In this issue of Blood, Kwon and colleagues1 describe the potent menin-KMT2A inhibitor JNJ-75276617 (bleximenib) and its preclinical efficacy in different leukemia models and demonstrate its activity in the presence of mutations that cause clinical resistance to other menin inhibitors.
The interaction between histone-lysine N-methyltransferase 2A (KMT2A; also referred to as MLL or mixed-lineage leukemia) and the scaffolding protein menin (encoded by the MEN1 gene) is an essential part of a chromatin complex that mediates aberrant gene expression in KMT2A-rearranged (KMT2A-r) and nucleophosmin 1 (NPM1)-altered leukemias.2,3 Disruption of the menin-KMT2A interaction has therefore become an appealing therapeutic strategy, with several inhibitors targeting the interaction now being developed.4
JNJ-75276617 is an orally bioavailable inhibitor that specifically binds to menin and disrupts the menin-KMT2A interaction. Kwon and colleagues found that JNJ-75276617 is highly active in vitro and in vivo in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) models with KMT2A and NPM1 alterations. In KMT2A-r and NPM1-mutated (NPM1c) AML cell lines, JNJ-75276617 prevented menin-KMT2A chromatin association and gene-regulatory region binding, and decreased expression of menin-KMT2A target genes. In KMT2A-r and NPM1c AML cell lines and cells of patients, JNJ-75276617 blocked proliferation and induced differentiation and apoptosis. Similarly, JNJ-75276617 inhibited proliferation and induced apoptosis in a KMT2A-r ALL cell line and in cells of patients. The efficacy of JNJ-75276617 was further demonstrated in a murine KMT2A-r AML cell–derived xenograft model, in both KMT2A-r and NPM1c AML patient–derived xenograft (PDX) models, and in a KMT2A-r B-ALL PDX model.
Although initial studies have reported the clinical efficacy of menin-KMT2A inhibitors as monotherapies,5 optimal response may be improved by strategic partnering with other therapies. The authors found that the combination of JNJ-75276617 with the specific FLT3 inhibitor gilteritinib was highly synergistic in a KMT2A- and FLT3-mutated AML cell line. In addition, the combinations of JNJ-75276617 with the BCL2 inhibitor venetoclax, alone or together with the hypomethylating agent azacitidine, were also effective in a KMT2A-r AML model in vitro and in vivo. These results are in concordance with observations from earlier investigations of similar combinations with other menin inhibitors.6-9
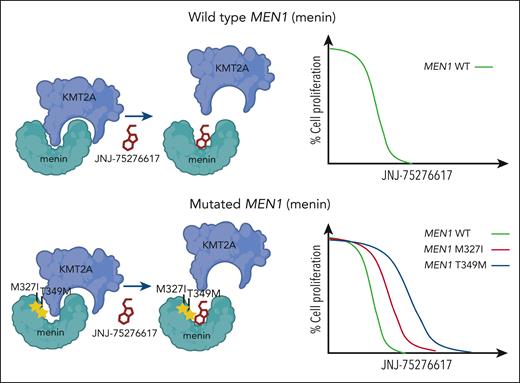
An emerging challenge that has become apparent from investigations of other menin inhibitors includes resistance mechanisms that involve mutation to MEN1. Recent studies have shown that somatic mutations to MEN1 are common in leukemia patients with acquired resistance to revumenib.10 Although the mutations prevent interference by revumenib, interaction between menin and KMT2A can still occur. Kwon et al show how JNJ-75276617 can still displace KMT2A and prevent its interaction with menin despite the presence of MEN1 mutations that block activity of other menin inhibitors (see figure). In addition, JNJ-75276617 remained effective and inhibited proliferation of KMT2A-r AML cell lines engineered to express mutated MEN1. Although this appears to be a unique feature of this menin-KMT2A inhibitor, additional studies are needed to demonstrate the clinical activity of JNJ-75276617, especially in patients with acquired MEN1 mutation after prior exposure to another menin-KMT2A inhibitor.
JNJ-75276617 (bleximenib) is active in the presence of wild-type and mutated menin. Mutations to MEN1 (menin), such as M327I and T349M, have been observed in patients with acquired clinical resistance to the menin-KMT2A inhibitor revumenib. Kwon et al show that the menin-KMT2A inhibitor JNJ-75276617 can block the interaction between KMT2A and wild-type menin or menin bearing the M327I or T349 mutation. JNJ-75276617 is also effective at inhibiting proliferation of cells expressing wild-type or mutant menin.
JNJ-75276617 (bleximenib) is active in the presence of wild-type and mutated menin. Mutations to MEN1 (menin), such as M327I and T349M, have been observed in patients with acquired clinical resistance to the menin-KMT2A inhibitor revumenib. Kwon et al show that the menin-KMT2A inhibitor JNJ-75276617 can block the interaction between KMT2A and wild-type menin or menin bearing the M327I or T349 mutation. JNJ-75276617 is also effective at inhibiting proliferation of cells expressing wild-type or mutant menin.
Clinical evaluation of JNJ-75276617 is ongoing in early-phase studies of acute leukemias as monotherapy (NCT04811560) and in combination (NCT05453903). Considering that patients exposed to other menin-KMT2A inhibitors can quickly develop resistance, novel inhibitors that can induce durable response and overcome the common mechanisms of acquired resistance will be needed.
Conflict-of-interest disclosure: C.A.H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal