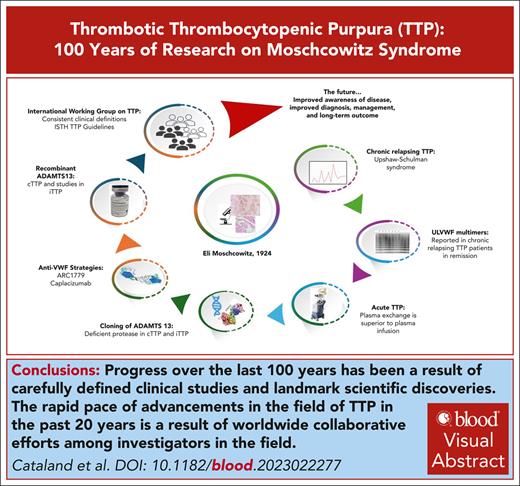
Visual Abstract
In the 100 years since Eli Moschcowitz reported the first case of thrombotic thrombocytopenic purpura (TTP), there has been remarkable awareness and progress in the diagnosis and management of this rare blood disorder. This progress initially was the result of careful clinical observations followed by well thought-out therapeutic interventions, with dual goals of both improving outcomes and discerning the pathophysiology of TTP. The discovery of the ADAMTS13 protease set in motion the efforts to more accurately define the specific etiologies of thrombotic microangiopathies (TMAs) based on objective, scientific data rather than clinical characterizations alone. This accurate differentiation led to better and more revealing clinical trials and advancements in the treatment of TTP and other TMAs. Further advances followed and included improvements in immune-suppressive therapy and targeted therapies of immune-mediated TTP (iTTP; caplacizumab) and congenital TTP (cTTP; recombinant ADAMTS13). The longitudinal study of patients with TTP revealed the unexpected risk for long-term complications in both patients with iTTP and those with cTTP in remission. Ongoing studies aim to further understand the prevalence, mechanisms, and appropriate screening for these mood disorders, neurocognitive deficits, and cardiovascular complications that develop at remarkably high rates and are associated with a decreased life expectancy. These discoveries are a result of the collaborative efforts of investigators worldwide that have been fostered by the frequent interactions of investigators via the International TTP Working Group meetings and TMA workshops held regularly at international meetings. These efforts will support the rapid pace of discovery and improved understanding of this rare disease.
Introduction
Because we mark 100 years since the description of the first thrombotic thrombocytopenic purpura (TTP) case by Eli Moschcowitz in 19241 (Box 1), it is appropriate to review the remarkable progress in our understanding and treatment of TTP. The index case was that of a 16-year-old female who became acutely ill from otherwise previously good health. She died after a short hospital stay, and the postmortem examination demonstrated widespread microvascular thrombi involving the heart, kidneys, and the spleen. Although it may be debated whether the 16-year-old female had congenital TTP (cTTP) or immune-mediated TTP (iTTP), there is no question that her case clearly demonstrates the course, progression, and life-threatening nature of TTP. This narrative will attempt to present the patients, physicians, and scientists who contributed to our stepwise understanding of TTP, distinguishing it from a more typical and detailed review article on TTP, of which there have been several comprehensive and informative review articles published.2-5 Advances in knowledge on rare diseases such as TTP commonly occur in leaps after astute observations, improvement of scientific methods, and clinical trials (Figure 1). Although historical reviews often omit debates and opposing viewpoints, we hope to capture these to descriptively “paint the picture of progress” in TTP over the past 100 years.
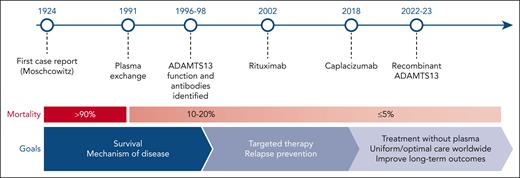
Timeline of important clinical and scientific landmarks over the past 100 years in the context of the remarkable improvements in the treatment outcomes in TTP and several key figures in the evolution in our understanding of both iTTP and cTTP. Figure adapted courtesy of Shruti Chaturvedi.
Timeline of important clinical and scientific landmarks over the past 100 years in the context of the remarkable improvements in the treatment outcomes in TTP and several key figures in the evolution in our understanding of both iTTP and cTTP. Figure adapted courtesy of Shruti Chaturvedi.
BOX 1
Eli Moschcowitz was born on 2 August 1879 in Girált, Kingdom of Hungary (today Giraltovce, Slovakia), into a Jewish family of 9 children. He was the youngest child of Rosa/Rezi (née Friedlander) and Moritz Moschcowitz. The family emigrated to the United States in 1881, sailing on board the ship called Gellert from Hamburg. Eli Moschcowitz graduated from the College of Physicians and Surgeons of Columbia University in 1900 and became an American citizen in 1902. After years of residency at the Mount Sinai Hospital, he traveled to Germany in 1903 to work in Ludwig Pick's laboratory. This training led to his appointment as a pathologist at the Beth Israel Hospital in New York. From 1920 until his retirement, he worked at The Mount Sinai Hospital. During his career, he authored dozens of publications on various fields on medical science. He was an excellent clinician-scientist, also being the first to describe eosinophilia with allergic reactions.
In addition to being a scholar, he was an epicure, a bibliophile, and a lover of music, art and traveling. He retired as one of the directors of Mount Sinai, as well as professor of clinical medicine at his alma mater. After retirement, he remained an active consultant and he also maintained his private practice up until the day of his death. He died at the age of 84 in New York City on 23 February 1964.
Courtesy of Andor Tóth
Eli Moschcowitz. Source: Journal of The Mount Sinai Hospital, volume 12, 1945 (https://archive.org/details/journalofmountsi1219moun/page/n5/mode/2up). © 1945 The Mount Sinai Hospital. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0).
Eli Moschcowitz. Source: Journal of The Mount Sinai Hospital, volume 12, 1945 (https://archive.org/details/journalofmountsi1219moun/page/n5/mode/2up). © 1945 The Mount Sinai Hospital. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0).
TTP as a disease
After Moschcowitz’s initial description, Singer et al in 1947 first noted that although thrombocytopenic purpura was relatively common, thrombocytopenic purpura associated with multiple platelet thrombi was sufficiently rare to justify the report of an additional case. They recognized this disorder as a definite disease and gave the name “thrombotic thrombocytopenic purpura.”6 Singer’s report stressed the clinical presentation and the postmortem histological pattern and put forth a theory that the thrombocytopenia resulted from a “disappearance of the platelets from the circulation due to the formation of the myriads of platelet thrombi.” Singer described the clinical features that eventually became the “pentad of TTP” as defined by Amorosi and Ultmann in 19667 in a review of 271 cases, including 16 of their own patients. Amorosi and Ultmann also hypothesized that TTP could be an autoimmune or vascular disease after recognizing the parallels between TTP and systemic lupus erythematosus. The “pentad of TTP” (thrombocytopenia, red cell fragmentation, neurologic injury, renal injury, and fever), although formerly helpful for the characterization and consideration of the diagnosis of TTP, was eventually replaced by more sensitive diagnostic criteria of thrombocytopenia and microangiopathic hemolytic anemia without any other explanation. These more streamlined diagnostic criteria became necessary after reports on the effectiveness of plasma-based treatment of TTP, to allow for the more rapid initiation of treatment for this once uniformly fatal disease. One expert has since stated that he “spent the first half of his career teaching the pentad of TTP, and the last half of his career teaching people to forget the pentad of TTP” to prevent delays in the initiation of treatment (James N. George, personal oral communication).
Early treatment of TTP
With no known therapy for TTP, the diagnosis was usually made at autopsy. Rubinstein et al initially reported the achievement of remission in a case of TTP treated with fresh, whole-blood exchange.8 Bukowski et al also reported 2 cases of TTP successfully treated with plasmapheresis after a series of patients with TTP had been treated at their institution with whole-blood exchange transfusions.9,10 The second of these 2 patients interestingly presented in the third trimester of pregnancy and responded rapidly to the initial therapy, suggesting this may have been a case of cTTP.
Two articles published jointly in the New England Journal of Medicine in 1991 began to define TTP more clearly and set the standard for the treatment of TTP that is still relevant today. Using the pentad to define the study population, Bell et al11 considered TTP and hemolytic uremic syndrome (HUS) as “parts of a spectrum” and used the term “thrombotic thrombocytopenic purpura–hemolytic uremic syndrome.” The degree of renal injury in enrolled patients suggests that there was a significant number of patients who today might be characterized as having a thrombotic microangiopathy (TMA) distinct from TTP. Patients with “minimal symptoms” received steroids alone (200 mg of prednisone), and symptomatic patients (neurologic symptoms and rapid decrease of hematocrit and platelet count) received steroids and plasma exchange. The study concluded that effective treatment was available for TTP-HUS, with a 91% overall survival and with 30 of 108 patients (28%) responding to prednisone alone. The heterogeneity of the study population limits any specific conclusions, but this report established several ideas in the field, including the following: the rationale for steroids, the effectiveness of plasma exchange, and the hypothesis that platelet infusions could be dangerous. Simultaneously, the landmark study by Rock et al included a cohort more representative of TTP based on the exclusion of patients with anuria.12 This Canadian study was the first prospective, randomized study in TTP and assigned patients to plasma infusion or plasma exchange, in addition to antiplatelet therapy (dipyridamole 400 mg daily and aspirin 325 mg daily) that was the standard of care at the time. This study defined plasma exchange to be superior to plasma infusion in terms of disease response and survival.
The use of aspirin and dipyridamole was incorporated into treatment regimens based more upon expert opinion and the presumption of efficacy in a disorder characterized by platelet-rich microthrombi. Subsequent to the study by Rock et al, a prospective, randomized study demonstrated that the addition of both aspirin and dipyridamole to plasma exchange and steroids resulted in a decreased 15-day mortality rate.13 Although this study was performed before the availability of ADAMTS13 testing to confirm the diagnosis of iTTP, the increased rates of cardiovascular complications (see “Long-term morbidity and mortality in TTP”) reported in iTTP survivors has raised again the question of the role of antiplatelet therapy as a preventive strategy for cardiovascular complications in patients with TTP.
Clinical studies as a tool to understand the pathophysiology
Clinical investigators astutely used clinical trials and data from patient observations to discern the pathophysiology of TTP. The debate as to whether it was the removal of a factor from the blood (plasmapheresis) or the transfusion of a missing factor (plasma infusion) that was important was in part addressed by the study from the Canadian apheresis group that suggested the superiority of plasma exchange over infusion.12 However, there was still clearly benefit for patients with TTP after plasma infusion alone. Much of the conflicting data may be explained by the intermixing of patients with cTTP and those with iTTP in studies, but the sequential treatment and perceptive observations of a pregnant patient (likely cTTP) reported by Byrnes et al14 accurately surmised that plasma contained the “missing factor” in patients with TTP. Additionally, in 1978, Jefferson Upshaw reported the case of a 29-year-old female who would today be diagnosed with cTTP, with 32 episodes of thrombocytopenia and precipitating factors that included infection, pregnancy, and pancreatitis. Upshaw’s studies included the observation that there was an increase in the survival of radiolabeled platelets and red blood cells after plasma infusions.15 The resulting condition, Upshaw-Schulman syndrome, today is synonymous with cTTP.
In studies of 4 patients clinically characterized with chronic relapsing TTP that included Upshaw’s and Schulman’s cases,16,17 Moake et al accurately hypothesized that a defect in a previously unrecognized regulatory process to metabolize released ultralarge plasma “factor VIII: von Willebrand factor (VWF) multimers” was the cause of the chronic relapsing TTP.18 In this seminal work, ultralarge VWF multimers were demonstrated to be present in remission but absent during acute relapse of disease, arguing that they were consumed in the development of the microvascular thrombotic disease. Furthermore, Moake et al also demonstrated the disappearance of circulating ultralarge VWF multimers (and the subsequent reappearance) in 3 patients from the same cohort with chronic relapsing TTP who received fresh frozen plasma, cryosupernatant plasma, or plasma exchange with fresh frozen plasma replacement.19 These prescient studies were the initial steps that culminated eventually in the discovery of what we know today as the metalloproteinase enzyme, ADAMTS13.
Although Furlan et al20 and Tsai21 simultaneously reported the purification of a new VWF-cleaving protease from normal human plasma, the work for this discovery was also paved by Ruggeri et al, who had identified the cleavage site for the VWF subunit by the protease to be Tyr1604-Met1605.22 Mild denaturation of VWF or increased shear forces were also reported to enhance VWF cleavage by this protease.20,21,23 The VWF-cleaving protease was subsequently purified, and the amino acid sequence was identified, leading to the designation of this protease as the 13th member of the ADAMTS family with the gene localized to chromosome 9q34.24-28 The identification of ADAMTS13 set into motion the rapid sequence of events and advances in the diagnosis and treatment of both the immune-mediated and congenital forms of TTP that we have seen over the last 20 or more years.
Differentiation of TTP and HUS
It had been argued that the differentiation of TTP from HUS was not necessary because plasma exchange was the best available initial treatment for both conditions. This would eventually change with a better understanding of the pathophysiology because atypical HUS or complement-mediated TMA was identified as a distinct disorder from TTP,29 driven by overactivation of the alternative pathway of the complement system, whereas TTP is a result of a congenital or acquired, severe deficiency of ADAMTS13 function. The development of remarkably efficient complement blockade therapy30 further solidified the need to differentiate this complement-mediated TMA from other TMAs.
Furlan et al and Tsai and Lian simultaneously reported that ADAMTS13 activity could be used to differentiate patients who were previously clinically characterized as either TTP or HUS based on the severity of renal injury.31-33 Although this could be seen as an oversimplification, the severity of renal injury has been shown to help predict the likelihood of severely deficient ADAMTS13 activity in patients with TMA34-38 and forms the basis for predictive models of ADAMTS13 activity including the French score36 and the PLASMIC score.39 Both Furlan et al and Tsai et al in these initial reports demonstrated that patients clinically classified as TTP indeed had severely deficient ADAMTS13 activity in contrast to patients characterized as atypical HUS who were not severely deficient.31,32 Similar data were also reported in pediatric patients with TTP.40 Additionally, both discovered the presence of immunoglobulin G inhibitors of ADAMTS13 as the cause of the acquired deficiency. From 6 familial cases with ADAMTS13 deficiency but no inhibitor, they suggested that this disorder was due to a “constitutional deficiency of the protease,” which today would be identified as cTTP.31 Subsequently, the investigation of genetic risk factors associated with iTTP identified loci in the HLA system promoting (DRB1∗11 and DQB1∗03) or preventing (DRB1∗04) anti-ADAMTS13 antibody production.41-44 In Japanese patients with iTTP, DRB1∗08:03, DRB3/4/5∗blank, and DQB1∗06:01 were found more frequently.45 Black patients did not have a specific iTTP-associated allele, and the protective HLA DRB1∗04 was often found significantly less compared with that of White patients.46
Recombinant ADAMTS13 protease
The discovery and characterization of ADAMTS13 led to intense efforts to produce a recombinant form of the protease. Plaimauer et al47 reported the expression of fully functional ADAMTS13 in HEK 293 cells that was shown to correct the defective VWF proteolytic activity when added in vitro to a plasma sample from a patient with cTTP.48 Years of work on recombinant human ADAMTS13 (rADAMTS13) culminated in the first-in-human safety and pharmacokinetics study of rADAMTS13 in 2017, in which a dose-dependent increase in the ADAMTS13 activity confirmed the catalytic activity of the rADAMTS13 toward VWF.49 Additional data from a compassionate access program before regulatory approval also reported the successful use of recombinant ADAMTS13 in a pregnant patient with cTTP, a neonate, and an adult patient with recurrent cerebrovascular accidents.50-52 A follow-up phase 3 study53 led to regulatory approval in the United States and by the European Medicines Agency for rADAMTS13 for the treatment of cTTP.
Equally interesting is the role of rADAMTS13 in iTTP. In this regard, in vitro studies from Plaimauer et al demonstrated the ability of rADAMTS13 to overcome neutralizing antibodies and restore ADAMTS13 protease activity,54 and preliminary data from a phase 2 study of recombinant ADAMTS13 in iTTP suggest efficacy in patients with acute iTTP when given in concert with plasma exchange.55
Second hit hypothesis in TTP
Although the discovery and understanding of the key role of ADAMTS13 in TTP pathophysiology was pivotal, severe ADAMTS13 deficiency alone is often not sufficient for the development of an acute TTP episode, which is particularly relevant to cTTP. An ADAMTS13 knockout mouse reported by Motto et al was remarkable in that the mice did neither spontaneously develop thrombocytopenia nor a clinical phenotype reminiscent of TTP. Only after the genetic introduction of a high basal VWF level did a subset of studied mice develop thrombocytopenia spontaneously.56 In this model, a trigger or “second hit” that included Shiga toxin or VWF infusion57 was required to more uniformly create disease in the mice. Although this may have been surprising, clinical observations also supported these observations. In a study from the French Thrombotic Microangiopathies Registry, 24% of women experiencing their first acute TTP during pregnancy were eventually diagnosed with cTTP for the first time in early adulthood.58 The “second hit” model also fits well with what is known about infections, excessive alcohol consumption, and pregnancy as common triggers of acute TTP episodes in patients with cTTP.59,60
During the recent COVID-19 pandemic, several cases of acute iTTP, either first manifestations or recurrent events, were reported during acute severe acute respiratory syndrome coronavirus 2 infection.61,62 Moreover, reports on acute TTP after COVID-19 vaccination were published, suggesting the latter as a trigger for acute TTP. Nevertheless, 2 well-conducted systematic epidemiologic studies63,64 provided reassurance that COVID-19 vaccination did not induce autoantibodies against ADAMTS13 and clinical TTP, with the possible theoretical exception of patients with a silent acquired ADAMTS13 deficiency. Similarly, a survey of patients with cTTP from the International hereditary TTP registry (86 of 122 adult patients with cTTP responded) showed that COVID-19 vaccination did not induce acute cTTP bouts, whereas 3 acute episodes occurred in 12 patients with cTTP during COVID-19 infection.65 Thus, the risk of acute iTTP or cTTP is much higher after severe acute respiratory syndrome coronavirus 2 infection than after COVID-19 vaccination, supporting the safety of COVID-19 vaccination in patients with TTP.
Immune-suppressive therapy and iTTP
Even before the rationale for the use of steroids was known,11 patients were routinely given corticosteroids. After Bell’s report that corticosteroids alone had efficacy in TTP (28% of patients responded to prednisone therapy alone), there are surprisingly few reported data that demonstrate the efficacy of steroids to suppress the production of the anti-ADAMTS13 antibodies. The summary of these studies suggested that patients treated with higher-dose steroids (methylprednisolone 10 mg/kg per day) for the first 3 days, followed by 2.5 mg/kg per day along with plasma exchange, had a significantly higher remission rate than those who received the standard dose (methylprednisolone 1 mg/kg per day) along with plasma exchange.66 Additional data from Cataland et al comparing prednisone 1 mg/kg per day with plasma exchange with cyclosporine with plasma exchange clearly demonstrated that there was suppression of the anti-ADAMTS13 antibodies and recovery of ADAMTS13 activity; in the majority of patients, however, recovery of ADAMTS13 activity to >20% did not begin for at least 2 to 3 weeks.67
The effectiveness of rituximab in the treatment of iTTP was first reported in 2002 in a case series of 3 patients with plasma-refractory iTTP.68 Soon thereafter, rituximab was increasingly used in TTP in association with plasma exchange and corticosteroids, with clinical trial data confirming the efficacy first as a salvage therapy and then as a frontline therapy to prevent or delay recurrences of iTTP.69-71 Concerns were initially raised regarding the potential risk of infection and other complications in patients with iTTP treated with rituximab that may have initially limited its more widespread use; however, rituximab has stood the test of time with increasing reports confirming both the efficacy and safety. Although there are no randomized, controlled trials, the mounting data suggested nonetheless that the use of rituximab as part of the initial therapy with corticosteroids and plasma exchange at least delayed relapses of iTTP, if not preventing them.72
In addition to the upfront use of rituximab in acute iTTP, there was an increasing recognition that patients with severely deficient ADAMTS13 activity in remission had an increased risk of relapse.69,73,74 The relapse risk was also shown to be greatest in younger patients with severe ADAMTS13 deficiency, with a 30% to 40% risk of relapse in the 3 months after the measurement.75 This recognition that relapse risk was tied to ADAMTS13 activity led investigators to treat patients preemptively with immune-suppressive therapy to improve ADAMTS13 activity and prevent relapse. In this context, the ease of use, efficacy, and favorable side-effect profile of rituximab led to its increased use to prevent relapses over other agents including cyclosporine and mycophenolate mofetil.67,73,74,76-78 Data from both the UK TTP Registry and the French TMA Registry consistently showed the ability of rituximab to improve ADAMTS13 activity and prevent clinical relapse, leading to the routine use of preemptive therapy with rituximab. Although extremely effective, it should not be surprising that there are data to suggest that there may be racial differences in the responses to rituximab.79 Black patients treated with rituximab at initial diagnosis experienced a shorter relapse-free survival than White patients; similarly, in patients with relapsed iTTP, Black patients did not achieve the same improvement in relapse-free survival seen in White patients. However, the reason for these inferior outcomes in Black patients treated with rituximab are not clear at this time.
Although splenectomy was one of the earliest attempted primary treatments for TTP before the reported effectiveness of plasma-based therapies, there are data that suggest the efficacy of splenectomy to prevent iTTP relapses.80 This retrospective series from Kappers-Klunne et al80 studied the effectiveness of splenectomy for both refractory iTTP as well as chronic relapsing iTTP as a preemptive therapy. This study demonstrated the benefit of splenectomy in chronic relapsing patients, decreasing the relapse rate from 0.74 relapses per patient-year to 0.10 relapses per patient-year after splenectomy in the chronic relapsing cohort after 10 years of follow-up. Although these data are retrospective, they support the hypothesis that splenectomy is a reasonable consideration in patients who have failed to respond to or were intolerant of preemptive immune-suppressive therapy. Interestingly, the first patient with iTTP in whom immunoglobulin G anti-ADAMTS13 antibodies were identified eventually underwent splenectomy and has since remained in clinical remission for over 20 years.81 Although the topic of splenectomy in iTTP can be guaranteed to stimulate a vigorous debate with diverging opinions (even among the authors of this paper), splenectomy as a mechanism of immune modulation in iTTP deserves additional study as a potentially more affordable and widely available form of immune modulation to prevent relapse in patients with iTTP.
Targeted therapies in TTP
Caplacizumab, a nanobody that binds to the A1 domain of VWF, thereby blocking VWF-platelet interactions, became the first regulatory-approved therapy for iTTP in 2018. The first in-patient study of an anti-VWF agent in iTTP, however, was with ARC1779, an aptamer that similarly targeted the A1 domain of VWF, blocking VWF-platelet interactions. Initially considered as a treatment for acute cardiac syndromes,82 it was administered IV as a continuous infusion. The pilot study of ARC1779 in iTTP enrolled only 9 patients before the study was stopped for financial reasons.83 Although the study demonstrated the safety and proof of concept of VWF inhibition in iTTP, enrollment in this study was slowed in part due to concerns regarding the potential for bleeding complications among some investigators regarding the use of an anti-VWF therapy in patients with severe thrombocytopenia.
Capitalizing on what was learned from the ARC1779 study, the development of ALX-0081 (Ablynx, Ghent, Belgium [now part of Sanofi]), or caplacizumab as it later became known, moved forward, bolstered by the safety data from the ARC1779 study, as well as the ease of subcutaneous administration in concert with plasma exchange. However, enrollment remained slow to the phase 2 Titan study due to remaining concerns regarding the risk of bleeding with ALX-0081 in patients with thrombocytopenia,84 which limited the number of participating sites and led to premature closure of the study. However, the remarkable clinical response data of the caplacizumab arm over the placebo arm (normalization of platelet count, 3.0 vs 4.9 days; exacerbation rate, 8% vs 28%) and safety profile in the Titan study increased the confidence and enthusiasm among investigators for this targeted approach to the treatment of iTTP. The phase 3, open-label, randomized controlled Hercules study of ALX-0081 that followed subsequently enrolled 145 patients over 17 months,85 whereas the previous Titan study took 39 months to enroll 75 patients. These studies led to caplacizumab becoming the first drug to receive regulatory approval for the treatment of acute iTTP. The ability of caplacizumab to block exacerbations of iTTP and the mounting evidence on its impact on acute mortality from “real-world” trials in iTTP86-88 have led to its increased use, despite questions raised regarding its cost effectiveness.89 Because current therapeutic regimens combining plasma exchange, immunosuppression, and caplacizumab have led to improved response and exacerbation rates in patients with iTTP,86-88 it is noteworthy that diagnostic delay now likely remains the primary limiting factor to further improve survival.
Long-term morbidity and mortality in TTP
Despite the remarkable advances in the diagnosis and treatment of iTTP over the past 40+ years, the recognition now that TTP (iTTP and cTTP) is a chronic disease with chronic sequelae that affect survival may be the most significant paradigm shift in recent years. Previously thought to be an acute disease that was treated and resolved, we now recognize the significant risks for long-term vascular and other complications that contribute to a decrease in the overall survival in patients previously diagnosed with iTTP.90,91
The very first studies looking at patient recovery and functioning after an acute iTTP episode had their genesis in TTP patient group meetings first held by the Oklahoma TTP Registry beginning in 1996. In these meetings (that were held 3 times per year), patients with TTP surprisingly reported that, despite clinical remission, they still had not recovered back to their previous level of functioning. The self-reported issues they experienced included difficulties with cognition and endurance.92 Since this first recognition of survivorship issues in patients with iTTP, numerous groups have reported an increased incidence of mood disorders, anxiety, post–traumatic stress disorder, and cardiovascular complications in patients in remission from a prior iTTP diagnosis.93,94
Cardiovascular disease is known to be a leading cause of death in TTP survivors,90,95 but the incidence and cumulative prevalence of these events in patients with TTP in remission remains to be clearly defined. The prevalence of major adverse cardiovascular events (MACEs; nonfatal or fatal myocardial infarction, stroke, and cardiac revascularization) in remission in 2 large iTTP cohorts was recently reported by Brodsky et al.96,97 In this study, 28.6% of patients experienced a MACE over a median of 7.6 years of follow-up, with cerebrovascular accidents occurring in 18.2%, nonfatal myocardial infarction in 6.6%, and cardiac revascularization occurring in 4.9%. Compared with the general US population, these events occurred earlier in life in both men and women. Although reduced ADAMTS13 activity has been associated with an increased risk for cerebrovascular accidents in patients with iTTP in remission,97 ADAMTS13 activity in remission was not significantly associated with the development of MACE in this study.96
Similar to patients with iTTP, patients with cTTP are also at risk for cardiovascular disease. These events in patients with cTTP may be related to both acute and subacute TMA episodes,59,60 but patients with cTTP are also at risk for long-term cardiovascular complications, which may be reduced with ADAMTS13 replacement therapy.98 It is possible that there is a unifying mechanism of injury leading to these cardiovascular complications in both patients with iTTP and those with cTTP, but it is also equally possible that etiology of these long-term complications differs in terms of pathogenesis. Ongoing studies attempting to better characterize the mechanism of injury, risk factors, and the “protective level” of ADAMTS13 protease activity that needs to be achieved will be essential to determine the optimal screening and management strategies.
IWG on TTP
The very first attempt at the formalization of clinical definitions was reported by the Oklahoma and Swiss research groups in 2003.99 Harmonization of the clinical definitions for research was clearly needed, given the increase in clinical trials, especially regarding the following terms: response, remission, exacerbation, and relapse, in which exacerbation defines the continuation of a previous TTP episode and relapse a new, distinct TTP episode.99 Led by Marie Scully, the International Working Group (IWG) was formed as a grassroots movement of investigators from around the world to collaborate and agree on standardized definitions in TTP. After group meetings at several international congresses and multiple draft revisions of the manuscript, the first consensus on definitions from the IWG were published in 2017.100 An updated version was subsequently published in 2021, with new definitions of exacerbation, relapse, and remission to account for new anti-VWF therapies. New definitions of ADAMTS13 remission and relapse were also established, given the evolving approach with preemptive rituximab therapy to prevent clinical relapse.101
These IWG collaborative efforts among TTP investigators from across the world have established relationships that have led to international prospective, therapeutic trials and cooperative research projects including the “Caplacizumab 500+” project. The Capla 500+ project was a study initiated and undertaken by many international colleagues to obtain real-world evidence regarding the efficacy and safety of caplacizumab in iTTP. Despite being the first FDA and EMA–approved therapy to treat TTP in concert with plasma exchange and immune-suppressive therapy, questions were still raised regarding its safety and efficacy. The results of the Capla 500+ study that were presented at the American Society of Hematology meeting in 2023 again demonstrated the efficacy, safety, and improved patient outcomes after treatment with caplacizumab.102 Most remarkable though was the efficiency and speed by which this study was completed. Data from >1000 patients were gathered, analyzed, and reported in <12 months in the absence of any financial or regulatory support from any source including the manufacturer of caplacizumab. Without question, the collaborative relationships established by the IWG have made this and future projects possible.
Future of TTP research
The remarkable rate of discovery in both bench and clinical research is a function as much of the collaborative relationships that mark the last 20 years of progress as the technologic and scientific discoveries. The contributing authors and numerous collaborators on this manuscript span up to 50 years of work in the field, and they are responsible for the productive working relationship of this close-knit group. In the past 10 years, TTP researchers from around the world have participated in the IWG that has met at international scientific meetings to develop consensus definitions100,101 as well as regular, international TMA workshops that have been held over this same period of time. These meetings have been essential for the open sharing of ideas and discussion of important topics in the field.
Going forward, there are clearly more questions than answers, but these are the challenges that lie ahead of investigators for the next 100 years. First on the list is the role of plasma exchange and whether it is required for the treatment of acute iTTP episodes. There are 2 ongoing studies using caplacizumab (NCT05468320) and rADAMTS13 (NCT05714969), initially without plasma exchange, in acute iTTP to determine whether plasma exchange can be safely omitted. Additional questions include, among many others, the following: the appropriate role, if any, of plasma-based therapy in acute iTTP; the most effective immune-suppressive regimen for both acute and preemptive treatment of iTTP; the potential role of novel biomarkers in TTP (ADAMTS13 open vs closed conformation; VWF multimer ratio) in addition to clinical factors to predict relapse; the incidence and pathophysiology of the long-term sequelae of TTP; the role of promising N-glycan–modified forms of ADAMTS13 to prevent binding of pathogenic autoantibodies; the reason for the particular susceptibility of ADAMTS13 to autoimmune recognition103-105; and the most appropriate goal for the ADAMTS13 activity in patients with cTTP and those with iTTP to prevent acute and chronic sequelae of the disease.
Acknowledgments
There are many who are no longer with us, who deserve to be remembered for their contributions to the field, but first among these are Miha Furlan and J. Evan Sadler, to whom this article is dedicated: Furlan for his discovery of the VWF-cleaving protease that was later to be named ADAMTS13 and its acquired or congenital deficiency in TTP; and Sadler for his innumerable contributions to our understanding of TTP and ADAMTS13; he taught by example with his hard work, collaboration, and gentle spirit that were so important to progress in the field.
Authorship
Contribution: S.R.C., P.C., M.S., and B.L. collectively wrote and edited the manuscript and figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the International Working Group on Thrombotic Thrombocytopenic Purpura appears in “Appendix.”
Correspondence: Spero R. Cataland, Ohio State University, 1800 Cannon Dr, 1150F Lincoln Tower, Columbus, OH 43210; email: spero.cataland@osumc.edu.
Appendix
The members of the International Working Group on Thrombotic Thrombocytopenic Purpura are as follows: Lorenzo Alberio, Ana Antun, Cihan Ay, Pasquale Agosti, Elie Azoulay, Ross Baker, Ygal Benhamou, Tiago Boechat, Paul Brinkkötter, Shruti Chaturvedi, James Crawley, Raimondo De Cristofaro, Julio del Río Garma, Tina Dutt, Rens De Groot, Javier de la Rubia, Tanja Falter, João Farias, Kenneth Friedman, Yoshihiro Fujimura, Eleni Gavriilaki, James N. George, Nuno A. G. Graça, Wolf-Achim Hassenpflug, Cristina Pascual Izquierdo, Bérangère Joly-Laffargue, Karim Kentouche, Paul Knoebl, Koichi Kokame, Johanna Kremer Hovinga, Lucas Kühne, Paul Kyrle, Will Lester, Ilaria Mancini, Camila Masias, Masanori Matsumoto, Marshall Mazepa, Wolfgang Miesbach, Ara Metjian, Maria-Eva Mingot-Castellano, Toshiyuki Miyata, Joel Moake, Joshua Muia, Chris Patriquin, Katerina Pavenski, Zoltan Prohaszka, Flora Peyvandi, Marienn Reti, Heidi Rossmann, Kazuya Sakai, Ravi Sarode, Reinhard Schneppenheim, Marissa Schraner, Deepak Singh, György Sinkovits, Matthew Stubbs, Jan-Dirk Studt, Senthil Sukumar, Mari Thomas, Andor Tóth, Karen Vanhoorelbeke, Agnes Veyradier, Linus Völker, Anne Sophie von Krogh, Jan Voorberg, Anders Waage, J. P. Westwood, Erica Wood, Hideo Yagi, and X. Long Zheng.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal