Key Points
KADs present with systemic inflammasome activation and distinct profiles between KAD subtypes.
Bystander monocyte activation in response to factors released by KSHV-latently infected cells may underlie inflammasome responses in KAD.
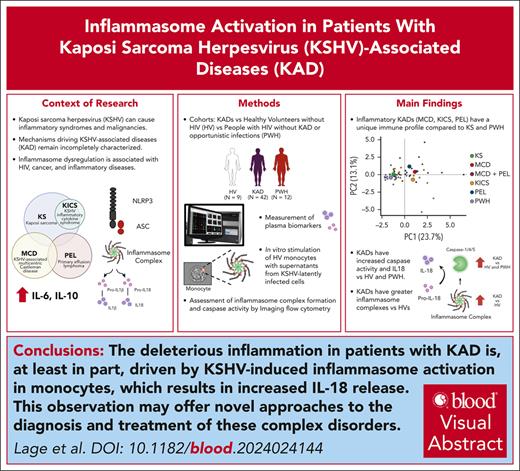
Visual Abstract
Kaposi sarcoma herpesvirus (KSHV)–associated diseases include Kaposi sarcoma (KS), primary effusion lymphoma (PEL), KSHV-associated multicentric Castleman disease (MCD), and KS inflammatory cytokine syndrome (KICS). PEL, MCD, and KICS are associated with elevated circulating inflammatory cytokines. However, activation of the inflammasome, which generates interleukin-1β (IL-1β) and IL-18 via active caspase-1/4/5, has not been evaluated in patients with KSHV-associated diseases (KADs). Herein we report that patients with HIV and ≥1 KAD present with higher plasma levels of IL-18 and increased caspase-1/4/5 activity in circulating monocytes compared with HIV-negative healthy volunteers (HVs) or people with HIV (PWH) without KAD. Within KAD subtypes, KICS and MCD shared enhanced caspase-1/4/5 activity and IL-18 production compared with HVs and PWH, whereas patients with PEL showed remarkably high levels of inflammasome complex formation (known as apoptosis–associated speck-like protein containing a caspase recruitment domain). Moreover, caspase-1/4/5 activity and IL-18 plasma levels correlated with KSHV viral load, indicating KSHV-driven inflammasome activation in KAD. Accordingly, factors released by cells latently infected with KSHV triggered inflammasome activation and cytokine production in bystander monocytes in vitro. Finally, both supervised and unsupervised analyses with inflammasome measurements and other inflammatory biomarkers demonstrate a unique inflammatory profile in patients with PEL, MCD, and KICS as compared with KS. Our data indicate that detrimental inflammation in patients with KAD is at least partially driven by KSHV-induced inflammasome activation in monocytes, thus offering novel approaches to diagnose and treat these complex disorders. These trials were registered at www.ClinicalTrials.gov as #NCT01419561, NCT00092222, NCT00006518, and NCT02147405.
Introduction
Kaposi sarcoma herpesvirus (KSHV) is the causative agent of 4 principal KSHV-associated diseases (KADs),1 which include malignancies such as Kaposi sarcoma (KS) and primary effusion lymphoma (PEL),2 a plasmablastic form of multicentric Castleman disease (MCD), and KS inflammatory cytokine syndrome (KICS).3 Collectively, these conditions, which can commonly develop among people with HIV (PWH) without active opportunistic infections or KAD, present either alone or concurrently within the same patient.1 Inflammation is a common characteristic in several KAD presentations and can confound the diagnostic process in patients with immunodeficiency who may also have concurrent infections. KAD, if not appropriately diagnosed and treated, contributes to significant morbidity and mortality.1,4
KS, the most common KSHV-associated cancer, presents as cutaneous lesions, or in severe cases, visceral disease, usually within the gastrointestinal tract, lymph nodes, or respiratory system. PEL, an aggressive B-cell non-Hodgkin lymphoma, usually develops as effusions of body cavities such as the pleural space or peritoneum, but can also manifest as extracavitary masses. MCD is a distinct B-cell lymphoproliferative disorder with a relapsing and remitting systemic course. KICS shares clinical features with PEL and MCD,5,6 but because these conditions are distinct pathologic entities with separate treatment paradigms, they must be excluded before diagnosing KICS.
Patients with KS alone may have limited systemic inflammation. KS can occur with concurrent MCD, PEL, and KICS; patients with these diagnoses often present with elevated serum cytokines, notably interleukin-6 (IL-6) and IL-10, as well as increased C-reactive protein (CRP) and KSHV viral load (KSHV-VL) in peripheral blood.7-9 Elevated circulating IL-6 and IL-10 have been associated with worse outcomes among patients with PEL.9 MCD flares can be treated with rituximab (an anti-CD20 monoclonal antibody), usually leading to remission of clinical symptoms along with decreases in inflammatory cytokines, KSHV-VL, and CRP.10,11 KSHV exists in latent and lytic phases and manipulates inflammatory and immune responses through mimicry of host cytokines and chemokines, which contributes to the pathogenesis of KAD.7,8
Another proinflammatory cytokine that is increased in patients with active MCD is IL-1β.7 IL-1β and its counterpart IL-18 play a pivotal role in the production of other inflammatory mediators such as IL-6, CRP, and interferon gamma (IFN-γ).12-17 Increased IL-18 is also associated with hyperferritinemia and cytopenia,18,19 both features found in certain KADs.5 IL-1β and IL-18 are produced as cytosolic precursors, and their activation and secretion are primarily controlled by inflammatory caspases, such as caspase-1, -4, and -5.20 These caspases are activated within inflammasomes, which are cytosolic aggregates that assemble to coordinate distinct immune responses to a variety of pathogenic or physiologic aberrations.21 The assembly of canonical inflammasomes requires cytosolic innate sensors and the adapter molecule ASC (apoptosis–associated speck-like protein containing a caspase activating and recruitment domain [CARD]), which form the inflammasome complex, also known as ASC-speck.22,23
In vitro studies with distinct cell types suggest that modulation of the IL-1 signaling by KSHV infection is a key event for life-long virus persistence and disease progression.24-27 Notably, KSHV has been shown to infect monocytes in vitro,28,29 with varying effects on inflammasome activation. Open reading frame 63, a tegument protein of KSHV, may be a homolog of NOD-like-receptor containing a Pyrin domain 1 (NLRP1) (nucleotide-binding and oligomerization, leucine-rich repeat (NLR) protein 3; NACHT, LRR, FIIND, CARD domain and PYD domains–containing protein 1), thus downmodulating its activity.30 In contrast, KSHV ORF K14 may have similar homology to cellular OX2, and was found to promote IL-1β secretion.31 These studies suggest that modulation of inflammasomes in monocytes may either help the virus to escape the host immune responses or promote inflammation and tumorigenesis. Given that the virus is detected primarily in B lymphocytes, endothelial cells, epithelial cells, and fibroblasts in infected individuals,32-34 the involvement of inflammasomes and the contribution of circulating monocytes in systemic inflammation observed in patients with KAD require further evaluation.
Herein, we evaluated inflammasome activation in circulating blood monocytes from patients with 1 or more KAD, and correlated these findings with other circulating inflammatory markers. Given that IL-1β can be alternatively produced in vivo by inflammasome-independent pathways,35 this study focuses on caspase-1/4/5 activity, ASC-speck formation, and plasma IL-18 measurements as readouts of inflammasome activation in patient samples. To investigate the mechanism for inflammasome activation in KAD pathogenesis, we further assessed inflammasome activity in monocytes in response to supernatants from KSHV-infected cells in vitro. Identifying unique pathways associated with inflammation in patients with KAD may improve our understanding of the pathogenesis of these diseases and inform novel diagnostic and therapeutic strategies.
Methods
Study participants
Patients with a diagnosis of HIV and ≥1concurrent KADs (KS and/or MCD, PEL, and KICS) who were evaluated at the HIV and AIDS Malignancy Branch within the Center for Cancer Research at the National Cancer Institute, National Institutes of Health36 between 2005 and 2021 were included. All patients with KAD were enrolled in natural history protocols for sample collection and management of KAD (NCT01419561, NCT00092222, NCT00006518, and NCT02147405), and their cryopreserved plasma and peripheral blood mononuclear cells (PBMCs) were obtained at the time of PEL or KICS diagnosis or presentation of KS before treatment and before MCD flare. The PWH subgroup was included from a separate protocol (NCT02147405) and was matched for CD4 T-cell count and HIV-VL with patients with KAD (Table 1). Deidentified blood samples from HIV-negative healthy volunteers (HVs) were obtained under the protocol 99-CC-0168. All listed protocols were approved by the National Institutes of Health Institutional Review Board. All participants provided written informed consent before any study procedures in accordance with the Declaration of Helsinki.
Clinical and laboratory characteristics of study participants
| Characteristic . | Patients with KAD (n = 42) . | PWH (n = 12) . | P value . |
|---|---|---|---|
| Age, y | 41 (32.3-54.0) | 35.5 (31.8-38.5) | .083 |
| Male, n (%) | 41 (97.6) | 11 (91.7) | .923 |
| Race, n (%) | |||
| Hispanic | 5 (11.9) | 5 (41.7) | .055 |
| African/African-American | 17 (40.5) | 5 (41.7) | 1.000 |
| White | 16 (38.1) | 1 (8.3) | .108 |
| Other | 4 (9.5) | 1 (8.3) | 1.000 |
| BMI (kg/m2) | 23.6 (21.1-26.5) | 23.8 (22.4-28.0) | .47 |
| Time from ART initiation to sample collection (wk) | 31.3 (5-263) | 48.6 (48-51) | .80 |
| CD4 T cells per μL | 208 (74-357) | 246 (227-304) | .440 |
| CD19 B cells per μL | 124 (50-242) | NA | NA |
| Hemoglobin (g/dL) | 10.0 (8.5-11.5) | 12.8 (11.4-13.8) | .001 |
| Platelets (×103/μL) | 219 (115-328) | 258 (218-287) | .448 |
| HIV-VL (log10 IU/mL) | 1.8 (0.7-3.0) | 0.9 (0.7-1.8) | .078 |
| Ferritin (ng/mL) | 696 (376-1520) | 29 (17-38) | <.001 |
| CRP (mg/L) | 33.7 (10.5-73.8) | 2.0 (1.3-2.3) | <.001 |
| KSHV-VL (log10 copies per million cells) | 3.6 (2.7-4.6) | NA | NA |
| KAD subgroups, n | NA | NA | |
| KS | 10 (23.8) | ||
| MCD ± KS | 11 (26.1) | ||
| MCD and PEL ± KS | 5 (11.9) | ||
| KICS + KS | 11 (26.1) | ||
| PEL | 5 (11.9) | ||
| Prior therapy | 18 (42.9) | NA | NA |
| Characteristic . | Patients with KAD (n = 42) . | PWH (n = 12) . | P value . |
|---|---|---|---|
| Age, y | 41 (32.3-54.0) | 35.5 (31.8-38.5) | .083 |
| Male, n (%) | 41 (97.6) | 11 (91.7) | .923 |
| Race, n (%) | |||
| Hispanic | 5 (11.9) | 5 (41.7) | .055 |
| African/African-American | 17 (40.5) | 5 (41.7) | 1.000 |
| White | 16 (38.1) | 1 (8.3) | .108 |
| Other | 4 (9.5) | 1 (8.3) | 1.000 |
| BMI (kg/m2) | 23.6 (21.1-26.5) | 23.8 (22.4-28.0) | .47 |
| Time from ART initiation to sample collection (wk) | 31.3 (5-263) | 48.6 (48-51) | .80 |
| CD4 T cells per μL | 208 (74-357) | 246 (227-304) | .440 |
| CD19 B cells per μL | 124 (50-242) | NA | NA |
| Hemoglobin (g/dL) | 10.0 (8.5-11.5) | 12.8 (11.4-13.8) | .001 |
| Platelets (×103/μL) | 219 (115-328) | 258 (218-287) | .448 |
| HIV-VL (log10 IU/mL) | 1.8 (0.7-3.0) | 0.9 (0.7-1.8) | .078 |
| Ferritin (ng/mL) | 696 (376-1520) | 29 (17-38) | <.001 |
| CRP (mg/L) | 33.7 (10.5-73.8) | 2.0 (1.3-2.3) | <.001 |
| KSHV-VL (log10 copies per million cells) | 3.6 (2.7-4.6) | NA | NA |
| KAD subgroups, n | NA | NA | |
| KS | 10 (23.8) | ||
| MCD ± KS | 11 (26.1) | ||
| MCD and PEL ± KS | 5 (11.9) | ||
| KICS + KS | 11 (26.1) | ||
| PEL | 5 (11.9) | ||
| Prior therapy | 18 (42.9) | NA | NA |
Data represent medians with interquartile range for continuous variables and frequencies for categorical variables. The Wilcoxon test was used to compare continuous variables, whereas the Pearson χ2 test was used to compare frequencies. KSHV-VL represents copies per million PBMCs. KAD subtype is reported as single disease or an overlapping presentation. Prior therapy refers to immune modulation therapies or chemotherapy in the year before sample collection: doxorubicin, pomalidomide, cyclophosphamide, corticosteroids, and rituximab.
ART, antiretroviral therapy; BMI, body mass index; NA, not applicable.
Plasma biomarkers, KSHV, and HIV-VL measurements
Inflammatory biomarkers were measured according to the manufacturer’s instructions. Kits used in this study are listed in supplemental Methods, available on the Blood website. The KSHV-VL per million PBMCs was measured in PBMC-extracted DNA by polymerase chain reaction using K6 primers as previously described.37 HIV-VL was measured using a TaqMan HIV-1 polymerase chain reaction assay.
Inflammasome assessment by imaging flow cytometry
Cryopreserved PBMCs were thawed and resuspended in complete medium (RPMI 1640; Corning, Corning, NY) supplemented with 10% heat-inactivated human AB serum (GeminiBio, West Sacramento, CA) and 0.05% Benzonase (MilliporeSigma, Burlington, MA). Cells rested for 1 hour at 37°C and 5% CO2 and were plated at 106 cells per well in round-bottom 96-well plates (Corning Costar, MilliporeSigma). For active caspase-1/4/5 detection, cells were incubated with the fluorochrome inhibitor of caspases-1/4/5 (FLICA; ImmunoChemistry Technologies, Bloomington, MN) according to the manufacturer’s instructions. Inflammasome assembly was evaluated by detection of caspase-1/4/5-positive–ASC-speck formation by imaging flow cytometry (denoted as FLICA+Speck+ cells), following the protocol previously described.38 The antibody panel used for monocyte phenotyping and ASC-speck identification is listed in supplemental Table 1. Image acquisition and analysis are detailed in the supplemental Methods.
In vitro exposure of human monocytes to supernatants from KSHV-infected cells
Human embryonic kidney (HEK) 293T cells infected with wild-type KSHV (Bac16) were a kind gift from Ting-Ting Wu.39,40 Uninfected and Bac16-infected HEK-293T cells were plated in 10% Dulbecco modified Eagle medium with penicillin-streptomycin (with puromycin for selection) at 20 000 cells per mL and grown for 5 days to confluency. After this period, supernatants were harvested and then stored at –70°C until further use. HV-derived circulating monocytes were isolated from fresh PBMCs using the EasySep Human Monocyte Enrichment Kit without CD16 Depletion (Stemcell Technologies) or by elutriation and plated at 1 × 106 cells per well. Cells were then exposed to complete media containing 50% of supernatant from either uninfected or KSHV-infected HEK-293T cells at 37°C overnight, in the presence or absence of the NLRP3 inflammasome inhibitor MCC950 (5 μM). After that, supernatants were harvested for quantification of IL-1β and IL-18 by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) following the manufacturer’s directions, and monocytes were processed for imaging flow cytometry, as described above.
Statistical analyses
Statistical analyses were performed in GraphPad Prism 9.3.1 software. Data are presented as median with interquartile ranges, and comparisons across groups were made with the Kruskal-Wallis test. Statistically significant (P < .05) differences were further evaluated with the Mann-Whitney test with Dunn correction for multiple comparisons. Spearman correlation analyses were performed using the corrplot package to create a correlation matrix. Principal component analysis and heat map analysis with unsupervised clustering were performed in R (version 4.2.1) using the FactoMineR, factoextra, and pheatmap packages.
Results
Participant characteristics
Overall, 42 cisgender men with KAD and 12 PWH were evaluated in this study (Table 1). Among patients with KAD, 23 (55%) had a single KAD (10 with KS, 9 with MCD, and 4 with PEL alone). Nineteen patients (45%) had >1 concurrent KAD at the time of study: 11 individuals with concurrent KS and KICS, 1 patient with KS and PEL, and 5 patients with a concurrent diagnosis of KS, PEL, and MCD. Clinical characteristics of the patients with KAD stratified by disease subtype are summarized in Table 2.
Characteristics of the cohort with KSHV-associated diseases stratified according to clinical classification into distinct KAD categories
| Characteristic . | KS . | MCD . | MCD + PEL . | KICS + KS . | PEL . |
|---|---|---|---|---|---|
| (n = 10) . | (n = 11) . | (n = 5) . | (n = 11) . | (n = 5) . | |
| Age, y | 29 (26.3-45.5) | 43 (40.5-44.8) | 55 (55-55) | 37 (30.5-49.5) | 39 (38-52) |
| Male, n | 10 | 10 | 5 | 10 | 5 |
| Race, n | |||||
| Hispanic | 0 | 1 | 0 | 3 | 1 |
| African/African-American | 5 | 4 | 2 | 5 | 1 |
| White | 4 | 5 | 3 | 2 | 2 |
| Other | 1 | 1 | 0 | 1 | 1 |
| BMI (kg/m2) | 22.6 (20.9-27.2) | 24.3 (22.6-27.1) | 23.2 (22.8-23.3) | 23.8 (20.5-27.4) | 24.6 (24.5-26.5) |
| Time from ART initiation to sample collection (wk) | 17.9 (3.8-32.6) | 125 (32-282) | 174 (59-260) | 6.9 (2.2-114) | 265 (22-650) |
| ECOG score | 0 (0-1) | 1 (1-3) | 2 (1-3) | 2 (1-2) | 2 (1-3) |
| KS stage, n | |||||
| Stage T0 | 2 | 1 | 0 | 2 | 0 |
| Stage T1 | 8 | 1 | 5 | 9 | 2 |
| CD4 T cells per μL | 208 (114-305) | 263 (190-531) | 318 (250-363) | 48 (33-111) | 401 (170-594) |
| CD19 B cells per μL | 113 (67-195) | 233 (104-377) | 174 (141-259) | 32 (11-62) | 197 (180-231) |
| Hemoglobin (g/dL) | 11.2 (9.9-14.0) | 10.3 (9.0-11.5) | 8.0 (7.9-9.1) | 8.6 (7.9-9.8) | 11.2 (9.6-12.2) |
| Platelets (×103/μL/μL) | 246 (197-284) | 171 (59-265) | 276 (253-347) | 212 (118-292) | 361 (176-371) |
| HIV-VL (log10 IU/mL) | 1.1 (0.7-2.1) | 1.0 (1.0-1.8) | 2.7 (1.7-2.8) | 2.9 (2.2-4.6) | 0.7 (0.7-3.0) |
| Ferritin (ng/mL) | 312 (117-554) | 833 (582-2920) | 1209 (366-2167) | 1219 (590-1678) | 565 (449-1143) |
| CRP (mg/L) | 9.15 (4.8-13.0) | 33.9 (17.0-109.4) | 51.0 (48.8-65.7) | 52.4 (14.6-83.2) | 61.4 (33.5-62.6) |
| KSHV-VL (log10 copies per million cells) | 0.0 (0.0-1.9) | 4.0 (3.4-5.0) | 3.6 (3.2-4.2) | 3.8 (3.4-4.6) | 4.4 (4.2-4.6) |
| Concurrent KS, n | N/A | 2 | 5 | 11 | 1 |
| Prior therapy, n | 4 | 6 | 3 | 4 | 1 |
| Chemotherapy (<1 mo) | 3 | 1 | 0 | 2 | 0 |
| Chemotherapy (<6 mo) | 1 | 1 | 3 | 2 | 1 |
| Corticosteroids (<6 mo) | 0 | 3 | 0 | 0 | 0 |
| Rituximab (<1 y) | 0 | 2 | 0 | 1 | 0 |
| Characteristic . | KS . | MCD . | MCD + PEL . | KICS + KS . | PEL . |
|---|---|---|---|---|---|
| (n = 10) . | (n = 11) . | (n = 5) . | (n = 11) . | (n = 5) . | |
| Age, y | 29 (26.3-45.5) | 43 (40.5-44.8) | 55 (55-55) | 37 (30.5-49.5) | 39 (38-52) |
| Male, n | 10 | 10 | 5 | 10 | 5 |
| Race, n | |||||
| Hispanic | 0 | 1 | 0 | 3 | 1 |
| African/African-American | 5 | 4 | 2 | 5 | 1 |
| White | 4 | 5 | 3 | 2 | 2 |
| Other | 1 | 1 | 0 | 1 | 1 |
| BMI (kg/m2) | 22.6 (20.9-27.2) | 24.3 (22.6-27.1) | 23.2 (22.8-23.3) | 23.8 (20.5-27.4) | 24.6 (24.5-26.5) |
| Time from ART initiation to sample collection (wk) | 17.9 (3.8-32.6) | 125 (32-282) | 174 (59-260) | 6.9 (2.2-114) | 265 (22-650) |
| ECOG score | 0 (0-1) | 1 (1-3) | 2 (1-3) | 2 (1-2) | 2 (1-3) |
| KS stage, n | |||||
| Stage T0 | 2 | 1 | 0 | 2 | 0 |
| Stage T1 | 8 | 1 | 5 | 9 | 2 |
| CD4 T cells per μL | 208 (114-305) | 263 (190-531) | 318 (250-363) | 48 (33-111) | 401 (170-594) |
| CD19 B cells per μL | 113 (67-195) | 233 (104-377) | 174 (141-259) | 32 (11-62) | 197 (180-231) |
| Hemoglobin (g/dL) | 11.2 (9.9-14.0) | 10.3 (9.0-11.5) | 8.0 (7.9-9.1) | 8.6 (7.9-9.8) | 11.2 (9.6-12.2) |
| Platelets (×103/μL/μL) | 246 (197-284) | 171 (59-265) | 276 (253-347) | 212 (118-292) | 361 (176-371) |
| HIV-VL (log10 IU/mL) | 1.1 (0.7-2.1) | 1.0 (1.0-1.8) | 2.7 (1.7-2.8) | 2.9 (2.2-4.6) | 0.7 (0.7-3.0) |
| Ferritin (ng/mL) | 312 (117-554) | 833 (582-2920) | 1209 (366-2167) | 1219 (590-1678) | 565 (449-1143) |
| CRP (mg/L) | 9.15 (4.8-13.0) | 33.9 (17.0-109.4) | 51.0 (48.8-65.7) | 52.4 (14.6-83.2) | 61.4 (33.5-62.6) |
| KSHV-VL (log10 copies per million cells) | 0.0 (0.0-1.9) | 4.0 (3.4-5.0) | 3.6 (3.2-4.2) | 3.8 (3.4-4.6) | 4.4 (4.2-4.6) |
| Concurrent KS, n | N/A | 2 | 5 | 11 | 1 |
| Prior therapy, n | 4 | 6 | 3 | 4 | 1 |
| Chemotherapy (<1 mo) | 3 | 1 | 0 | 2 | 0 |
| Chemotherapy (<6 mo) | 1 | 1 | 3 | 2 | 1 |
| Corticosteroids (<6 mo) | 0 | 3 | 0 | 0 | 0 |
| Rituximab (<1 y) | 0 | 2 | 0 | 1 | 0 |
Data represent medians with interquartile range for continuous variables and frequencies for categorical variables. KSHV-VL represents copies per million PBMCs. Prior therapy refers to immune modulation therapies or chemotherapy in the year before sample collection: doxorubicin, pomalidomide, cyclophosphamide, corticosteroids, and rituximab.
ECOG, Eastern Cooperative Oncology Group.
Patients with KAD had a lower hemoglobin (10.0 vs 12.8 g/dL; P = .001) and higher CRP (33.7 vs 2.0 mg/L; P < .001) and ferritin levels (696 vs 29 ng/mL; P < .001) compared with PWH (Table 1). Excluding patients with KS alone, the remaining patients with KICS, MCD, and/or PEL had evidence of systemic inflammation at the time of sample collection. Cytopenia and inflammatory markers were significantly increased in those with KAD compared with PWH (supplemental Figure 1).
Systemic inflammasome activation in individuals with KAD compared with PWH and HV
Given that both KSHV and HIV-1 have been shown to activate inflammasome sensors,24,31,41 we investigated whether inflammasome activation was distinct in 3 groups of participants: KAD (all had concurrent HIV), PWH (without active opportunistic infections or KAD), and HV (all HIV-negative).
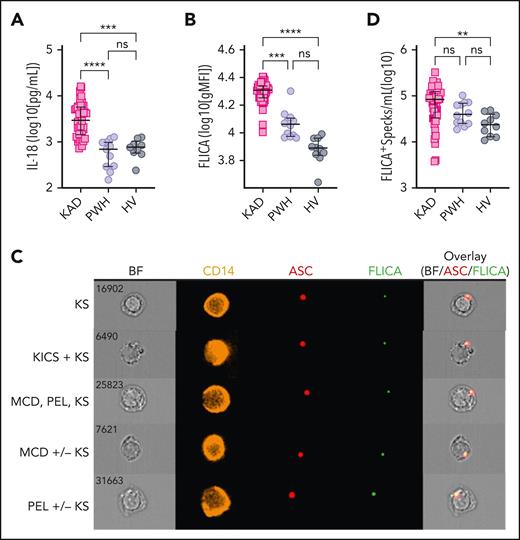
Participants with KAD had significantly higher IL-18 plasma levels compared with PWH and HV (Figure 1A). Accordingly, circulating monocytes from patients with KAD presented increased caspase-1/4/5 activity compared with monocytes from PWH and HV (Figure 1B), consistent with KSHV-related inflammasome activation in patients with HIV and concurrent KAD. Furthermore, we observed higher levels of monocytes presenting cytosolic ASC-speck formation, associated with active caspase-1/4/5, identified as FLICA+Speck+ cells (Figure 1C, Merged panel), in patients with KAD compared with HV (Figure 1D). Despite the increased proportion of monocytes presenting FLICA+Specks observed in patients with KAD compared with PWH, these values did not reach statistical significance (Figure 1D).
Exacerbated inflammasome activation is found in participants with KAD when compared with PWH and HVs. (A) Plasma levels of IL-18 were compared among participants with KAD (n = 42), PWH (n = 12), and HVs (n = 9). Lines represent median values and interquartile ranges. PBMCs from the same patients and distinct HVs (n = 10) were incubated with the FLICA, stained for monocyte identification and intracellular ASC, and acquired by imaging flow cytometry. (B) gMFI of FLICA within total circulating blood monocytes was assessed. Data are presented as median with interquartile range. (C) Representative images showing respectively CD14, ASC, and FLICA fluorescence followed by a composite image containing brightfield (BF), and the fluorescence of ASC and FLICA merged, were randomly selected from representative samples of the distinct KAD conditions. In these CD14+ cells, FLICA and ASC fluorescence are superimposed. (D) The number of monocytes showing spontaneous FLICA+ ASC-speck formation was quantified after application of Modulation_Morphology (M11,Ch11)_11-ASC feature, followed by Bright Detail Similarity R3_MC_11-ASC_2-FLICA, inside the monocyte gate, by using IDEAS software. Lines represent median values and interquartile ranges. Comparisons were made using the Kruskal-Wallis test followed by the Dunn multiple test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Exacerbated inflammasome activation is found in participants with KAD when compared with PWH and HVs. (A) Plasma levels of IL-18 were compared among participants with KAD (n = 42), PWH (n = 12), and HVs (n = 9). Lines represent median values and interquartile ranges. PBMCs from the same patients and distinct HVs (n = 10) were incubated with the FLICA, stained for monocyte identification and intracellular ASC, and acquired by imaging flow cytometry. (B) gMFI of FLICA within total circulating blood monocytes was assessed. Data are presented as median with interquartile range. (C) Representative images showing respectively CD14, ASC, and FLICA fluorescence followed by a composite image containing brightfield (BF), and the fluorescence of ASC and FLICA merged, were randomly selected from representative samples of the distinct KAD conditions. In these CD14+ cells, FLICA and ASC fluorescence are superimposed. (D) The number of monocytes showing spontaneous FLICA+ ASC-speck formation was quantified after application of Modulation_Morphology (M11,Ch11)_11-ASC feature, followed by Bright Detail Similarity R3_MC_11-ASC_2-FLICA, inside the monocyte gate, by using IDEAS software. Lines represent median values and interquartile ranges. Comparisons were made using the Kruskal-Wallis test followed by the Dunn multiple test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
We then evaluated inflammasome activation within the distinct circulating monocyte subsets, which among healthy controls are typically 70% to 90% classical (CD14highCD16–) monocytes and ∼7% to 15% nonclassical or patrolling (CD14lowCD16+) cells plus an intermediate monocyte subset (CD14+CD16+)42,43 (supplemental Figure 2A). These monocyte populations have distinct functional roles, and a change in the subset distribution and activation has been shown in a range of inflammatory disorders.44-47 We observed that patients with KAD display increased proportion of intermediate monocytes compared with PWH and HV (supplemental Figure 2B). Although higher caspase-1/4/5 activity was broadly detected in all subsets in patients with KAD compared with PWH and HV, increased ASC-speck formation was observed solely within intermediate monocytes when compared with PWH (supplemental Figure 2C-E), possibly due to the enrichment of this subset found in KAD. Finally, PWH and HV presented with similar levels of IL-18 (Figure 1A), caspase-1/4/5 activity, and monocytes with ASC-specks regardless of the subset of monocytes tested (supplemental Figure 2).
Therefore, our data demonstrate that inflammation among patients with KAD is distinct from that observed with HIV alone, with features of systemic inflammasome activation characterized by increased monocyte caspase-1/4/5 activity and IL-18 production and increased proportion of intermediate monocytes with inflammasome complex formation.
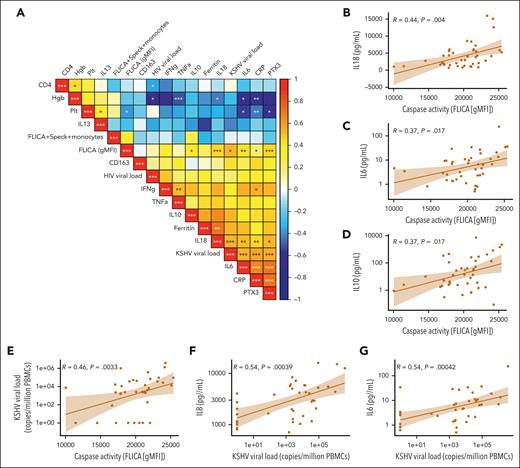
Inflammasome activation correlates with KSHV-VL and plasma inflammatory markers in patients with KAD
To further characterize inflammasome activation in patients with KAD, we performed Spearman correlation analyses between inflammasome-related variables (plasma IL-18 and caspase-1/4/5 activity on monocytes), HIV, and KSHV-VL, as well as other plasma inflammatory markers. There were positive correlations between monocyte-derived caspase-1/4/5 activity (FLICA geometric mean fluorescence intensity [gMFI]) and several inflammatory markers (Figure 2A). Statistically significant correlations were identified with the downstream inflammasome mediator IL-18 and with IL-6 and IL-10, with the latter 2 cytokines consistently associated with KAD pathogenesis (Figure 2B-D). Furthermore, caspase-1/4/5 activity in circulating monocytes and the levels of IL-18 and IL-6 demonstrate a significant positive association with KSHV-VL in patients with KAD (Figure 2E-G), whereas HIV-VL (mostly suppressed) was not significantly associated with the inflammatory profiles (Figure 2A). Significant associations of KSHV-VL with caspase-1/4/5 activity and IL-6 were maintained even after excluding patient time points with an undetectable KSHV-VL (supplemental Figure 3). Overall, our data suggest that inflammasome activation observed in patients with KAD is positively associated with the increased KSHV-VL and systemic inflammatory cytokine levels.
Correlation matrix of inflammasome activation flow cytometry markers, soluble biomarkers, and clinical laboratory tests within the KAD cohort. (A) Multiparameter Spearman correlation matrix of inflammasome activation markers, soluble biomarkers, and clinical laboratory tests from all participants with KAD. KSHV-VL represents copies per million PBMCs. (B-G) Individual correlation plots highlighting significant associations by Spearman r between caspase activity, KSHV-VL, and key KAD-related inflammatory cytokines. Hgb, hemoglobin; Plt, platelets; PTX3, pentraxin-3.
Correlation matrix of inflammasome activation flow cytometry markers, soluble biomarkers, and clinical laboratory tests within the KAD cohort. (A) Multiparameter Spearman correlation matrix of inflammasome activation markers, soluble biomarkers, and clinical laboratory tests from all participants with KAD. KSHV-VL represents copies per million PBMCs. (B-G) Individual correlation plots highlighting significant associations by Spearman r between caspase activity, KSHV-VL, and key KAD-related inflammatory cytokines. Hgb, hemoglobin; Plt, platelets; PTX3, pentraxin-3.
Patients with PEL, MCD, and KICS are distinguished from PWH and HV by enhanced inflammasome activation and show a unique inflammatory profile compared with patients with KS alone
We next sought to evaluate inflammasome activation by KAD subtype compared with PWH and HV. We observed that patients with KICS + KS, MCD (with or without KS or PEL), or PEL (with or without concurrent KS) were distinct from HVs with regard to their plasma levels of IL-18 and caspase-1/4/5 activity on circulating monocytes (Figure 3A-B, respectively). The KICS + KS and MCD groups also differed from the PWH group in both markers, whereas the PEL group only had significantly higher levels of IL-18 compared with the PWH group (Figure 3A-B). In contrast, there was no difference between patients with KS alone and PWH or HVs when comparing IL-18 and caspase-1/4/5 activity (Figure 3A-B). These differences in caspase-1/4/5 activation levels were found in all monocyte subsets, reaching statistical significance for patients with MCD and those with KICS when compared with PWH, within the CD14highCD16– and CD14highCD16+ subsets (supplemental Figure 4A-C). Furthermore, only patients with PEL with or without KS demonstrated significantly higher numbers of monocytes with ASC-speck compared with HVs (Figure 3C), which was restricted to the classical monocyte subset (supplemental Figure 4D-F). Interestingly, those with MCD showed higher numbers of monocytes with ASC-speck compared with PWH within the intermediate group of monocytes (supplemental Figure 4E), which does not seem to be due to a proportional expansion of this subset (supplemental Figure 4G-H).
Inflammasome activation across different groups of patients presenting with KSHV-associated diseases stratified according to their clinical classification. (A) Plasma levels of IL-18 were compared among participants with KAD (n = 42, comprising patients with KS alone [10], MCD [11], MCD + PEL [5], KICS + KS [11], and PEL ± KS [5]; ∗means with or without KS), PWH (n = 12), and HV (n = 10). (B) PBMCs from the previously mentioned groups were incubated with the FLICA, and the gMFI of FLICA within total circulating blood monocytes was compared among groups. (C) Alternatively, PBMCs were also stained for monocyte identification and intracellular ASC and acquired by imaging flow cytometry. Data are presented as median with interquartile range. Data were analyzed using the Kruskal-Wallis test followed by Dunn multiple comparisons. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Inflammasome activation across different groups of patients presenting with KSHV-associated diseases stratified according to their clinical classification. (A) Plasma levels of IL-18 were compared among participants with KAD (n = 42, comprising patients with KS alone [10], MCD [11], MCD + PEL [5], KICS + KS [11], and PEL ± KS [5]; ∗means with or without KS), PWH (n = 12), and HV (n = 10). (B) PBMCs from the previously mentioned groups were incubated with the FLICA, and the gMFI of FLICA within total circulating blood monocytes was compared among groups. (C) Alternatively, PBMCs were also stained for monocyte identification and intracellular ASC and acquired by imaging flow cytometry. Data are presented as median with interquartile range. Data were analyzed using the Kruskal-Wallis test followed by Dunn multiple comparisons. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Inflammasome measurements in total circulating monocytes (Figure 3) or within each subset (supplemental Figure 4) as single variables could generally not distinguish among the different KADs when patients were grouped based on their clinical category at the time of sampling. IL-1β, IL-6, IL-10, IL-13, IL18BP, Tumor necrosis factor-α (TNF-α), IFN-γ, C-X-C motif chemokine ligand 9 (CXCL9), and CD163 also showed minimal differences across KADs (supplemental Figure 5). Notably, plasma IL-1β levels were mostly below the limit of detection in this cohort, which may be an assay limitation.48 IL-10 was significantly increased in all groups compared with patients with KS alone (P = .0049), and there was a trend toward higher ferritin levels in patients with MCD with or without PEL when compared with those with KS alone (P = .02) (supplemental Figure 5).
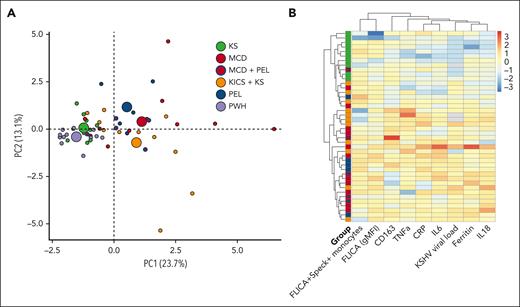
A principal component analysis incorporating inflammasome measurements (FLICA+Speck+ monocytes and FLICA [gMFI]) and a broad set of inflammatory biomarkers showed an overlap among all KAD groups (Figure 4A). However, patients with KS alone clustered near the PWH subgroup. Those with MCD, KICS, or PEL extended across a broad area, but clearly separated from PWH or KS alone, indicating a unique inflammatory pattern. The greatest separation was observed among those with MCD or KICS, which is consistent with the pathologic inflammatory state clinically observed in these patients.
Multidimensional analysis and heat map with unsupervised clustering of caspase-1/4/5 activity, speck formation, and inflammasome-associated biomarkers. (A) Principal component analysis incorporating inflammasome components (caspase-1/4/5 activity, FLICA+ ASC-speck formation) and 11 inflammatory biomarkers (ferritin, CRP, IFN-γ, IL-6, IL-10, IL-13, IL-18, IL18BP, CXCL9, CD163, and TNF-α) demonstrates significant overlap in clusters when defined by clinical syndrome. Individuals are represented by small colored circles for each group, whereas the overall group is represented by large colored circles. Participants with MCD and concomitant PEL are depicted as red circles with a blue outline. (B) Heat map with unsupervised clustering of inflammasome components, KSHV-VL (copies per million PBMCs), and monocyte-associated biomarkers identified that those with MCD primarily cluster together, including those with concomitant PEL (depicted with blue circle inside red box). Those with KS alone form a separate distinct cluster. Patients with KICS are distributed throughout. Individuals with MCD, PEL, and KICS may or may not have had concomitant KS. Analysis performed in R (version 4.1.2) using the FactoMineR, factoextra, and heatmap packages.
Multidimensional analysis and heat map with unsupervised clustering of caspase-1/4/5 activity, speck formation, and inflammasome-associated biomarkers. (A) Principal component analysis incorporating inflammasome components (caspase-1/4/5 activity, FLICA+ ASC-speck formation) and 11 inflammatory biomarkers (ferritin, CRP, IFN-γ, IL-6, IL-10, IL-13, IL-18, IL18BP, CXCL9, CD163, and TNF-α) demonstrates significant overlap in clusters when defined by clinical syndrome. Individuals are represented by small colored circles for each group, whereas the overall group is represented by large colored circles. Participants with MCD and concomitant PEL are depicted as red circles with a blue outline. (B) Heat map with unsupervised clustering of inflammasome components, KSHV-VL (copies per million PBMCs), and monocyte-associated biomarkers identified that those with MCD primarily cluster together, including those with concomitant PEL (depicted with blue circle inside red box). Those with KS alone form a separate distinct cluster. Patients with KICS are distributed throughout. Individuals with MCD, PEL, and KICS may or may not have had concomitant KS. Analysis performed in R (version 4.1.2) using the FactoMineR, factoextra, and heatmap packages.
Finally, using an unsupervised clustering algorithm including inflammasome variables (FLICA+Speck+ monocytes and FLICA [gMFI]), inflammatory markers (CRP, IL-6, ferritin, IL-18, soluble CD163 [sCD163], TNF-α), and KSHV-VL, we identified that patients with MCD with or without KS primarily clustered together along with several patients with KICS (Figure 4B), consistent with a unique inflammatory profile. Individuals with MCD and concurrent PEL also localized more closely to those with MCD (Figure 4B). Most of these patients demonstrated relative increases in myeloid activation markers including IL-6, TNF-α, sCD163, ferritin, and IL-18. Patients with KS alone were found within a separate cluster driven by FLICA+Speck+ monocytes and lower levels of ferritin and IL-18. Overall, patients with KAD had clear evidence of inflammasome activation. However, distinct patterns within the spectrum of KAD exist, which may help delineate these entities for both diagnostic and therapeutic purposes.
Supernatants from cells latently infected with KSHV trigger inflammasome activation in bystander monocytes
Although KSHV has been shown to infect monocytes in vitro, few PBMCs are infected with KSHV in vivo.32 This indicates that inflammasome activation in circulating monocytes from patients with KAD may reflect their responses to systemic inflammation rather than direct infection. Monocytes can react to systemic inflammation by activating the NLRP3 inflammasome in response to several damage-associated molecular patterns released by infected/injured cells, thus contributing to the development of several inflammatory diseases and cancers.49
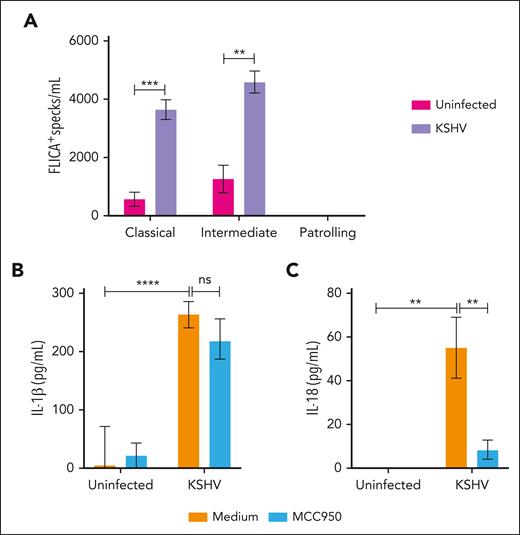
Therefore, we hypothesized that inflammasome activation of monocytes in KAD could indicate bystander activation from factors released by KSHV-infected cells. To test this hypothesis, we evaluated inflammasome activation in HV-derived monocytes following in vitro exposure to supernatants from uninfected or KSHV latently infected HEK293T cells. We observed that overnight exposure to supernatants from infected (KSHV) but not from uninfected cultures (Uninfected) induced inflammasome complex formation in monocytes from HV within the classical and intermediate subsets (Figure 5A).
In vitro stimulation of monocytes with supernatants from KSHV-infected cells. HV-isolated monocytes were plated (1 × 106 cells per well) and incubated overnight with complete media containing 50% of supernatant from either uninfected or KSHV-infected HEK293T cells, in the presence or absence of the NLRP3 inflammasome inhibitor MCC950 (5 μM). After stimulation, culture supernatants were harvested for quantification of IL-1β and IL-18 by enzyme-linked immunosorbent assay (ELISA), and monocytes were stained for detection of inflammasome complex formation by imaging flow cytometry. (A) Numbers of monocytes showing FLICA+ ASC-speck formation per mL were quantified inside the monocyte gate. Lines represent mean values with standard error of the mean (SEM). Data were analyzed using the Welch t test to compare the uninfected with the KSHV-infected group within each monocyte subset. ∗∗P < .01; ∗∗∗P < .001. (B) The levels of (B) IL-1β and (C) IL-18 produced by stimulated monocytes were quantified by ELISA and compared across the different groups. Lines represent mean values with SEM. Data were analyzed using the Kruskal-Wallis test. ∗∗P < .01; ∗∗∗∗P < .0001. Data are presented as a pool of 2 different batches of HEK293T culture–derived supernatants.
In vitro stimulation of monocytes with supernatants from KSHV-infected cells. HV-isolated monocytes were plated (1 × 106 cells per well) and incubated overnight with complete media containing 50% of supernatant from either uninfected or KSHV-infected HEK293T cells, in the presence or absence of the NLRP3 inflammasome inhibitor MCC950 (5 μM). After stimulation, culture supernatants were harvested for quantification of IL-1β and IL-18 by enzyme-linked immunosorbent assay (ELISA), and monocytes were stained for detection of inflammasome complex formation by imaging flow cytometry. (A) Numbers of monocytes showing FLICA+ ASC-speck formation per mL were quantified inside the monocyte gate. Lines represent mean values with standard error of the mean (SEM). Data were analyzed using the Welch t test to compare the uninfected with the KSHV-infected group within each monocyte subset. ∗∗P < .01; ∗∗∗P < .001. (B) The levels of (B) IL-1β and (C) IL-18 produced by stimulated monocytes were quantified by ELISA and compared across the different groups. Lines represent mean values with SEM. Data were analyzed using the Kruskal-Wallis test. ∗∗P < .01; ∗∗∗∗P < .0001. Data are presented as a pool of 2 different batches of HEK293T culture–derived supernatants.
Accordingly, we found that KSHV-derived supernatants induced both IL-1β and IL-18 production by monocytes when compared with supernatants from uninfected cells (Figure 5B-C). Culture levels of IL-18 , but not of IL-1β, were significantly reduced when monocytes were also treated with MCC950, a specific NLRP3 inhibitor (Figure 5B-C). Supernatants from both uninfected and infected cells were previously tested and had very low levels of IL-1β and IL-18, without significant differences in cytokine levels between these 2 types of supernatants (IL-1β, P = .8805; IL-18, P = .1174; supplemental Figure 6), indicating that our results were not affected by cytokines from the HEK293T cell culture media. Taken together, our data demonstrate that factors released by cells latently infected with KSHV can induce inflammasome complex formation and cytokine production in bystander monocytes through a mechanism partially dependent on NLRP3.
Discussion
Dysregulated inflammasome activation leads to a wide range of acute and chronic inflammatory diseases. Although serum inflammatory signatures are elevated in active PEL, MCD, and KICS, activation of the inflammasome has not been addressed in patients with KAD to date. Herein, we demonstrate systemic inflammasome activation in a group of patients with HIV and heterogeneous KAD, including individual and concurrent malignancies, an inflammatory cytokine syndrome, and a lymphoproliferative disorder. Overall, inflammasome activation in those patients was characterized by increased activity of caspase-1/4/5 in circulating blood monocytes, the presence of ASC-specks, and elevated plasma levels of IL-18 that were distinct from HV. The association of these measurements with KSHV-VL and other innate immune markers (CRP, IL-6, ferritin, sCD163, and TNF-α) also identified a distinct inflammatory profile for a subset of patients with PEL, MCD (with or without PEL), and KICS compared with those with KS alone.
Patients with KS alone demonstrated the weakest inflammasome signal among all KADs, with markedly lower levels of IL-18. Robust IL-1β and IL-18 secretion requires “priming” (signal-1), leading to transcriptional upregulation of their inactive forms via TLR/cytokine/chemokine pathways20 that can be activated in response to KSHV and a number of inflammatory mediators. Given that those factors are found at lower levels in patients with KS alone than in patients with other KADs, our data support that reduced IL-18 production in these patients reflects their limited systemic inflammation. Alternatively, it is possible that prior treatment received by this group of patients (ie, immune modulators and chemotherapy) may have downmodulated IL-18 production at a transcriptional level while preserving monocytes with preformed inflammasome complexes.
Altogether, patients with KAD (and HIV infection) demonstrated significantly higher plasma levels of IL-18 and caspase-1/4/5 activity in circulating monocytes compared with PWH. This is consistent with KSHV-driven inflammasome activation in KAD, and is corroborated by significant correlations between IL-18 and caspase activity with KSHV-VL, but not with HIV-VL. KSHV-VL and caspase-1/4/5 activity also correlated with IL-6 and IL-10, 2 cytokines known to be involved in KAD–associated inflammation and disease progression.7,9,11 Conversely, patients within the KAD and PWH groups presented overall similar levels of ASC-specks, and in those with KAD, there was a weak correlation between KSHV-VL and the proportion of monocytes with ASC-speck. These findings might suggest the involvement of other cell types, such as tissue-resident macrophages and epithelial cells, as alternative sources of ASC-specks in KAD pathogenesis. Otherwise, the noncanonical inflammasome pathway, which activates inflammatory caspase-4 to induce pyroptosis and IL-18 secretion without ASC-complex formation,50,51 may also contribute to this phenomenon. Given that KSHV is not known to activate noncanonical caspases, the inflammasome trigger in this context remains to be elucidated.
Alternatively, these data may also suggest lingering, yet subtle, HIV-related canonical inflammasome responses in KAD. In fact, inflammasomes were implicated in persistent low-grade inflammation in virally suppressed PWH,52 which may also partially contribute to KAD pathogenesis. Given that this study did not include HIV-negative individuals with KAD, the magnitude of inflammasome activation that is driven by chronic HIV infection in KAD pathophysiology requires further investigation. It is noteworthy, however, that patients with KAD presented an increased proportion of intermediate monocytes showing ASC-speck compared with PWH, likely due to an enrichment of those cells in their monocyte compartment. Intermediate monocytes are known to secrete proinflammatory cytokines,53 to present antigens,36 and to migrate into inflamed tissues,54 and are found expanded in circulation in a variety of conditions.46,55-62 Cros et al63 showed that isolated intermediate monocytes were the greatest producers of TNF-α, IL-1β, and IL-6 among all 3 subsets in response to lipopolysaccharides (LPS) in vitro. Therefore, future studies should consider the contribution of this specific monocyte subset to KAD-associated inflammation.
Our in vitro data have shown that monocytes are able to engage the NLRP3 inflammasome and form ASC-specks in response to factors released from cells latently infected with KSHV to produce IL-18, in vitro, further emphasizing a role for KSHV in KAD-associated inflammation. IL-1β secretion, however, seems to be downstream of a different inflammasome sensor, thus highlighting that distinct inflammasome pathways may play a role in KAD. Given that cells latently infected with KSHV do not release a significant number of viral particles, it is reasonable to suggest that other factors are involved in the activation of uninfected monocytes in this context. In line with this, it has been shown that mitochondrial DNA on the surface of extracellular vesicles released by KSHV-infected cells can induce IFN-stimulated genes in bystander cells.64 IFN-γ-inducible protein-16 (IFI16), an innate immune sensor for intracellular DNA, and cleaved IL-1β were also detected in the exosomes released from KSHV-infected BCBL-1 cells.24 Given that our supernatants were not treated to eliminate exosomes, their uptake by monocytes is a strong potential candidate mechanism driving inflammasome activation in those cells. Finally, other mediators such as cytokines produced by KSHV-infected cells may act in concert to fully activate the inflammasome.
The limitations of our study include the small sample size in each KAD subgroup, lack of women, and the variability in type and timing of prior treatments across the groups. Specifically, the use of corticosteroids, chemotherapy, and immunotherapy may impact the assessment of inflammatory pathways and the group comparisons between PWH and HV. Future prospective studies with larger sample sizes before receiving therapy will be necessary to evaluate KAD subtypes. Despite these limitations, this is the first study to our knowledge that provides a careful evaluation of inflammasome activation in this complex group of patients.
In summary, our findings suggest a role for inflammasome activation in the pathologic inflammation associated with KAD. The role of dysregulated caspase activity and IL-18 production appeared greatest in patients with MCD and KICS, whereas ASC-speck formation was more pronounced in patients with PEL. It is noteworthy that most patients with MCD and concomitant PEL cluster along with the MCD group, which may suggest that MCD drives the inflammatory process in patients with concurrent PEL and emphasizes the importance of careful evaluation of individuals diagnosed with MCD for a concomitant histologic diagnosis of PEL. Therapies targeting IL-1β and IL-18 have been successfully used to manage hyperinflammatory syndromes driven by pathologic inflammasome activation,65,66 and could therefore be considered to mitigate the exacerbated inflammatory responses in those with KAD. Targeting NLRP3 and its adapter ASC with recently generated effective inhibitors could also potentially help manage these conditions in the future. Further study on the role of KSHV and HIV in inflammasome signaling may lead to new treatment options for this complex group of diseases.
Acknowledgments
The authors are grateful to the study participants and blood bank donors for making this study possible. They also thank the outpatient 8 clinical staff and the research staff at the HIV and AIDS Malignancy Branch for their assistance in patient recruitment and evaluation, and Allison Sedlock for help designing the graphical abstract.
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute (NCI), and in part with federal funds from the NIH, NCI (contract numbers 75N91019D00024/HHSN261201500003I).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: S.L.L., R.R., and I.S. were involved in the conception and design of the study; R.Y. was involved in conceptualizing in vitro experiments; S.L.L., A.R., and D.A.D. performed experiments; S.L.L., R.R., and J.M.R. performed data analysis; R.R., K.L., and M.M. recruited patients, assisted in clinical care, and/or carried out patient data curation; D.W. analyzed and provided KSHV-VL results from patients with KSHV-associated disorder; A.R., R.Y., and I.S. provided resources and reagents; S.L.L., R.R., J.M.R., and I.S. were involved in drafting the manuscript; and all authors have reviewed and approved the final manuscript.
Conflict-of-interest disclosure: D.W. and A.R. are employed by Leidos Biomedical Research, Inc. R.R., K.L., and R.Y. report receiving research support from Celgene/Bristol Myers Squibb, CTI BioPharma (a Sobi A.B. company), PBS Biotech, and Janssen Pharmaceuticals through Cooperative Research and Development Agreements (CRADAs) with the National Cancer Institute (NCI). R.R., K.L., and R.Y. report receiving drugs for a clinical trial from Merck, EMD Serono, and Eli Lilly through CRADAs with the NCI. I.S., R.Y., and D.W. are coinventors on US patent 10,001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds and uses of biomarkers.” An immediate family member of R.Y. is a coinventor on patents or patent applications related to internalization of target receptors, epigenetic analysis, and ephrin tyrosine kinase inhibitors. All rights, title, and interest to these patents have been assigned to the US Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing financial interests.
Correspondence: Ramya Ramaswami, National Cancer Institute, National Institutes of Health, 10 Center Dr, Bldg 10, Room 6N106, Bethesda, MD 20892; email: ramya.ramaswami@nih.gov; and Silvia Lucena Lage, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Dr, Bldg 10, Room 11B15, Bethesda, MD 20892; email: silvia.lage@nih.gov.
References
Author notes
S.L.L. and R.R. are joint first authors.
Data are available on request from corresponding author Silvia Lucena Lage (silvia.lage@nih.gov).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Inflammasome activation across different groups of patients presenting with KSHV-associated diseases stratified according to their clinical classification. (A) Plasma levels of IL-18 were compared among participants with KAD (n = 42, comprising patients with KS alone [10], MCD [11], MCD + PEL [5], KICS + KS [11], and PEL ± KS [5]; ∗means with or without KS), PWH (n = 12), and HV (n = 10). (B) PBMCs from the previously mentioned groups were incubated with the FLICA, and the gMFI of FLICA within total circulating blood monocytes was compared among groups. (C) Alternatively, PBMCs were also stained for monocyte identification and intracellular ASC and acquired by imaging flow cytometry. Data are presented as median with interquartile range. Data were analyzed using the Kruskal-Wallis test followed by Dunn multiple comparisons. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/14/10.1182_blood.2024024144/2/m_blood_bld-2024-024144-gr3.jpeg?Expires=1769085626&Signature=gNhmv01S-KjeIFBRPE0OCmGE9w25hpV38XZX1wG19GGa-jLaHtYzhTtZqqDJmGOcqKFSR0HpBYVh0cZRzxZsFdGacwySgybSPHui4M4ouPjAgHMtxRpECH~cAvb7CT24nSt3EDcoCJ4ItthHmOzgUsKun9eHH3XEH3BidpfsGT9YJPJ4yMyKP5J6JlHEU0o4Z68E-J-LsNRVE8Rc2NhR~zQl8qmlKA7RxibQURkS09xG0wOf8NaKKUyV~8Rmj1LiZjAFsOA9pSD9xPUlgXOiIsWoq9d78D-Kwfh3rrq68zlwNeRKwjdYHWbqC~r3gCIThZzkfGGx1hdXgyzRC6SQbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal