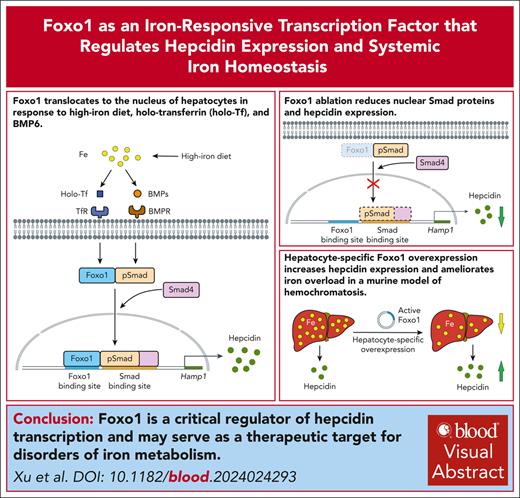
Key Points
Foxo1 regulates hepcidin expression and systemic iron homeostasis.
Foxo1 could serve as a therapeutic target for hereditary hemochromatosis.
Visual Abstract
The liver plays a crucial role in maintaining systemic iron homeostasis by secreting hepcidin, which is essential for coordinating iron levels in the body. Imbalances in iron homeostasis are associated with various clinical disorders related to iron deficiency or iron overload. Despite the clinical significance, the mechanisms underlying how hepatocytes sense extracellular iron levels to regulate hepcidin synthesis and iron storage are not fully understood. In this study, we identified Foxo1, a well-known regulator of macronutrient metabolism, which translocates to the nucleus of hepatocytes in response to high-iron feeding, holo-transferrin, and bone morphogenetic protein 6 (BMP6) treatment. Furthermore, Foxo1 plays a crucial role in mediating hepcidin induction in response to both iron and BMP signals by directly interacting with evolutionally conserved Foxo binding sites within the hepcidin promoter region. These binding sites were found to colocalize with Smad-binding sites. To investigate the physiological relevance of Foxo1 in iron metabolism, we generated mice with hepatocyte-specific deletion of Foxo1. These mice exhibited reduced hepatic hepcidin expression and serum hepcidin levels, accompanied by elevated serum iron and liver nonheme iron concentrations. Moreover, high-iron diet further exacerbated these abnormalities in iron metabolism in mice lacking hepatic Foxo1. Conversely, hepatocyte-specific Foxo1 overexpression increased hepatic hepcidin expression and serum hepcidin levels, thereby ameliorating iron overload in a murine model of hereditary hemochromatosis (Hfe−/− mice). In summary, our study identifies Foxo1 as a critical regulator of hepcidin and systemic iron homeostasis. Targeting Foxo1 may offer therapeutic opportunities for managing conditions associated with aberrant iron metabolism.
Introduction
Iron is a vital micronutrient for numerous biological processes essential to maintaining overall health. However, both deficiency and excess iron can lead to health complications. Absolute or functional iron deficiency will limit red blood cell production, causing iron-deficient erythropoiesis and anemia.1 Iron overload is present in individuals with hemochromatosis or red cell disorders, in whom iron deposits in hepatocytes, β-cells, and cardiomyocytes, increasing the risk of diseases such as type 2 diabetes and cardiovascular diseases.2 Therefore, the regulation of iron metabolism is meticulously orchestrated to ensure optimal circulating and storage levels within the body.
The liver is the primary organ responsible for iron sensing and storage.3 Hepatocyte-secreted hepcidin is a pivotal hormone controlling systemic iron homeostasis by modulating iron absorption from the intestine and iron recycling from the reticuloendothelial system.4 Hepcidin interacts with the iron exporter ferroportin in the enterocytes and macrophages, leading to the internalization and degradation of ferroportin, thereby reducing circulating iron levels and promoting cellular iron retention.5
Two signaling pathways are involved in synthesizing hepcidin, including the iron-induced bone morphogenetic protein-Smad (BMP-Smad) and inflammation-induced JAK-STAT pathways.4,6 Smad proteins modulate hepcidin synthesis through 2 pathways: one involves increased liver iron levels, which upregulates the expression of BMPs in the sinusoidal endothelial cells,7,8 leading to increased Smad phosphorylation and hepcidin transcription in the hepatocytes; the other pathway involves transferrin-bound iron, which stimulates hepcidin expression by interacting with HFE/TFR1/TFR2 complex on the cell membrane of hepatocytes, leading to increased Smad phosphorylation.9,10 Erythroferrone, secreted under enhanced erythropoiesis, inhibits BMP-Smad signaling pathway via trapping BMP ligands.11 Mutations in genes involved in BMP-Smad signaling pathways can lead to human hereditary hemochromatosis, characterized by insufficient hepcidin synthesis and excessive iron absorption and accumulation in primary organs such as the liver, pancreas, and heart.12
Despite understanding these signaling pathways, how hepatocytes sense extracellular iron to regulate hepcidin synthesis and iron stores remains incompletely understood. Here, we identified that Forkhead box proteins 1 (FOXO1), a well-known regulator of macronutrient metabolism, as an iron-responsive transcriptional factor in hepatocytes that regulates liver hepcidin induction and systemic iron homeostasis. FOXOs are primary transcriptional factors responsible for genes regulating glucose homeostasis, cell differentiation and proliferation, and angiogenesis.13 In hepatocytes, FOXO1 controls the expression of key enzymes involved in gluconeogenesis, such as phosphoenolpyruvate carboxykinase and glucose 6-phosphatase, promoting the conversion of gluconeogenic substrates to glucose during fasting or starvation.14 Insulin signaling inhibits FOXO1 activity by phosphorylation via AKT. Phosphorylated FOXO1 will be excluded from the nucleus, reducing hepatic glucose output under fed conditions.15
In this study, we observed that high-iron feeding, transferrin-bound iron, or BMP ligands led to the nuclear translocation of FOXO1 in hepatocytes. Our results suggest that FOXO1 plays a significant role as a transcriptional factor in modulating hepcidin expression in response to fluctuations of dietary iron levels.
Materials and methods
Mice
Mice with Foxo1 floxed alleles (Foxo1flox/flox; C57BL/6J background; Shanghai Model Organisms; catalog no. nm-cko-200177) were bred with the Albumin-Cre transgenic mice (C57BL/6J background; GemPharmatech, Nanjing, China; catalog no. T003814)16 to generate the hepatocyte-specific Foxo1 gene knockout mice (Foxo1flox/flox; Albumin-Cre, Foxo1LKO). Foxo1flox/flox mice were bred with Albumin-Cre/ERT2 transgenic mice (C57BL/6J background; Shanghai Model Organisms; catalog no. NM-KI-00002, China) to generate the hepatocyte-specific Foxo1 gene inducible knockout mice (Foxo1flox/flox; Albumin-Cre/ERT2). Hfe gene knockout mice (Hfe−/−, C57BL/6J background; supplemental Figure 6A, available on the Blood website) were obtained from GemPharmatech (Nanjing, China; catalog no. T028372). All animal experiments received approval and were performed in compliance with the guidelines approved by the ethics committee of the China Agricultural University (approval number AW91503202-5-1).
Tissue nonheme iron concentration and iron staining
Measurement of tissue nonheme iron concentration was performed as previously described.17 Perls iron staining was performed using a Prussian Blue Iron Stain Kit with nuclear fast red solution (Solarbio, Beijing, China; catalog no. G1422). Full methods are available in the supplemental Materials and methods.
Primary cell isolation and cell culture
Primary hepatocytes were purified using the modified Seglen 2-step perfusion method combined with Percoll density gradient centrifugation.18,19 Mice used for primary hepatocytes isolation were fed a normal diet (ND), except when indicated. Full methods are available in the supplemental Materials and methods.
Plasmids and reagents
Murine Foxo1-AAA, a constitutively active Foxo1 containing T24A, S253A, and S316A mutations,20 was synthesized and inserted into the pLIVE vector (Mirus Bio, Madison, Wisconsin) to achieve hepatocyte-specific Foxo1 overexpressing in mice. Human FOXO1-AAA, a constitutively active FOXO1 containing T24A, S256A, and S319A mutations,21 was synthesized and inserted into pcDNA3.1 vector to achieve FOXO1 overexpression in cultured cells. Full methods are available in the supplemental Materials and methods.
Cellular fractionation
Cellular fractionation was performed according to a published method to obtain the nuclear and cytoplasmic components.22 Full methods are available in the supplemental Materials and methods.
Immunofluorescence
After overnight incubation with primary antibodies, the coverslips were stained using appropriate secondary antibodies. Finally, 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO; catalog no. 10 236 276 001) was added as a nuclear dye in the immunofluorescence staining slide. The slide was visualized using a Zeiss LSM900 (Carl Zeiss, Germany) confocal laser scanning microscope.
Hamp1 promoter cloning and luciferase assay
Cells were transfected with the wild-type or mutated luciferase reporter plasmids respectively. After transfection, cells were collected and assayed using a Dual-Luciferase Reporter Gene Assay Kit (Yeasen Biotechnology, Shanghai, China; catalog no. 11402ES60). Subsequently, autoluminescence changes in each group were detected using a microplate reader (BioTek Instruments, Wnooski, VT).
ChIP assay
Chromatin immunoprecipitation (ChIP) assays were performed using a ChromataChIPTM Kit (Novus Biologicals, Centennial, CO; catalog no. NBP1-71709). More details are outlined in the supplemental Materials and methods.
Statistical analysis
All data were presented as means ± standard deviations and compared by the Student t test or the 1-way analysis of variance with the Sidak post hoc test using GraphPad Prism (version 8). P values were 2-sided, and P value <.05 was considered statistically significant.
Results
Iron induces the nuclear translocation of Foxo1 in hepatocytes
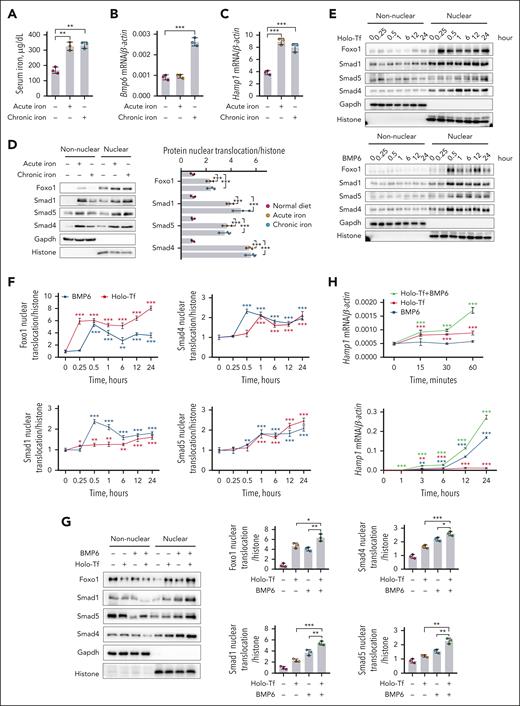
In mice receiving a high-iron diet (HID), both 3-hour acute and 3-day chronic iron challenge elevated serum iron (Figure 1A) and transferrin saturation (supplemental Figure 1A), inducing elevated hepatic Bmp6 expression (Figure 1B), serum Bmp6 (supplemental Figure 1B), and hepatic hepcidin (Hamp1) expression (Figure 1C) in the liver tissue. Consistent with a prior research,23 acute iron exposure appeared to directly regulate hepcidin expression without a corresponding induction of hepatic Bmp6 messenger RNA (mRNA) expression (Figure 1B) and serum Bmp6 concentration (supplemental Figure 1B). Acute or chronic iron challenge increased nuclear Smad1, Smad4, and Smad5 (Figure 1D). Simultaneously, nuclear Foxo1 was enhanced (Figure 1D). In the whole-cell lysate of these hepatocytes, decreased Foxo1 phosphorylation (pFoxo1) and enhanced Smad1/5/8 phosphorylation (pSmad1/5/8) signals were observed (supplemental Figure 1C).
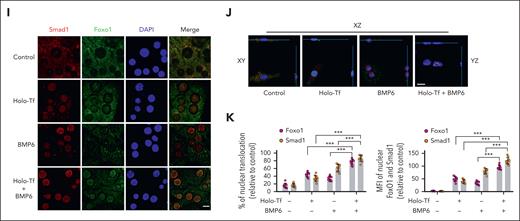
Iron induces the nuclear translocation of Foxo1 and Smad in hepatocytes. (A-D) Eight-week-old male wild-type C57BL/6J mice received a 3-hour acute (ferrous sulfate, 2 mg/kg body weight, oral gavage) or 3-day chronic iron challenge (rodent diet containing 8.24 g carbonyl iron per kg). (A-C) Serum iron levels (A), Bmp6 mRNA (B), and Hamp1 mRNA (C) were measured in the liver tissues. (D) Nuclear Foxo1 and Smad proteins were detected (left) and quantified (right) in the hepatocytes. (E-F) Primary hepatocytes treated with holo-Tf or BMP6 at indicated time duration. (E-F) Foxo1 and Smad proteins were measured by nucleocytoplasmic separation (E) and quantified (F). (G) Primary hepatocytes treated with holo-Tf or/and BMP6 for 7 hours; Foxo1 and Smad proteins were measured by nucleocytoplasmic separation (left panel) and quantified (right panel). (H) Hepcidin (Hamp1) mRNA expression levels in the primary hepatocytes treated with holo-Tf and/or BMP6 at indicated time duration. (I-K) Primary hepatocytes treated with holo-Tf and/or BMP6 for 7 hours; the immunofluorescence (I), corresponding 3-dimensional visualization (J), percentage (K) (left panel) and mean fluorescence intensity (MFI, right panel) of nuclear Foxo1 and Smad1 were analyzed. Nuclear translocation and MFI of Foxo1 and Smad1 were quantified using 10 representative images from 3 independent experiments (scale bar, 20 μm). Primary hepatocytes in panels E-K were isolated from 8-week-old male wild-type 129S2/SvPasCrl mice. The concentration of holo-Tf was 30 μM and BMP6 was 20 ng/mL. Results from quantitative polymerase chain reaction (qPCR) were obtained from 3 biological replicates. Quantification of western blot was obtained from 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way analysis of variance (ANOVA) with the Sidak multiple comparison test. For panels A-D, significance label indicates the comparison between treated group and untreated group. For panels F,H, significance label indicates the comparison with the 0-time point, a negative control cultured for 24 hours without holo-Tf or BMP6 treatment.
Iron induces the nuclear translocation of Foxo1 and Smad in hepatocytes. (A-D) Eight-week-old male wild-type C57BL/6J mice received a 3-hour acute (ferrous sulfate, 2 mg/kg body weight, oral gavage) or 3-day chronic iron challenge (rodent diet containing 8.24 g carbonyl iron per kg). (A-C) Serum iron levels (A), Bmp6 mRNA (B), and Hamp1 mRNA (C) were measured in the liver tissues. (D) Nuclear Foxo1 and Smad proteins were detected (left) and quantified (right) in the hepatocytes. (E-F) Primary hepatocytes treated with holo-Tf or BMP6 at indicated time duration. (E-F) Foxo1 and Smad proteins were measured by nucleocytoplasmic separation (E) and quantified (F). (G) Primary hepatocytes treated with holo-Tf or/and BMP6 for 7 hours; Foxo1 and Smad proteins were measured by nucleocytoplasmic separation (left panel) and quantified (right panel). (H) Hepcidin (Hamp1) mRNA expression levels in the primary hepatocytes treated with holo-Tf and/or BMP6 at indicated time duration. (I-K) Primary hepatocytes treated with holo-Tf and/or BMP6 for 7 hours; the immunofluorescence (I), corresponding 3-dimensional visualization (J), percentage (K) (left panel) and mean fluorescence intensity (MFI, right panel) of nuclear Foxo1 and Smad1 were analyzed. Nuclear translocation and MFI of Foxo1 and Smad1 were quantified using 10 representative images from 3 independent experiments (scale bar, 20 μm). Primary hepatocytes in panels E-K were isolated from 8-week-old male wild-type 129S2/SvPasCrl mice. The concentration of holo-Tf was 30 μM and BMP6 was 20 ng/mL. Results from quantitative polymerase chain reaction (qPCR) were obtained from 3 biological replicates. Quantification of western blot was obtained from 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way analysis of variance (ANOVA) with the Sidak multiple comparison test. For panels A-D, significance label indicates the comparison between treated group and untreated group. For panels F,H, significance label indicates the comparison with the 0-time point, a negative control cultured for 24 hours without holo-Tf or BMP6 treatment.
To investigate how Foxo1 responded to iron fluctuation in hepatocytes, cells were treated with extracellular ligands holo-transferrin (holo-Tf) and BMP6 to mimic dietary iron challenge in mice. Holo-Tf and BMP6 induced the nuclear translocation of Foxo1 at 0.25 and 0.5 hours, respectively, and holo-Tf and BMP6 induced the nuclear translocation of Smad4 at 1 and 0.5 hours, respectively (Figure 1E-F). Simultaneously, decreased pFoxo1 and enhanced pSmad1/5/8 were found in the whole-cell lysate of hepatocytes treated with holo-Tf (supplemental Figure 1D) or BMP6 (supplemental Figure 1E). When hepatocytes were treated with both holo-Tf and BMP6, nuclear Foxo1 and Smad proteins were further increased than those treated with holo-Tf or BMP6 alone (Figure 1G). Consistently, this cumulative effect was also observed in pFoxo1 (supplemental Figure 1F), pSmad1/5/8 (supplemental Figure 1F), and hepcidin expression (Hamp1; Figure 1H) of hepatocytes treated with both holo-Tf or BMP6. These results were further validated by the immunofluorescent signals of Foxo1 and Smad proteins, in which intensified nuclear translocation of these proteins were triggered upon holo-Tf and/or BMP6 addition (Figure 1I-K; supplemental Figure 1G).
Foxo1 modulates hepcidin expression in hepatocytes
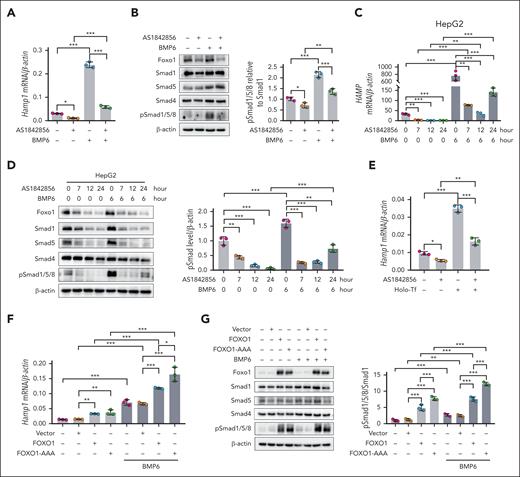
To examine whether Foxo1 was involved in the regulation of hepcidin expression, murine hepatocytes were treated with the Foxo1-specific inhibitor AS1842856.24 Foxo1 inhibition decreased basal and BMP6-induced hepcidin expression (Hamp1; Figure 2A) and pSmad1/5/8 levels (Figure 2B) in hepatocytes. Foxo1 inhibition also repressed BMP6-induced hepcidin mRNA expression (HAMP; Figure 2C) and pSmad1/5/8 levels (Figure 2D) in HepG2 cells. Consistent results were observed using small interfering RNA-mediated downregulation of FOXO1 in HepG2 cells (supplemental Figure 2A). Hepatocytes treated with the Foxo1 inhibitor also displayed reduced holo-Tf–induced hepcidin expression (Figure 2E).
Foxo1 modulates hepcidin expression in hepatocytes. (A-B) Primary hepatocytes treated with the Foxo1 specific inhibitor AS1842856; (A) hepcidin (Hamp1) mRNA expression (A) and pSmad1/5/8 (B) were measured under basal or BMP6 treated condition (AS1842856, 10 μM, 7 hours; BMP6, 20 ng/mL, 6 hours; cells were treated with AS1842856 1 hour before the addition of BMP6). (C-D) HepG2 cells were treated with the Foxo1 specific inhibitor AS1842856; hepcidin (HAMP) mRNA (C) and pSmad1/5/8 (D) were measured at indicated time duration (left) and quantified (right). Cells were treated with 10 μM AS1842856 1 hour before the addition of 20 ng/mL BMP6. (E) Primary hepatocytes were treated with the Foxo1-specific inhibitor AS1842856; holo-Tf-induced Hamp1 mRNA expression was measured (AS1842856, 10 μM, 7 hours; holo-Tf, 30 μM, 6 hours; cells were treated with AS1842856 1 hour before the addition of holo-Tf). (F-G) Primary hepatocytes were transfected with the pCDNA3.1 vector containing human wild-type FOXO1 or the constitutively active form of FOXO1 (FOXO1-AAA) for 24 hours; Hamp1 expression (F) and pSmad1/5/8 (G) were measured under basal and BMP6 treated conditions. Cells were treated with 20 ng/mL BMP6 for 6 hours before the end of plasmids transfection. Primary hepatocytes were isolated from 8-week-old male wild-type 129S2/SvPasCrl mice. Results from qPCR were obtained from 3 biological replicates. Quantification of western blot was obtained from 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test.
Foxo1 modulates hepcidin expression in hepatocytes. (A-B) Primary hepatocytes treated with the Foxo1 specific inhibitor AS1842856; (A) hepcidin (Hamp1) mRNA expression (A) and pSmad1/5/8 (B) were measured under basal or BMP6 treated condition (AS1842856, 10 μM, 7 hours; BMP6, 20 ng/mL, 6 hours; cells were treated with AS1842856 1 hour before the addition of BMP6). (C-D) HepG2 cells were treated with the Foxo1 specific inhibitor AS1842856; hepcidin (HAMP) mRNA (C) and pSmad1/5/8 (D) were measured at indicated time duration (left) and quantified (right). Cells were treated with 10 μM AS1842856 1 hour before the addition of 20 ng/mL BMP6. (E) Primary hepatocytes were treated with the Foxo1-specific inhibitor AS1842856; holo-Tf-induced Hamp1 mRNA expression was measured (AS1842856, 10 μM, 7 hours; holo-Tf, 30 μM, 6 hours; cells were treated with AS1842856 1 hour before the addition of holo-Tf). (F-G) Primary hepatocytes were transfected with the pCDNA3.1 vector containing human wild-type FOXO1 or the constitutively active form of FOXO1 (FOXO1-AAA) for 24 hours; Hamp1 expression (F) and pSmad1/5/8 (G) were measured under basal and BMP6 treated conditions. Cells were treated with 20 ng/mL BMP6 for 6 hours before the end of plasmids transfection. Primary hepatocytes were isolated from 8-week-old male wild-type 129S2/SvPasCrl mice. Results from qPCR were obtained from 3 biological replicates. Quantification of western blot was obtained from 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test.
When wild-type FOXO1 or the constitutively active form of FOXO1 (FOXO1-AAA) was overexpressed in murine hepatocytes, either wild-type FOXO1 or FOXO1-AAA overexpression remarkably increased nuclear Foxo1 (supplemental Figure 2B). Wild-type FOXO1 or FOXO1-AAA overexpression upregulated hepcidin expression and pSmad1/5/8 levels at basal or BMP6-treated condition (Figure 2F-G). Treatment of primary hepatocytes with FOXO1 agonist LOM61225 increased hepcidin expression (supplemental Figure 2C). We observed concurrently increased Bmp2, Bmp6, and Smad4 expressions in hepatocytes overexpressing FOXO1 (supplemental Figure 2D), which may increase pSmad1/5/8 via an autocrine manner.
Hepatic Foxo1 ablation disrupts iron homeostasis
To exclude the metabolic abnormalities caused by hepatic Foxo1 deletion at embryonic stage,26 we constructed a tamoxifen-induced, hepatocyte-specific Foxo1 gene knockout mouse by crossing Foxo1flox/flox mice with Albumin-Cre/ERT2 transgenic mice (Foxo1flox/flox; Albumin-Cre/ERT2). To obtain Foxo1iLKO or Foxo1iCon mice, tamoxifen or corn oil was administered to Foxo1flox/flox; Albumin-Cre/ERT2 mice via intraperitoneal injection at age 4 weeks for 5 consecutive days (supplemental Figure 3A-B). Foxo1 knockout efficiency was validated by mRNA and protein expressions in the hepatocytes of Foxo1iLKO mice (supplemental Figure 3C-D).
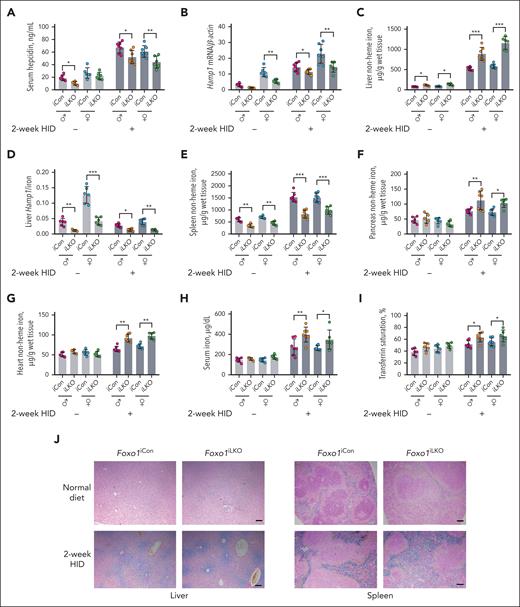
Foxo1iLKO and Foxo1iCon mice fed ND or 2-week HID were euthanized at 8 weeks for phenotype analysis. Compared with Foxo1iCon mice, male Foxo1iLKO mice displayed reduced serum hepcidin levels when fed either ND or HID (Figure 3A). Female Foxo1iLKO mice displayed reduced hepatic Hamp1 (Figure 3B) and Id1 expressions (supplemental Figure 3E) when fed either ND or HID. Foxo1iLKO mice displayed increased liver nonheme iron concentrations (Figure 3C), decreased liver Hamp1/iron ratios (Figure 3D), and decreased spleen nonheme iron concentrations (Figure 3E) under both ND and HID conditions. Increased pancreas nonheme iron concentrations, heart nonheme iron concentrations, serum iron and transferrin saturation levels were seen in Foxo1iLKO mice fed a 2-week HID (Figure 3F-I). Increased ferroportin protein expression was observed in the small intestine of Foxo1iLKO mice under ND (supplemental Figure 3F). Increased liver tissue iron and decreased spleen tissue iron levels of Foxo1iLKO mice were confirmed by inductively coupled plasma-optical emission spectrometry (supplemental Figure 3G-H), ferritin heavy chain protein (supplemental Figure 3I-J), and Perls iron staining (Figure 3J). Foxo1iLKO mice displayed comparable hematological parameters with Foxo1iCon mice (supplemental Table 1).
Foxo1iLKO mice display disturbed iron metabolism under both ND and HID.Foxo1iLKO and Foxo1iCon mice were maintained on an ND or a 2-week HID before they were euthanized at age 8 weeks. Iron-related phenotypes were assessed. (A) Serum hepcidin levels. (B) Liver hepcidin (Hamp1) mRNA expressions. (C) Liver nonheme iron concentrations. (D) Liver Hamp1 mRNA/liver nonheme iron concentration ratios (Hamp1/iron). (E) Spleen nonheme iron concentrations. (F) Pancreas nonheme iron concentrations. (G) Heart nonheme iron concentrations. (H) Serum iron levels. (I) Transferrin saturation levels. (J) Perls iron staining of the liver and spleen tissues (scale bar, 100 μm). ♂, male mice; ♀, female mice; n = 5 to 7 in each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test in sex- and diet-matched data.
Foxo1iLKO mice display disturbed iron metabolism under both ND and HID.Foxo1iLKO and Foxo1iCon mice were maintained on an ND or a 2-week HID before they were euthanized at age 8 weeks. Iron-related phenotypes were assessed. (A) Serum hepcidin levels. (B) Liver hepcidin (Hamp1) mRNA expressions. (C) Liver nonheme iron concentrations. (D) Liver Hamp1 mRNA/liver nonheme iron concentration ratios (Hamp1/iron). (E) Spleen nonheme iron concentrations. (F) Pancreas nonheme iron concentrations. (G) Heart nonheme iron concentrations. (H) Serum iron levels. (I) Transferrin saturation levels. (J) Perls iron staining of the liver and spleen tissues (scale bar, 100 μm). ♂, male mice; ♀, female mice; n = 5 to 7 in each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test in sex- and diet-matched data.
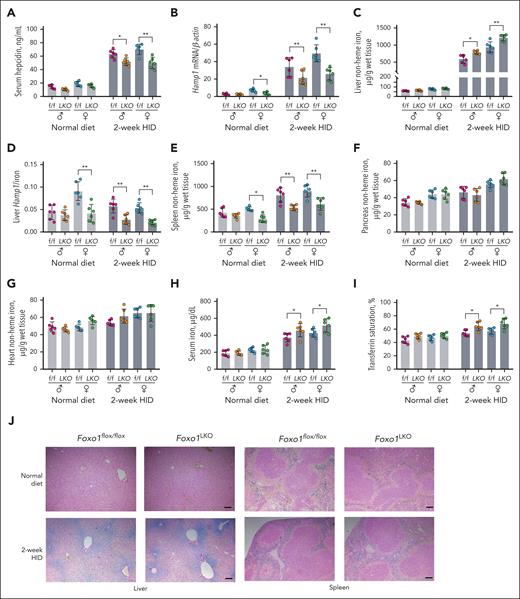
We also constructed a mouse model with embryonic Foxo1 gene deletion in hepatocytes (Foxo1LKO) by crossing Foxo1flox/flox mice with Albumin-Cre transgenic mice (supplemental Figure 4A), with knockout efficiency validated in hepatocytes (supplemental Figure 4B). Foxo1LKO and Foxo1flox/flox mice fed ND or a 2-week HID were euthanized at age 8 weeks for phenotypic analysis. Compared with Foxo1flox/flox mice, Foxo1LKO mice displayed reduced serum hepcidin levels (Figure 4A), hepatic Hamp1 expressions (Figure 4B), and Id1 expressions (supplemental Figure 4C) under HID. Foxo1LKO mice also displayed increased liver nonheme iron concentrations (Figure 4C), decreased liver Hamp1/iron ratios (Figure 4D), and decreased spleen nonheme iron concentrations (Figure 4E) under HID. Under ND, female Foxo1LKO mice displayed decreased Hamp1 expressions (Figure 4B), liver Hamp1/iron (Figure 4D), and spleen nonheme iron concentrations (Figure 4E) compared with Foxo1flox/flox mice. No change was observed in the nonheme iron concentration of pancreas and heart tissues between Foxo1LKO mice and Foxo1flox/flox mice (Figure 4F-G). Foxo1LKO mice displayed increased serum iron (Figure 4H) and transferrin saturation (Figure 4I) levels when fed a 2-week HID. Compared with Foxo1flox/flox mice, increased ferroportin protein expression was observed in the small intestine of Foxo1LKO mice under ND (supplemental Figure 4D). A consistent increase of ferroportin protein expression was seen in the liver of Foxo1LKO mice (supplemental Figure 4E). The liver and spleen tissue iron levels were validated through Perls iron staining. Increased liver tissue iron was observed in Foxo1LKO mice under a 2-week HID (Figure 4J, left panel). A modest decrease in spleen tissue iron was found in Foxo1iLKO mice under both ND and HID (Figure 4J, right panel). Foxo1LKO displayed comparable hematological parameters with Foxo1flox/flox mice (supplemental Table 1).
Foxo1LKO mice display disturbed iron metabolism under HID.Foxo1LKO and Foxo1flox/flox mice were maintained on an ND or a 2-week HID before they were euthanized at age 8 weeks. Iron-related phenotypes were assessed. (A) Serum hepcidin levels. (B) Liver hepcidin (Hamp1) mRNA expressions. (C) Liver nonheme iron concentrations. (D) Liver Hamp1 mRNA/liver nonheme iron concentration ratios (Hamp1/iron). (E) Spleen nonheme iron concentrations. (F) Pancreas nonheme iron concentrations. (G) Heart nonheme iron concentrations. (H) Serum iron levels. (I) Transferrin saturation levels. (J) Perls iron staining of liver and spleen tissue (scale bar, 100 μm). ♂, male mice; ♀, female mice; n = 6 in each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test in sex- and diet-matched data.
Foxo1LKO mice display disturbed iron metabolism under HID.Foxo1LKO and Foxo1flox/flox mice were maintained on an ND or a 2-week HID before they were euthanized at age 8 weeks. Iron-related phenotypes were assessed. (A) Serum hepcidin levels. (B) Liver hepcidin (Hamp1) mRNA expressions. (C) Liver nonheme iron concentrations. (D) Liver Hamp1 mRNA/liver nonheme iron concentration ratios (Hamp1/iron). (E) Spleen nonheme iron concentrations. (F) Pancreas nonheme iron concentrations. (G) Heart nonheme iron concentrations. (H) Serum iron levels. (I) Transferrin saturation levels. (J) Perls iron staining of liver and spleen tissue (scale bar, 100 μm). ♂, male mice; ♀, female mice; n = 6 in each group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test in sex- and diet-matched data.
Foxo1 ablation impairs the response of Smad proteins to dietary iron
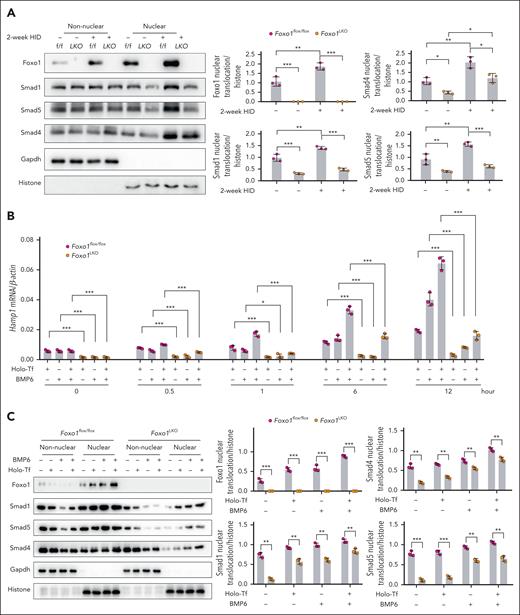
To investigate how Foxo1 modulates hepcidin expression in response to dietary iron fluctuation, primary hepatocytes were isolated from Foxo1LKO mice and Foxo1flox/flox mice. HID enhanced pSmad1/5/8 (supplemental Figure 5A), nuclear Smad1, Smad4, and Smad5 in hepatocytes, whereas Foxo1 ablation reduced these signals in the nucleus (Figure 5A). Foxo1 ablation also remarkably reduced holo-Tf- or BMP6-induced Hamp1 (Figure 5B), pSmad1/5/8 (supplemental Figure 5B), nuclear Smad1, Smad4, and Smad5 (Figure 5C) in untreated hepatocytes, as well as their levels in holo-Tf- or BMP6-treated hepatocytes.
Foxo1 ablation impairs the response of hepcidin signaling to iron. (A) Nuclear Foxo1, Smad1, Smad4, and Smad5 were measured in the primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice receiving normal or a 2-week HID (left). Quantification of the nuclear Foxo1, Smad1, Smad4, and Smad5 proteins (right). (B) Primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice were treated with holo-Tf and/or BMP6; hepcidin (Hamp1) mRNA expression was measured by qPCR at indicated time point. (C) Primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice were treated with holo-Tf and/or BMP6 for 7 hours; the nuclear translocation of Foxo1, Smad1, Smad4 and Smad5 were measured by nucleocytoplasmic separation (left) and quantified (right). The concentration of holo-Tf was 30 μM and BMP6 was 20 ng/mL. Results from qPCR were obtained from 3 biological replicates. Quantification of western blot was obtained from 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test. (B) Multiple comparison test was performed in groups with the same treatment duration. (C) Multiple comparison test was performed in groups with the same treatment condition.
Foxo1 ablation impairs the response of hepcidin signaling to iron. (A) Nuclear Foxo1, Smad1, Smad4, and Smad5 were measured in the primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice receiving normal or a 2-week HID (left). Quantification of the nuclear Foxo1, Smad1, Smad4, and Smad5 proteins (right). (B) Primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice were treated with holo-Tf and/or BMP6; hepcidin (Hamp1) mRNA expression was measured by qPCR at indicated time point. (C) Primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice were treated with holo-Tf and/or BMP6 for 7 hours; the nuclear translocation of Foxo1, Smad1, Smad4 and Smad5 were measured by nucleocytoplasmic separation (left) and quantified (right). The concentration of holo-Tf was 30 μM and BMP6 was 20 ng/mL. Results from qPCR were obtained from 3 biological replicates. Quantification of western blot was obtained from 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the 1-way ANOVA with the Sidak multiple comparison test. (B) Multiple comparison test was performed in groups with the same treatment duration. (C) Multiple comparison test was performed in groups with the same treatment condition.
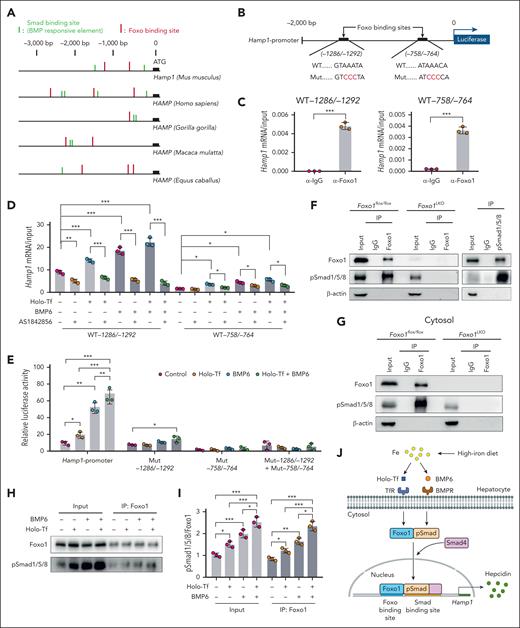
Foxo1 and Smad proteins cooperatively regulate hepcidin transcription
After investigations into the promoter region of hepcidin gene in mammals, we revealed multiple binding sites for Foxo1 (Foxo binding sites) and Smad proteins (Smad binding sites; Figure 6A). Interestingly, Foxo and Smad binding sites are typically colocalized within the 3000 base pairs (bp) upstream of the hepcidin gene start codon (Figure 6A). In the murine Hamp1 promoter, 2 Foxo binding sites were characterized at position −1286/−1292 bp (GTAAATA) and −758/−764 bp (ATAAACA) (Figure 6B). The interaction between Foxo1 and the 2 predicted Foxo binding sites in the murine Hamp1 promoter region was confirmed by ChIP–quantitative polymerase chain reaction (Figure 6C). The interaction between Foxo1 and Foxo binding sites was enhanced when treated with holo-Tf or BMP6 but repressed when treated with Foxo1 inhibitor in hepatocytes (Figure 6D).
Foxo1 and Smad proteins coregulate hepcidin transcription. (A) Analysis of Foxo binding sites (red) and Smad binding sites/BMP-responsive elements (green) in the 3000 bp upstream region of hepcidin gene transcription start site in different mammals. (B) In the murine Hamp1 promoter, a scheme summarizing the 2 predicted Foxo binding sites and their corresponding mutant forms. WT, wild-type Hamp1 promoter; Mut, Hamp1 promoter with mutated Foxo binding site (red). (C-D) ChIP-qPCR analysis of the interaction between Foxo1 and 2 Foxo binding sites in the murine Hamp1 promoter region under basal condition (C) or holo-Tf/BMP6/AS1842856–treated condition (D). Cells were treated with 10 μM AS1842856 1 hour before the addition of 20 ng/mL BMP6 and/or 30 μM holo-Tf (AS1842856, 7 hours; BMP6, 6 hours; holo-Tf, 6 hours). (E) As indicated in panel B, Hamp1 promoter luciferase reporter plasmids containing wild-type (WT) or mutated (Mut) Foxo binding sites were transfected into primary hepatocytes and assayed for their luciferase reporter activities (holo-Tf, 30 μM; BMP6, 20 ng/mL; 7 hours). (F) Immunoprecipitation (IP) and western blot analysis of the Foxo1-pSmad1/5/8 interaction in primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice (left) and 8-week-old male WT 129S2/SvPasCrl mice (right). (G) IP/western blot analysis of the Foxo1-pSmad1/5/8 interaction in the cytosol of primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice. (H) IP/western blot analysis of the Foxo1-pSmad1/5/8 interaction in the primary hepatocytes treated with holo-Tf and/or BMP6 (7 hours; holo-Tf, 30 μM; BMP6, 20 ng/mL). (I) Quantifications of Foxo1-pSmad1/5/8 interaction from results displayed in panel H. (J) A working model to elucidate the role of Foxo1 in hepatic hepcidin regulation. Primary hepatocytes were isolated from 8-week-old male WT 129S2/SvPasCrl mice. Results from ChIP-qPCR, luciferase assay, IP/western blot, and corresponding quantification analysis were all obtained from 3 independent biological replicates. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. The P value was calculated by the Student t test for 2 group comparison or the 1-way ANOVA with the Sidak post hoc test for multiple comparison. For panels D-E, multiple comparison test was separately performed in different Foxo binding sites or their mutations.
Foxo1 and Smad proteins coregulate hepcidin transcription. (A) Analysis of Foxo binding sites (red) and Smad binding sites/BMP-responsive elements (green) in the 3000 bp upstream region of hepcidin gene transcription start site in different mammals. (B) In the murine Hamp1 promoter, a scheme summarizing the 2 predicted Foxo binding sites and their corresponding mutant forms. WT, wild-type Hamp1 promoter; Mut, Hamp1 promoter with mutated Foxo binding site (red). (C-D) ChIP-qPCR analysis of the interaction between Foxo1 and 2 Foxo binding sites in the murine Hamp1 promoter region under basal condition (C) or holo-Tf/BMP6/AS1842856–treated condition (D). Cells were treated with 10 μM AS1842856 1 hour before the addition of 20 ng/mL BMP6 and/or 30 μM holo-Tf (AS1842856, 7 hours; BMP6, 6 hours; holo-Tf, 6 hours). (E) As indicated in panel B, Hamp1 promoter luciferase reporter plasmids containing wild-type (WT) or mutated (Mut) Foxo binding sites were transfected into primary hepatocytes and assayed for their luciferase reporter activities (holo-Tf, 30 μM; BMP6, 20 ng/mL; 7 hours). (F) Immunoprecipitation (IP) and western blot analysis of the Foxo1-pSmad1/5/8 interaction in primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice (left) and 8-week-old male WT 129S2/SvPasCrl mice (right). (G) IP/western blot analysis of the Foxo1-pSmad1/5/8 interaction in the cytosol of primary hepatocytes from 8-week-old male Foxo1LKO and Foxo1flox/flox mice. (H) IP/western blot analysis of the Foxo1-pSmad1/5/8 interaction in the primary hepatocytes treated with holo-Tf and/or BMP6 (7 hours; holo-Tf, 30 μM; BMP6, 20 ng/mL). (I) Quantifications of Foxo1-pSmad1/5/8 interaction from results displayed in panel H. (J) A working model to elucidate the role of Foxo1 in hepatic hepcidin regulation. Primary hepatocytes were isolated from 8-week-old male WT 129S2/SvPasCrl mice. Results from ChIP-qPCR, luciferase assay, IP/western blot, and corresponding quantification analysis were all obtained from 3 independent biological replicates. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. The P value was calculated by the Student t test for 2 group comparison or the 1-way ANOVA with the Sidak post hoc test for multiple comparison. For panels D-E, multiple comparison test was separately performed in different Foxo binding sites or their mutations.
To further confirm the interaction between Foxo1 and Foxo binding sites, the 2000 bp upstream sequence in the murine Hamp1 promoter region containing the 2 Foxo binding sites and corresponding loss-of-function mutations (AAA to CCC mutations in the Foxo binding consensus sequence) were cloned into the luciferase reporter vectors (illustrated in Figure 6B). The wild-type murine Hamp1 promoter was enhanced by holo-Tf or BMP6 in primary hepatocytes (Figure 6E), whereas reporters containing mutated Foxo binding consensus sequence at either or both Foxo binding sites failed to respond to holo-Tf and/or BMP6 (Figure 6E).
We speculated that Foxo1 may interact with Smad proteins to regulate hepcidin transcription. In the immunoprecipitates of endogenous Foxo1, pSmad1/5/8 was detected, and vice versa (Figure 6F). The interaction between Foxo1 and pSmad1/5/8 was also identified in the cytoplasmic fraction of murine primary hepatocytes (Figure 6G). This interaction was enhanced by holo-Tf or BMP6 treatment (Figure 6H-I). According to these findings, a working model was proposed (Figure 6J). Foxo1 and pSmad proteins seem to interact with each other in the cytosol and then collectively bind to the colocalized Foxo binding sites and Smad binding sites in the Hamp1 promoter to initiate hepcidin transcription.
Hepatocyte-specific Foxo1 overexpression alleviates iron overload in hereditary hemochromatosis
We next investigated whether Foxo1-induced hepcidin expression could improve iron overload in the murine model of hemochromatosis (Hfe−/− mice; supplemental Figure 6A). A hepatocyte-specific Foxo1 overexpression plasmid encoding murine constitutively active form of Foxo1 was constructed (pLIVE-Foxo1-AAA). Primary hepatocytes transfected with pLIVE-Foxo1-AAA displayed elevated Foxo1 and Hamp1 expressions (supplemental Figure 6B-D).
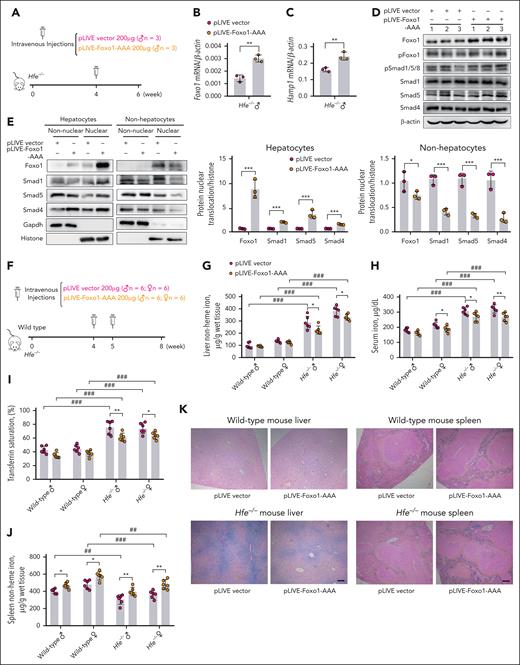
At first, to validate the specificity of Foxo1 overexpression in vivo, we performed a single injection of pLIVE-Foxo1-AAA (200 μg) in Hfe−/− mice via the tail vein for 2 weeks (Figure 7A). Compared with Hfe−/− mice receiving an empty vector, mice receiving pLIVE-Foxo1-AAA displayed increased Foxo1 (Figure 7B), alongside mildly increased Hamp1 (Figure 7C) and Id1 expressions (supplemental Figure 6E) in hepatocytes. Enhanced pSmad1/5/8 (Figure 7D), nuclear Foxo1, Smad1, Smad4, and Smad5 were observed in hepatocytes with high specificity (Figure 7E). These results were further confirmed by the immunofluorescence staining of Foxo1 and Smad1 proteins in the primary hepatocytes from Hfe−/− mice receiving pLIVE-Foxo1-AAA or empty vector (supplemental Figure 6F-G). Different from wild-type mice, Hfe−/− mice displayed enhanced baseline Foxo1 levels in the cytoplasm and nucleus of hepatocytes (supplemental Figure 6H), likely due to liver iron accumulation. This may explain the modest increase in Hamp1 that was found when Foxo1 was overexpressed in Hfe−/− mice (Figure 7C).
Hepatocyte-specific Foxo1 overexpression alleviates iron overload in Hfe−/− mice. (A) A scheme to illustrate the validation of specificity of pLIVE-Foxo1-AAA in vivo. Four-week-old male Hfe−/− mice received a single tail vein injection of 200 μg pLIVE-Foxo1-AAA or an empty vector for 2 weeks (n = 3 mice in each group). (B-D) In the primary hepatocytes from Hfe−/− mice receiving 200 μg pLIVE-Foxo1-AAA or an empty vector for 2 weeks, Foxo1 mRNA (B), Hamp1 mRNA (C), Foxo1 protein, and pSmad1/5/8 (D) were measured. (E) The expression specificity of pLIVE-Foxo1-AAA was analyzed in the hepatocytes and nonhepatocytes from Hfe−/− mice receiving 200 μg pLIVE-Foxo1-AAA or an empty vector for 2 weeks (left). The nuclear Foxo1, Smad1, Smad4, and Smad5 in hepatocytes and nonhepatocytes were quantified (right). Quantification of western blot was obtained from 3 independent experiments. (F) A scheme to illustrate the validation of effectiveness in reducing iron overload in vivo. Four-week-old WT and Hfe−/− mice received the tail vein injection of 200 μg pLIVE-Foxo1-AAA or an empty vector at ages 4 and 5 weeks, respectively (2 times, 200 μg each time). Mice were euthanized at age 8 weeks (n = 6 in each group). (G-N) Iron-related phenotypes of WT and Hfe−/− mice received pLIVE-Foxo1-AAA or an empty vector treatment in panel F. (G-J) Liver nonheme iron (G), serum iron (H), transferrin saturation (I), and spleen nonheme iron levels (J) were measured. (K) Perls iron staining of the liver and spleen tissues. (L-N) Liver end point Foxo1 mRNA (L), Hamp1 mRNA (M), and serum hepcidin levels (N) were measured in WT and Hfe−/− mice receiving pLIVE-Foxo1-AAA or an empty vector in panel F. WT and Hfe−/− mice were C57BL/6J background. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the Student t test for 2 group comparison or the 1-way ANOVA with the Sidak post hoc test for multiple comparisons in sex- and genotype-matched data. #P < .05; ##P < .01; ###P < .001. The P values were calculated by the 1-way ANOVA with the Sidak post hoc test for multiple comparisons between WT mice and Hfe−/− mice in sex-matched data.
Hepatocyte-specific Foxo1 overexpression alleviates iron overload in Hfe−/− mice. (A) A scheme to illustrate the validation of specificity of pLIVE-Foxo1-AAA in vivo. Four-week-old male Hfe−/− mice received a single tail vein injection of 200 μg pLIVE-Foxo1-AAA or an empty vector for 2 weeks (n = 3 mice in each group). (B-D) In the primary hepatocytes from Hfe−/− mice receiving 200 μg pLIVE-Foxo1-AAA or an empty vector for 2 weeks, Foxo1 mRNA (B), Hamp1 mRNA (C), Foxo1 protein, and pSmad1/5/8 (D) were measured. (E) The expression specificity of pLIVE-Foxo1-AAA was analyzed in the hepatocytes and nonhepatocytes from Hfe−/− mice receiving 200 μg pLIVE-Foxo1-AAA or an empty vector for 2 weeks (left). The nuclear Foxo1, Smad1, Smad4, and Smad5 in hepatocytes and nonhepatocytes were quantified (right). Quantification of western blot was obtained from 3 independent experiments. (F) A scheme to illustrate the validation of effectiveness in reducing iron overload in vivo. Four-week-old WT and Hfe−/− mice received the tail vein injection of 200 μg pLIVE-Foxo1-AAA or an empty vector at ages 4 and 5 weeks, respectively (2 times, 200 μg each time). Mice were euthanized at age 8 weeks (n = 6 in each group). (G-N) Iron-related phenotypes of WT and Hfe−/− mice received pLIVE-Foxo1-AAA or an empty vector treatment in panel F. (G-J) Liver nonheme iron (G), serum iron (H), transferrin saturation (I), and spleen nonheme iron levels (J) were measured. (K) Perls iron staining of the liver and spleen tissues. (L-N) Liver end point Foxo1 mRNA (L), Hamp1 mRNA (M), and serum hepcidin levels (N) were measured in WT and Hfe−/− mice receiving pLIVE-Foxo1-AAA or an empty vector in panel F. WT and Hfe−/− mice were C57BL/6J background. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. The P value was calculated by the Student t test for 2 group comparison or the 1-way ANOVA with the Sidak post hoc test for multiple comparisons in sex- and genotype-matched data. #P < .05; ##P < .01; ###P < .001. The P values were calculated by the 1-way ANOVA with the Sidak post hoc test for multiple comparisons between WT mice and Hfe−/− mice in sex-matched data.
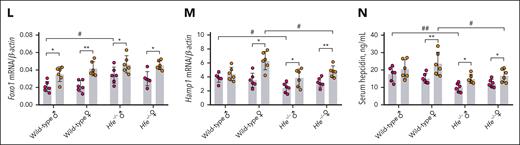
Next, to assess the effectiveness of Foxo1 overexpression in reducing iron overload in vivo, a larger dose of pLIVE-Foxo1-AAA (2 times; 200 μg each time) was administered to Hfe−/− mice for a longer time (4 week; Figure 7F). A larger dose and longer expression duration of pLIVE-Foxo1-AAA significantly decreased liver nonheme iron (Figure 7G), serum iron (Figure 7H), and transferrin saturation levels (Figure 7I) in Hfe−/− mice. Wild-type and Hfe−/− mice receiving pLIVE-Foxo1-AAA displayed increased spleen nonheme iron concentrations (Figure 7J). The changes in liver and spleen iron levels were confirmed by Perls iron staining (Figure 7K). Unchanged heart or pancreas nonheme iron concentration (supplemental Figure 6I-J) was observed in either wild-type or Hfe−/− mice. After the 4-week treatment, Hfe−/− mice receiving pLIVE-Foxo1-AAA still maintained higher levels of hepatic Foxo1 mRNA (Figure 7L), Hamp1 mRNA (Figure 7M), Id1 mRNA (supplemental Figure 6K), Foxo1 protein (supplemental Figure 6L), pSmad1/5/8 (supplemental Figure 6L), and serum hepcidin levels (Figure 7N) than mice receiving an empty vector.
Discussion
Hepcidin levels are regulated by multiple pathophysiological conditions, including but not limited to iron deficiency, oral iron administration, infectious or inflammatory diseases, hypoxia, ineffective erythropoiesis, and sex hormones. These conditions, sometimes existing simultaneously, collectively determine the output level of hepcidin through modulation of genes involved in hepcidin transcription or iron utilization.27,28
Hepcidin expression can be induced by starvation, resulting in hypoferremia and tissue iron sequestration.29,30 Hepatic hepcidin expression was correlated with the magnitude of food deprivation, as well as hepatic gluconeogenic gene expressions under food deprivation.30 Starvation-induced transcriptional factors such as PGC1α (PPARGC1A)29 and CREBH,31 were identified to regulate hepcidin expression. PGC1α is a key factor regulating energy metabolism, and it activates a variety of transcription factors promoting hepatic gluconeogenesis, including CREBH32 and FOXO1.33 CREBH binds to the human hepcidin promoter and activates hepcidin expression.31 In mice lacking Creb3l3 (CREBH), hepcidin failed to be activated under food deprivation, but serum hepcidin and hepatic Hamp1 expression levels of Creb3l3−/− mice were unchanged at basal condition under fed condition.29
Similar to Creb3l3−/− mice,29 Foxo1 was also observed to be involved in the transcriptional activation of hepcidin under food deprivation in this study. Hepatic Foxo1 ablation blunted the starvation-induced hepcidin expression in mice (supplemental Figure 7A-C). Under fed condition, alterations in hepcidin and iron-related phenotypes in Foxo1LKO mice were relatively modest compared with those of mice with BMP-SMAD pathway genes knockout. The transcriptional regulation of Foxo1 on hepcidin becomes more important under HID. It may explain why no alteration of iron metabolism was identified in previous studies of Foxo1LKO mice maintained on ND.26,34,35Foxo1 gene ablation at different stages may produce nuanced differences in iron-related phenotypes. Knockout of the Foxo1 gene at age 4 weeks (Foxo1iLKO mice) yielded more severe iron accumulation than embryonic knockout (Foxo1LKO mice). We cannot overlook the compensation potentially offered by other Foxo family members (eg, Foxo3, Foxo4, and Foxa2) in mice undergoing embryonic knockout of the Foxo1 gene.36 In the luciferase reporter vector lacking Foxo binding sties, a clearer reduction in hepcidin expression activities was found (Figure 6E). The mutation in Foxo binding sites may influence the binding of multiple Foxo proteins, providing evidence to support the existence of functional redundancy across Foxo proteins at the transcriptional level. Major metabolic alterations in the embryonic knockout of Foxo1 in the liver include a 40% reduction of blood glucose at birth and a 30% reduction in adult mice after 48 hours of fasting.26 In primary hepatocytes under glucose starvation from 3 to 36 hours, no difference was found in hepcidin expressions (supplemental Figure 8), excluding the possibility that hepcidin can be affected by low glucose level in Foxo1LKO mice.
Foxo binding sites within hepcidin promoter are critical for the maintenance of both basal and ligand-induced hepcidin transcription activity. Gene transcription coregulated by Foxo1 and Smad proteins has been characterized in previous studies. For instance, 1 Foxo binding site lies 25 bp upstream of a Smad binding site in the proximal region of the myostatin gene promoter, and Foxo1 (Foxo1 binding site as well) is required for basal and TGF-β-Smad2/3/4–mediated myostatin expression in myoblasts.37 The proximal Foxo and Smad binding sites in the myostatin gene are fully conserved across 5 mammal species.37 In another study conducting an RNA interference screen in human epithelial cells, 11 genes involved in stress and adaptive responses were identified to be jointly controlled by Foxo and Smad proteins.38 However, the number and relative position of colocalized Foxo and Smad binding sites are substantially differed across these gene promoters.38 In the murine Hamp1 promoter, mutation of the distal Foxo binding site (−1286/−1292) caused a more modest decrease in Hamp1 reporter activity than the mutation of the proximal Foxo binding site (−758/−764; Figure 6E). The mutation of the proximal Foxo binding site completely abolished the response of hepcidin to holo-Tf or BMP6 (Figure 6E). The difference between distal and proximal Foxo binding sites was consistent with the regulatory activity of the 2 Smad binding sites within the Hamp1 promoter; that is, deletion of the distal Smad binding sites resulted in a more modest decrease in the reporter activity than the proximal Smad binding site deletion.39
The function of Foxo1 as an iron regulator allows for a novel perspective on the intricate correlation between iron and glucose metabolism. Dietary iron appears to instigate both hepatic hepcidin synthesis and glucose production, suggesting a direct influence on blood glucose levels through Foxo1-mediated hepatic glucose output. This hypothesis is supported by data from mice receiving an iron dextran injection, in which an augmentation in gluconeogenesis via the hepatic protein kinase A and Foxo1 axis was evident.40 Similarly, mice fed a HID exhibited amplified hepatic hepcidin levels, alongside heightened gluconeogenesis and fasting glucose concentrations.41 These findings resonate with the hypothesis that the metabolism of energy substrates and iron sensing are synchronized to modulate cellular energy utilization.42
The complete iron-regulatory signaling of Foxo1 in hepatocytes remains to be defined. Foxo1 is a cytosolic transcription factor but not a cell membrane receptor, and therefore, it is not likely that it directly responds to extracellular iron. Therefore, Foxo1 may act as a downstream effector, whose activity was activated upon the engagement of extracellular iron or BMP6, as well as BMP2 (supplemental Figure 9). Conversely, insulin, known to promote pFoxo1 and reduce the nuclear Foxo1 levels, was shown to suppress hepcidin expression in hepatocytes (supplemental Figure 10), indicating that extracellular iron and insulin signaling imposed an opposite regulation on hepcidin via Foxo1. Unlike hepatocytes, the iron treatment decreased FOXO1 acetylation without altering its phosphorylation in human 3T3-L1 adipocytes.43 This discrepancy suggests a possible tissue specificity in the regulatory pattern of Foxo1, with distinct regulatory mechanisms and downstream targets. Despite the protein modification by iron, it is still unclear how chronic iron treatment (3 days and 2 weeks) increased the total Foxo1 protein levels in hepatocytes (supplemental Figure 1C; Figure 5A), which differs in acute iron treatment (3 hours; supplemental Figure 1C) and short-term treatments in cultured hepatocytes (supplemental Figure 1D-F).
Foxo1 overexpression enhanced pSmad1/5/8 and Hamp1 mRNA expressions in the hepatocytes of Hfe−/− mice (Figure 7). However, the marked nuclear translocation of Foxo1 and Smad proteins did not yield a comparable elevation in hepatic hepcidin expression (Figure 7C). Hfe−/− mice exhibited an enhanced nuclear Foxo1 in hepatocytes (supplemental Figure 6H), which may blunt hepcidin induction when Foxo1 was further expressed. In addition, male wild-type mice tended to have a blunted increase of Hamp1 (Figure 7M), Id1 (supplemental Figure 6K), and serum hepcidin (Figure 7N) when pLIVE-Foxo1-AAA was administered. Testosterone was reported to repress hepcidin expression, and hepcidin expression was found to be more repressed in the males than females.44 Therefore, the interaction between testosterone and hepcidin may attenuate hepcidin induction. However, iron overload may offset the inhibitory influence of testosterone on hepcidin expression, because iron overload in mice tends to present as hypogonadism with a significant reduction in serum testosterone.45,46 This mechanism may also explain why some phenotypes were only seen in the female Foxo1LKO mice (Figures 3 and 4).
In summary, this study identified a critical role of Foxo1 in hepcidin regulation and systemic iron homeostasis. The iron-modulating property of Foxo1 also confers its therapeutic value in treating iron disorders. Future research should focus on elucidating the specific iron-sensing pathways of Foxo1 and discerning the contributions of various Foxo proteins in iron metabolism.
Acknowledgments
This work was supported by the National Key R&D Program of China (2023YFF1105201; P.A.), National Natural Science Foundation of China (32371229 to P.A.; 31970717 and 82170429 to Y.L.; 81900786 to H.G.; 32171171 to H.W.; and 32000820 to L.J.), the Chinese Universities Scientific Fund (2020TC015; Y.L.), the Beijing Municipal Natural Science Foundation (7222111; Y.L.), CACMS Innovation Fund (CI2021A04708; M.S.), the Pinduoduo-China Agricultural University Research Fund (PC2023B01014; Y.L.), the 111 project from the Education Ministry of China (B18053), and the State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, Chinese Academy of Medical Sciences (2024GZkf-05; J.L.).
Authorship
Contribution: P.A., Y.L., J.L., and H.G. conceived the study; T.X., X.Z., W.Z., S.W., Y.Z., J.H., L.J., J.S., M.S., and H.W. performed the animal and cell experiments; T.X., X.Z., J.H., P.A., Y.L., J.S., M.S., and J.L. analyzed the data; P.A., T.X., Y.L., and H.G. drafted manuscript; and Y.H. and J.L. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hong Gao, College of Life Sciences, Wuhan University, Wuchang District, Wuhan 430072, China; email: honggao@whu.edu.cn; Junjie Luo, Department of Nutrition and Health, China Agricultural University, 10 Tianxiu Rd, Haidian District, Beijing 100193, China; email: luojj@cau.edu.cn; Yongting Luo, Department of Nutrition and Health, China Agricultural University, 10 Tianxiu Rd, Haidian District, Beijing 100193, China; email: luo.yongting@cau.edu.cn; and Peng An, Department of Nutrition and Health, China Agricultural University, 10 Tianxiu Rd, Haidian District, Beijing 100193, China; email: an-peng@cau.edu.cn.
References
Author notes
T.X. and X.Z. contributed equally to this work.
Original data are available on request from the corresponding authors, Hong Gao (honggao@whu.edu.cn), Junjie Luo (luojj@cau.edu.cn), Yongting Luo (luo.yongting@cau.edu.cn), and Peng An (an-peng@cau.edu.cn).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal