overlapping aplastic anemia as well as patients failing both drugs. A recent retrospective study including 60 patients in second line reported a 70% ORR with cyclophosphamide. By contrast, methotrexate was not effective in patients failing cyclophosphamide.103
Treatment in relapsed/refractory diseases
Several approaches have been evaluated in patient refractory to the 3 main immunosuppressive drugs. Alemtuzumab has been used with promising results (ORR, 56%; 95% confidence interval, 35-76) but high toxicity (7 deaths of 25 patients).104 Bendamustine and purine analogues such as fludarabine and cladribine have shown interesting response rates in small retrospective studies.100,105 In patients with LGLL with aplastic anemia, antithymocyte globulins associated or not with cyclosporine A led to some responses.70 Autologous and allogeneic stem cell transplantations have been rarely used in highly pretreated and refractory patients.106
Given the key role of JAK-STAT pathway in the pathogenesis of LGLL, JAK inhibitors such as ruxolitinib represent an attractive option. We initially reported the efficacy of ruxolitinib in 2 patients with refractory T-LGLL.107 The efficacy of ruxolitinib was recently confirmed in a prospective phase 2 clinical trial.108,109 In 20 patients with refractory/relapsed LGLL evaluable, the ORR and complete response rate were 55% and 30%, respectively. Interestingly, STAT3 mutational status was predictive of the event-free survival with a 14-month event-free survival of 100% in patients with mutations compared with 40% in those without mutation. We confirmed these impressive results in a series of 21 refractory/relapsed patients with an ORR of 85%.110 These results lead us to consider ruxolitinib as 1 of the best second-line options. Similarly, tofacitinib, a JAK3 inhibitor, demonstrated partial efficacy in patients with LGLL with associated RA but yet with a significant toxicity.111
Future therapeutic directions
BNZ-1, a pegylated peptide selectively inhibiting the binding of IL-15 and other γc cytokines to their receptors has been recently prospectively evaluated in a phase 1/2 clinical trial after promising preclinical results.112,113 In 20 highly pretreated patients, 20% had an objective clinical response. Futures studies are warranted to confirm the efficacy of this agent as a monotherapy or in combination. A phase 1 study evaluating siltuximab is ongoing (ClinicalTrials.gov identifier: NTC05316116). Azacitidine, an hypomethylating agent, inhibits STAT3 activation by SHP1 restoration and DNMT1 downregulation.29 A phase 1 study with oral azacitidine is ongoing (ClinicalTrials.gov identifier: NCT05141682). Efficacy of decitabine was reported in a refractory patient.114 A phase 1/2 trial assessing the safety and efficacy of DR-01, a nonfucosylated antibody targeting the CD94 lectin receptor is now recruiting (ClinicalTrials.gov identifier: NCT05475925). Similarly, ABC008 is a first-in-class monoclonal antibody targeting the coinhibitory TCR killer cell lectin-like receptor G1. A phase 1/2 trial is ongoing in the United States (ClinicalTrials.gov identifier: NCT05532722). Specific STAT inhibitors have been developed. KT-333 is a STAT3 degrader currently evaluated in a phase 1a/1b study (ClinicalTrials.gov identifier: NCT05225584). Drugs targeting IL-6 and its receptor such as tocilizumab and siltuximab could also represent potential candidates. Considering the multiple pathways involved in the pathogenesis of LGLL and the clonal heterogeneity of the disease, it is conceivable that a multidrug approach combining a JAK-STAT inhibitor and a cytokine-targeting agent may be efficient based on a personalized therapy approach.
Innovative research strategies
There remain several research areas in need of additional study to enhance our understanding of LGLL pathogenesis and clinical management. Multiple recent studies have defined the somatic variants in coding genes as well as transcriptomic profiles for large cohorts of T- and NK-LGLL samples.32,38,53,61 Thus, the field must now fully characterize the functional consequences of recurrent molecular events, the impact of cooccurring somatic variants, and the contribution of mutant acquisition sequence to LGLL biology. Refined studies are needed to define the biochemical, molecular, and clinical consequences for the large list of STAT3 somatic variants that have been reported in LGLL.
Further single-cell transcriptomic analyses with associated definitions of TCR clonality have refined our understanding of LGL-specific gene expression programs and pathways.16,17 Knowledge gaps include a lack of single-cell transcriptomics for NK-LGLL, integrated single-cell chromatin accessibility and gene expression data, and detailed analyses of samples with varying mutational status in STAT3, TET2, CCL22, and other recurrently mutated genes. New insights will be gained by comparing LGL single-cell data sets with those acquired from T cells during acute or chronic viral infection as well as tumor infiltration. New spatial transcriptomic platforms also provide the opportunity to explore single-cell gene expression along with tissue localization and cell–cell communication of LGLs in the context of bone marrow or other tissue microenvironments relevant to disease symptoms and progression.
Beyond somatic variants and gene expression, the field lacks a detailed catalog of chromatic accessibility, DNA methylation, histone modifications, long-range enhancer interactions, transcription factor networks, large-scale duplications and deletions, and the impact of noncoding variants on these parameters. STAT3 variants are linked to comutation of chromatin and epigenetic modifier genes, thus a full picture of the genomic architecture of LGLL requires that each of these data types be acquired and integrated in samples of varying STAT3 mutational subtypes. Finally, each of these technologies should be applied to longitudinal samples that span the course of disease progression and treatment to characterize molecular events associated with clonal evolution, clinical features, and treatment response. The refined molecular characterization provided by these new data sets, especially single-cell and spatial transcriptomics studies, is likely to yield new LGL-specific therapeutic targets and strategies.
Conclusion
LGLL is a rare lymphoproliferative disorder characterized by large spectrum of clinicobiological manifestations and a peculiar association with autoimmune disorders. Although single-agent immunosuppressive drugs induce responses in the majority of patients, relapses are frequent and the disease remains uncurable. Advances in the understanding of the pathophysiology with the identification of recurrent mutations open the way to the development of new therapeutic strategies.
Authorship
Contribution: T.M., T.P.L. Jr, and T.L. wrote and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thierry Lamy, Service d’Hématologie Clinique, Centre Hospitalier Universitaire de Rennes, 2 rue Henri Le Guilloux, 35033 Rennes Cedex, France; email: thierry.lamy@chu-rennes.fr; and Thomas P. Loughran Jr, Emily Couric Clinical Cancer Center, 1240 Lee St, Charlottesville, VA 22903; email: tl7cs@uvahealth.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal