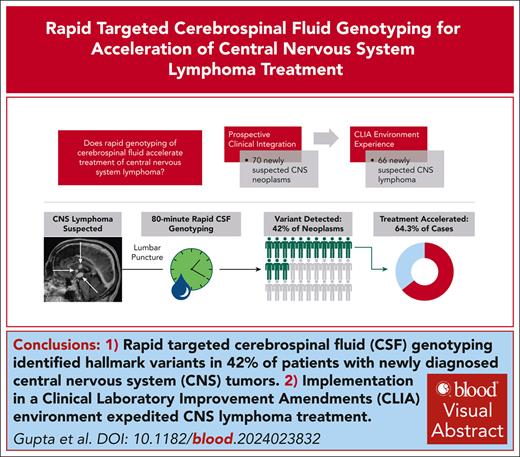
Key Points
Rapid targeted genotyping of CSF prospectively detected hallmark single-nucleotide variants in 42% of patients with new CNS tumors.
Integrating this approach in a CLIA environment accelerated times to diagnosis and treatment for patients with newly diagnosed CNS lymphoma.
Visual Abstract
Delays and risks associated with neurosurgical biopsies preclude timely diagnosis and treatment of central nervous system (CNS) lymphoma and other CNS neoplasms. We prospectively integrated targeted rapid genotyping of cerebrospinal fluid (CSF) into the evaluation of 70 patients with CNS lesions of unknown cause. Participants underwent genotyping of CSF-derived DNA using a quantitative polymerase chain reaction–based approach for parallel detection of single-nucleotide variants in the MYD88, TERT promoter, IDH1, IDH2, BRAF, and H3F3A genes within 80 minutes of sample acquisition. Canonical mutations were detected in 42% of patients with neoplasms, including cases of primary and secondary CNS lymphoma, glioblastoma, IDH-mutant brainstem glioma, and H3K27M-mutant diffuse midline glioma. Genotyping results eliminated the need for surgical biopsies in 7 of 33 cases (21.2%) of newly diagnosed neoplasms, resulting in significantly accelerated initiation of disease-directed treatment (median, 3 vs 12 days; P = .027). This assay was then implemented in a Clinical Laboratory Improvement Amendments environment, with 2-day median turnaround for diagnosis of CNS lymphoma from 66 patients across 4 clinical sites. Our study prospectively demonstrates that targeted rapid CSF genotyping influences oncologic management for suspected CNS tumors.
Introduction
The sensitivity of traditional cerebrospinal fluid (CSF) liquid-based assays for diagnosing central nervous system (CNS) neoplasms, such as CNS lymphoma, ranges from 13.3% for cytology to 23.3% for flow cytometry and 26.9% for IgH gene rearrangement (supplemental Table 1, available on the Blood website).1 CNS tumor diagnosis thus frequently necessitates invasive neurosurgical biopsy.2-4 Although histomolecular tissue analysis remains the gold standard, diagnostic yields and risks depend on lesion characteristics, patient comorbidities, and neurosurgeon experience.5-7
CSF-based approaches have been proposed to enable less invasive CNS tumor diagnosis. Digital droplet polymerase chain reaction (PCR) offers increased sensitivity, but examines a limited number of variants and requires manual threshold determination.8 Tumor-specific DNA alterations have been detected by next-generation sequencing (NGS) of CSF-derived DNA.9-11 Studies of CSF from patients with known CNS lymphoma showed enhanced sensitivity by incorporating machine learning–assisted circulating tumor DNA sequencing,12 clonotypic immunoglobulin gene rearrangement detection,13 and characterization of multiple biomarkers.14 Residual circulating tumor DNA in the peripheral blood after induction treatment for CNS lymphoma has also shown prognostic value that may guide personalized therapy.15 However, current turnaround times hinder the utility of these approaches because most new CNS neoplasms are diagnosed via emergency admissions, necessitating urgent diagnostic information.16
We previously described a rapid CSF genomic analysis technique, targeted rapid sequencing (TetRS),2,17,18 consisting of quantitative PCR–based parallel detection of highly recurrent somatic mutations defining CNS neoplasms in the TERT promoter, MYD88, IDH1/2, H3F3A, and BRAF genes within 80 minutes of specimen acquisition, with detection limits of 0.15% variant allele fraction in tissue.2 Here, we prospectively implemented rapid genotyping of CSF-derived DNA in suspected CNS neoplasms and analyzed the impact of this approach on diagnosis and treatment.
Study design
The prospective cohort in this study comprised patients at least 18 years old admitted to Massachusetts General Hospital with new CNS lesions of unknown cause for which neoplasm was suspected. Excess CSF that would otherwise be discarded was collected from clinically indicated procedures between 1 December 2020 and 10 December 2021; a total of 70 patients meeting inclusion criteria were designated the rapid genotyping cohort.
Informed consent was obtained under an institutional review board–approved protocol. Consistent with US Food and Drug Administration guidance for returning potentially actionable research findings,19 results were reported directly to treating physicians, who conducted all communication of findings via shared decision-making with patients. We evaluated real-time TetRS result integration by analyzing documented decision-making and clinical trajectories, and comparing these with historical cohorts.
To assess somatic alteration prevalence, genotyping was performed retrospectively from archived specimens of patients with primary CNS lymphoma (PCNSL) and other brain tumor specimens (supplemental Methods and supplemental Figure 1).
Rapid genotyping assay
TetRS was used to detect hallmark mutations in the TERT promoter, MYD88, IDH1/2, H3F3A, and BRAF genes. Multiplexed GAPDH detection was an internal control for reaction integrity. Assays were performed separately on supernatant and pelleted CSF fractions.2,17,18 Assay modifications, protocols, and validation are described in the supplemental Methods.
Written informed consent was obtained, and specimens were prospectively collected and analyzed under Massachusetts General Hospital (MGH) Institutional Review Board (IRB) protocol 2017P001581. The IRBs at MGH, Brigham and Women's Hospital, and Dana-Farber Cancer Institute approved analyses of electronic health records under MGH IRB protocol 2019P002400. The Dana-Farber Cancer Institute protocol 10-454 covered analysis of archived clinical specimens and genomic DNA extracts.
Results and discussion
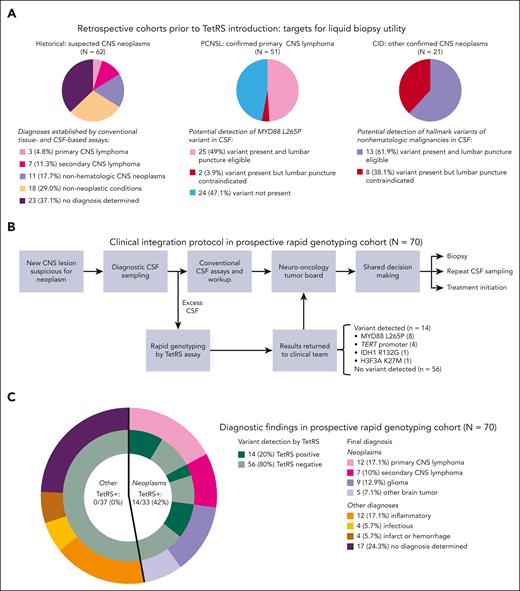
We first detailed the diverse diagnoses and historical variability in diagnostic trajectories among patients with newly suspected CNS neoplasms at our multidisciplinary referral center (historical cohort, Figure 1A). Although diagnostic evaluation was ongoing, empiric treatment regimens were initiated in 40 of 62 patients (64.5%). Median time to diagnosis was 9 days, with lymphoma diagnosed in 10 of 62 patients (16.1%), nonhematologic malignancies in 11 of 62 patients (17.7%), and no diagnosis achieved in 23 of 62 patients (37.1%) (supplemental Figure 2).
Clinical integration of rapid CSF genotyping for diagnosis of suspected CNS neoplasms. (A) Retrospective patient cohorts from before rapid genotyping introduction were included in this study to serve as benchmarks for diagnostic trajectories of CNS neoplasms and potential for detection of hallmark variants in CSF. Diverse neoplastic and nonneoplastic diagnoses were encountered in 62 patients with new CNS lesions with radiographic suspicion for neoplasms (historical cohort, left panel). Among 52 patients with PCNSL (PCNSL cohort, middle panel) and 21 patients with nonhematologic CNS malignancies (CID cohort with known variants present in diagnostic biopsy site tissue, right panel), analyzing variant status and safety of lumbar puncture revealed the maximum proportion of patients with potentially detectable variants in CSF. (B) Prospective clinical workflow for returning TetRS rapid genotyping assay results to providers during real-time diagnosis of suspected CNS neoplasms. Excess CSF was used to perform the TetRS assay in a research laboratory setting. Results were reported to the clinical team, who then included them in tumor board discussions and collaborative treatment decisions with patients. Conventional CSF assays included chemistries, cell counts, cytology, flow cytometry, cultures, microbiologic, serologic (antibody), and antigen testing. (C) The categories and proportions of final diagnoses secured in the 70 patients in the rapid genotyping cohort. The inner circles adjacent to each final diagnosis display the detection of variants in CSF by TetRS.
Clinical integration of rapid CSF genotyping for diagnosis of suspected CNS neoplasms. (A) Retrospective patient cohorts from before rapid genotyping introduction were included in this study to serve as benchmarks for diagnostic trajectories of CNS neoplasms and potential for detection of hallmark variants in CSF. Diverse neoplastic and nonneoplastic diagnoses were encountered in 62 patients with new CNS lesions with radiographic suspicion for neoplasms (historical cohort, left panel). Among 52 patients with PCNSL (PCNSL cohort, middle panel) and 21 patients with nonhematologic CNS malignancies (CID cohort with known variants present in diagnostic biopsy site tissue, right panel), analyzing variant status and safety of lumbar puncture revealed the maximum proportion of patients with potentially detectable variants in CSF. (B) Prospective clinical workflow for returning TetRS rapid genotyping assay results to providers during real-time diagnosis of suspected CNS neoplasms. Excess CSF was used to perform the TetRS assay in a research laboratory setting. Results were reported to the clinical team, who then included them in tumor board discussions and collaborative treatment decisions with patients. Conventional CSF assays included chemistries, cell counts, cytology, flow cytometry, cultures, microbiologic, serologic (antibody), and antigen testing. (C) The categories and proportions of final diagnoses secured in the 70 patients in the rapid genotyping cohort. The inner circles adjacent to each final diagnosis display the detection of variants in CSF by TetRS.
We then identified patients who established new diagnoses of CNS neoplasms before TetRS assay introduction (cohorts PCNSL and Center for Integrated Diagnostics [CID], Figure 1A), and analyzed genotyping findings alongside clinical trajectories to determine whether detection of variants in the TetRS panel from CSF could have facilitated diagnosis or treatment. Accounting for variant frequency and lumbar puncture eligibility, we found potential benefit of CSF genotyping for facilitating diagnosis or treatment in 38 of 72 patients (52.8%) (25/51 [49.0%] with PCNSL and 13/21 [61.9%] with CID, Figure 1A).
We then performed a prospective, single-center study involving hospitalized patients with new CNS lesions of unknown cause for which neoplasm was in the differential diagnosis. Excess CSF from 88 patients undergoing CSF sampling was submitted by providers for rapid genotyping in clinical real time; 70 patients meeting inclusion criteria comprised the rapid genotyping cohort (Figure 1B-C).
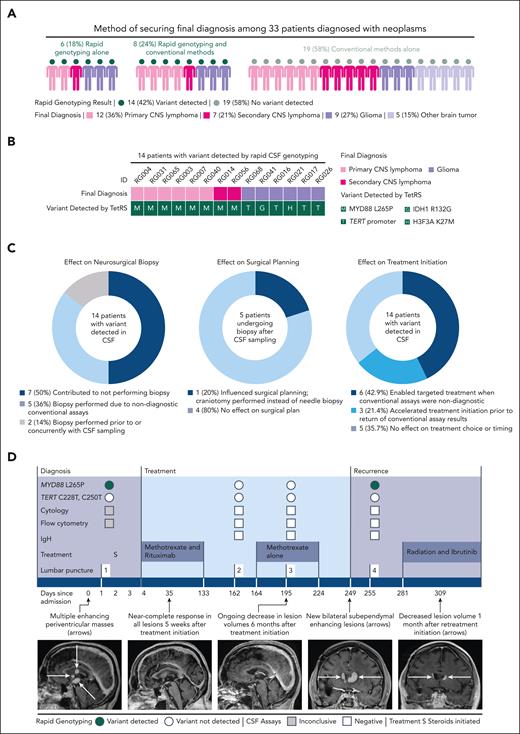
In the rapid genotyping cohort, detection of a mutation by TetRS shortened turnaround time of diagnostic information compared with conventional assays (median [interquartile range], 0.5 [0.5-not estimable (NE)] vs 25 [11-34] days, respectively; P < .001) (supplemental Figure 4). TetRS identified a mutation in 14 of 33 patients (42.4%) who were ultimately diagnosed with a neoplasm, including primary and secondary CNS lymphoma, glioblastoma, IDH-mutant brainstem glioma, and H3K27M-mutant diffuse midline glioma. No false-positive detection in 37 patients with nonneoplastic diagnoses was encountered (Figure 1C). In 6 of 33 patients (18.2%) diagnosed with a neoplasm, diagnoses were secured by TetRS results alone (Figure 2A-B). A number needed to diagnose (inverse of the Youden index)20 analysis revealed that the number of patients needed to undergo a diagnostic assay to accurately detect a hematologic CNS malignancy was 1.18 by neurosurgical biopsy, 1.74 by TetRS, and 4.74 to 15.9 by conventional diagnostics (supplemental Table 1).
Clinical impact of rapid CSF genotyping. (A) This shows the method of securing final diagnoses among 33 patients diagnosed with a neoplasm in the rapid genotyping cohort. Each illustrated person represents 1 patient; the color of the head represents whether a variant was detected in CSF by rapid genotyping, and the color of the body represents the final diagnosis reached. (B) Among 14 patients in the rapid genotyping cohort with a variant detected in CSF by TetRS, the variant detected is displayed below the final diagnosis established in each case. (C) Plots show the number and percentages of patients with clinical effects of positive variant detection by TetRS. (D) The clinical course of a representative patient with a new CNS neoplasm diagnosed by rapid CSF genotyping without neurosurgical biopsy is shown. A 69-year-old woman (patient RG003) presented with deep periventricular-enhancing lesions concerning for CNS lymphoma, glioma, or metastasis. TetRS detected the MYD88 L265P variant and was negative for TERT promoter variants. Positron-emission tomography computed tomography scan, magnetic resonance imaging of the spine, and ophthalmologic examination were negative for other sites of disease. Primary CNS lymphoma was diagnosed on the basis of TetRS variant detection, enabling methotrexate and rituximab initiation within 4 days. After completion of induction chemotherapy and during consolidation, lumbar punctures were negative for MYD88 L265P, correlating with radiographic response to treatment. MYD88 L265P was again detected in CSF at the time of recurrence; retreatment with radiation and ibrutinib was initiated, with ongoing response 1 month later. Additional TetRS and orthogonal sequencing results are displayed in the supplemental Data. IgH, immunoglobulin heavy chain rearrangement; TERT denotes the TERT C228T and C250T variants.
Clinical impact of rapid CSF genotyping. (A) This shows the method of securing final diagnoses among 33 patients diagnosed with a neoplasm in the rapid genotyping cohort. Each illustrated person represents 1 patient; the color of the head represents whether a variant was detected in CSF by rapid genotyping, and the color of the body represents the final diagnosis reached. (B) Among 14 patients in the rapid genotyping cohort with a variant detected in CSF by TetRS, the variant detected is displayed below the final diagnosis established in each case. (C) Plots show the number and percentages of patients with clinical effects of positive variant detection by TetRS. (D) The clinical course of a representative patient with a new CNS neoplasm diagnosed by rapid CSF genotyping without neurosurgical biopsy is shown. A 69-year-old woman (patient RG003) presented with deep periventricular-enhancing lesions concerning for CNS lymphoma, glioma, or metastasis. TetRS detected the MYD88 L265P variant and was negative for TERT promoter variants. Positron-emission tomography computed tomography scan, magnetic resonance imaging of the spine, and ophthalmologic examination were negative for other sites of disease. Primary CNS lymphoma was diagnosed on the basis of TetRS variant detection, enabling methotrexate and rituximab initiation within 4 days. After completion of induction chemotherapy and during consolidation, lumbar punctures were negative for MYD88 L265P, correlating with radiographic response to treatment. MYD88 L265P was again detected in CSF at the time of recurrence; retreatment with radiation and ibrutinib was initiated, with ongoing response 1 month later. Additional TetRS and orthogonal sequencing results are displayed in the supplemental Data. IgH, immunoglobulin heavy chain rearrangement; TERT denotes the TERT C228T and C250T variants.
Although maximal safe neurosurgical resection may confer benefit for glioma and metastases, biopsy alone is indicated for hematologic malignancies, which may alternatively be diagnosed by CSF or vitreous studies and are initially treated with chemotherapy.2-4,21 TetRS influenced adjuvant treatment initiation in 9 of 14 cases (64.3%) where a mutation was prospectively detected in the rapid genotyping cohort. This included eliminating the need for biopsies in 7 of 14 cases (50%) of neoplasms following tumor board discussion and shared decision-making (Figure 2C-D). In cases where a variant was detected by TetRS, treatment initiation was significantly accelerated by obviating surgical biopsies (median [interquartile range], 3 [3-NE] vs 12 [6-NE] days, respectively; P = .027) (supplemental Figure 4). No consistent differences were observed in lesional anatomy, clinical presentation, or conventional CSF-based assay performance between patients offered biopsy compared with patients in whom biopsy was not pursued (supplemental Figure 4; supplemental Results).
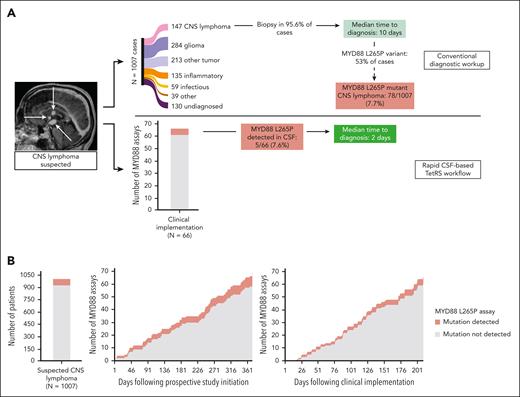
Recognizing this potential to impact clinical workflows in CNS lymphoma, MYD88 L265P detection from CSF was validated in a Clinical Laboratory Improvement Amendments environment, and 66 CSF specimens were then analyzed over 210 days from 4 clinical sites. MYD88 L265P was detected in 5 of 66 cases (7.6%) (Figure 3A), consistent with published estimates of 7.7% MYD88 L265P-mutant CNS lymphoma when lymphoma is suspected (77 MYD88-L265P mutated PCNSL cases of 1007 radiographically suspected cases2; P = .960), and 8 of 70 cases (11.4%) in the rapid genotyping cohort (P = .445) (Figure 3B). Median result reporting time was 2 days (range, 0-6 days).
Clinical implementation of rapid genotyping of cell-free DNA in CSF accelerates diagnosis of CNS lymphoma (CNSL). (A) Analysis of a historical cohort of 1007 patients with new CNS lesions of unknown cause for which CNSL was in the differential diagnosis showed that only 14.7% are ultimately diagnosed with CNSL. Securing a CNSL diagnosis required 95.6% of patients to undergo neurosurgical biopsy, resulting in a median time to diagnosis of 10 days (top pathway, adapted from Gupta et al2). Because 53% of CNS lymphoma cases in our institutional experience were positive for the MYD88 L265P variant (Figure 1), we estimated that 7.7% of the initial cohort would harbor this variant. Following clinical implementation of the Clinical Laboratory Improvement Amendments–certified cell-free DNA assay, a similar percentage of CNSL bearing the MYD88 L265P mutation was identified, with a median turnaround time of 2 days (bottom pathway). (B) The rate of accrual of CSF specimens was 1 sample/5.3 days in the prospective rapid genotyping cohort (middle panel) vs 1 sample/3.2 days following clinical implementation (right panel) (P = .002). The frequency of MYD88 L265P detection in these study populations was 8 of 70 patients (11.4%) and 5 of 66 patients (7.6%), respectively, similar to the 78 of 1007 patients (7.7%) found in the historical cohort (left panel).
Clinical implementation of rapid genotyping of cell-free DNA in CSF accelerates diagnosis of CNS lymphoma (CNSL). (A) Analysis of a historical cohort of 1007 patients with new CNS lesions of unknown cause for which CNSL was in the differential diagnosis showed that only 14.7% are ultimately diagnosed with CNSL. Securing a CNSL diagnosis required 95.6% of patients to undergo neurosurgical biopsy, resulting in a median time to diagnosis of 10 days (top pathway, adapted from Gupta et al2). Because 53% of CNS lymphoma cases in our institutional experience were positive for the MYD88 L265P variant (Figure 1), we estimated that 7.7% of the initial cohort would harbor this variant. Following clinical implementation of the Clinical Laboratory Improvement Amendments–certified cell-free DNA assay, a similar percentage of CNSL bearing the MYD88 L265P mutation was identified, with a median turnaround time of 2 days (bottom pathway). (B) The rate of accrual of CSF specimens was 1 sample/5.3 days in the prospective rapid genotyping cohort (middle panel) vs 1 sample/3.2 days following clinical implementation (right panel) (P = .002). The frequency of MYD88 L265P detection in these study populations was 8 of 70 patients (11.4%) and 5 of 66 patients (7.6%), respectively, similar to the 78 of 1007 patients (7.7%) found in the historical cohort (left panel).
Rapid genotyping of CSF can potentially accelerate CNS neoplasm workup by characterizing hallmark molecular features22 within 80 minutes, using universally available infrastructure in clinical molecular pathology laboratories.2,23 Although rapid, targeted information cannot subtype lymphoma or glioma, it can focus differential diagnoses, guide surgical decisions, and inform treatment in selected cases. Detecting MYD88 L265P raises suspicion for lymphoproliferative or plasmacytic processes,24,25 whereas negative results are counterbalanced by parallel detection of mutations indicative of common alternate diagnoses; for instance, identifying TERT promoter alterations in the absence of IDH mutations suggests glioblastoma.26
TetRS guides surgical planning for biopsy vs resection, particularly when the differential diagnosis includes lymphoma vs glioma (case RG026; supplemental Results) or if tissue analysis is required for glioma classification,22 lymphoma subtyping, or confirmation of a neoplastic diagnosis. Our findings that 37 of 70 patients (52.9%) in the rapid genotyping cohort with suspected neoplasms ultimately had nonneoplastic or indeterminate diagnoses also highlights that resource-intensive sequencing methods may not be cost-effective upfront diagnostic approaches12; importantly, however, DNA isolated in the TetRS workflow is suitable for NGS analysis (case RG041; supplemental Figure 10), permitting a step-wise workflow.
Clinical integration of this approach enables critical subsequent studies. First, TetRS may improve cost-effectiveness by uniquely offering rapid turnaround from a Clinical Laboratory Improvement Amendments–certified assay using commercially available reagents and publicly available methods at <$9 per reaction2; comparably scalable quantitative PCR–based diagnostics have been deployed in resource-limited settings.27 We observed that costs of incorporating rapid genotyping were offset by reductions in other CSF-based assays for suspected nonneoplastic causes (supplemental Figure 9). Second, clonal variants detected by TetRS may constitute treatment response, minimal residual disease, and recurrence biomarkers, such as MYD88 L265P correlating with disease burden (Figure 2D).
Larger studies are needed to determine the clinical impact of earlier therapy initiation, and whether our findings in a limited number of patients with CNS lymphoma are broadly applicable. We observed high specificity of this approach (supplemental Table 1), with 42% sensitivity for CNS tumor detection. Although adding more tumor-defining variants to this panel may increase sensitivity and ability to subclassify selected tumors, there may be inherent limitations of CSF genotyping by lumbar puncture. For instance, NGS-based analysis of CSF has 74% to 82.5% circulating tumor DNA (ctDNA) detection rates when CSF is sampled from intracranial compartments, but 49% to 63% detection from lumbar puncture. Tumor burden, grade, and location also correlate with ctDNA detectability.9-11,28
Although cases in which brain biopsies were obviated by CSF genotyping were associated with shorter times to treatment initiation (supplemental Figure 4), multiple factors influence treatment timing, because of the heterogeneity and complexity of patients with CNS tumors. Because conventional CSF flow cytometry and cytomorphologic assessment are standards accepted in hematologic malignancy diagnosis and monitoring, the impact of our approach compared with conventional assays is most apparent in patients with CNS lymphoma.
We prospectively demonstrate that targeted hallmark genomic alteration detection from CSF influences oncologic management of patients with suspected CNS tumors. Unlike prior studies demonstrating the theoretical promise of NGS-based detection of somatic alterations from CSF ctDNA among patients with known neoplasms,2,8,9,11,28 we examined patients without established diagnoses. This cohort is more representative of the challenges unique to securing a diagnosis of a hematologic malignancy affecting the CNS to guide timely treatment. To our knowledge, our data provide the first comparisons between sequencing-based and conventional methods, prospectively calculated performance benchmarks, and multicenter results after Clinical Laboratory Improvement Amendments certification.
Acknowledgments
The authors deeply appreciate the collaborative support for these studies by many parties at the Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital, including the following: Departments of Neuro-Oncology and Neurology clinical providers, nurses, and staff; Department of Pathology tissue banks; and clinical core laboratories. This work would not have been possible without the research community at the MGH Brain Tumor Research Center. The authors additionally thank Donald Glazer for critical review of the manuscript.
This work was supported by grants from the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke 1K08NS107634-01A1 (to G.M.S.), The Darwin Project (to G.M.S.), the Brian D. Silber Memorial Fund (to G.M.S.), and the NIH, National Cancer Institute 5U01CA230697 (to B.S.C.).
Authorship
Contribution: G.M.S., B.S.C., D.P.C., T.K., J.D., M.M., and J.K.L. designed and supervised the study; M.G., J.D.B., E.M., E.J.B., N.Z.G., G.E.M., A. Gallagher, and G.M.S. acquired, analyzed, and interpreted data; M.G., E.M., and G.M.S. performed statistical analysis; G.M.S., B.S.C., D.P.C., and J.K.L. provided administrative, technical, and material support; N.N., J.T., P.K.B., and K.T. contributed vital reagents; M.G., J.D.B., E.M., E.J.B., F.I., D.P.C., J.K.L., B.S.C., and G.M.S. wrote the manuscript; J.M.B., A. Gordon, S.S.J., M.P., Y.S., P.S.J., F.G.B., W.T.C., R.G., J.M.R., N.W., M.M.-L., D.A.F., B.V.N., T.T.B., L.L.R., J.T.J., D.P.C., J.K.L., B.S.C., and G.M.S. identified patients for study enrollment and analyzed and interpreted clinical data for inclusion in the manuscript; G.M.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors participated in critically revising the manuscript for important intellectual content.
Conflict-of-interest disclosure: D.P.C. and G.M.S. hold a patent pertaining to the use of peptide and locked nucleic acids as used in this study during the amplification of target DNA sequences (patent number US20170369939A1). Outside the scope of this work, the authors report the following. R.G. serves as a paid consultant for Idorsia, Inc, and Mosaic Research Management; is an advisory board member of Braintale, Inc, and the University of Wisconsin’s computed tomography board; provided expert testimony to Mary Hitchcock Memorial Hospital; has received speaker honoraria from Siemens; provided legal consulting to the Harvard Medical Institutions Risk Management Foundation; and received research funding from Samsung and the US National Institutes of Health. N.W. has received royalties from Wolters Kluwer and research funding from Merck, and was previously on the advisory board of Seattle Genetics (ended 2021). P.K.B. has consulted for Merck, Genentech-Roche, Eli Lilly, Tesaro, ElevateBio, Dantari, Voyager Therapeutics, SK Life Sciences, Pfizer, ACI, Atavistik, Sintetica, Kazia, MPM, CraniUS, Axiom, and InCephalo; serves on the Scientific Advisory Board for Kazia and CraniUS; has received grant/research support (to institution) from Merck, GlaxoSmithKline, AstraZeneca, Kazia, Genentech-Roche, Eli Lilly, Mirati, Bristol Myers Squibb, and Kinnate; and has received speaker’s honoraria from Merck, Genentech-Roche, Eli Lilly, and Medscape. D.A.F. is a shareholder for Eli Lilly. B.V.N. has consulted for Robeaute, BK Medical, Merck, BrainLab, Zeta, and Enclear. He has received grant funding from the US National Institutes of Health. T.T.B. has received publishing royalties from UpToDate, Inc, and Oxford University Press; and has received research institutional grant/research support related to a clinical trial (to Brigham and Women’s Hospital) from ONO Pharmaceuticals. L.L.R. has received honoraria from PeerView, Medscape, and Clinical Care Options; and has received consulting honoraria from AbbVie, Personal Genome Diagnostics, Bristol Myers Squibb, Loxo Oncology at Lilly, Amgen, Merck, AstraZeneca, Sanofi-Genzyme, and EMD Serono. J.T.J. holds equity in Navio Theragnostics, Akeila Bio, and The Doctor Lounge; and has participated in paid consulting from Navio Theragnostics, Recursion Pharmaceuticals, Alexion Pharmaceuticals, Springworks Pharmaceuticals, Merck Pharmaceuticals, Children’s Tumor Foundation, CEC Oncology, and Elsevier. J.D. is a consultant for Amgen, Blue Earth Diagnostics, and Unum Therapeutics; and has received research support from Novartis and publishing royalties from Wolters for UpToDate, Inc. M.M. consults for Bayer, DelveBio, Interline, and Isabl; holds equity in DelveBio, Interline, and Isabl; is an inventor of patents licensed to LabCorp and Bayer; and receives research funding from Bayer and Janssen. D.P.C. has consulted for the Massachusetts Institute of Technology, Advise Connect Inspire, Lilly, GlaxoSmithKline, Iconovir, Boston Pharmaceuticals, and Boston Scientific; serves on the advisory board of Pyramid Biosciences, which includes an equity interest; and has received honoraria and travel reimbursement from Merck for invited lectures, and from the US National Institutes of Health and Department of Defense for clinical trial and grant review. B.S.C. is a consultant for Koh Young. The remaining authors declare no competing financial interests.
Correspondence: Ganesh M. Shankar, Department of Neurosurgery, Massachusetts General Hospital, 15 Parkman St, WAC 745, Boston, MA 02114; email: gshankar@mgh.harvard.edu.
References
Author notes
The authors confirm that the data supporting the findings presented are available in the article and accompanying supplemental content. Requests for additional data should be sent to the corresponding author, Ganesh M. Shankar (gshankar@mgh.harvard.edu). All data and materials shared will be deidentified.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal