Using perfused human kidneys awaiting transplantation, Dumbill et al show in this issue of Blood that kidney oxygen consumption and energy-dependent tubular secretion are reduced when the perfusing stored red blood cells (RBCs) demonstrate prolonged oxygen off-loading.1 This finding is at variance with the conventional cardiovascular physiology view that oxygen delivery to tissues is only perfusion limited because oxygen off-loading from RBCs is faster than the capillary transit time.2 The existence of diffusion-limited O2 delivery should not come as a surprise to hematologists who know that high-affinity hemoglobins and hereditary spherocytosis3 cause erythrocytosis despite carrying equivalent amounts of oxygen to normal cells. The variability in oxygen off-loading observed among stored RBCs from different donors suggests that more than 1 RBC storage lesion may be involved.4
The oxygen affinity of hemoglobin is the ratio of the oxygen binding on-rate and the oxygen binding off-rate.5 The on-rate can be measured by flash photolysis, using a pulse of laser light to knock the oxygen off the hemoglobin and watching the bulk rate of color change as the oxygen rebinds. The on-rate is fast, with a half-time for free hemoglobin of the order of a millisecond, and RBCs are “fully” saturated on a quick pass through the lung. The off-rate is more difficult to measure, but mixing studies of oxygenated hemoglobin and degassed water suggest that the off-rate is rapid too. Measurements of maximum oxygen delivery in athletes suggest that cardiac output and oxygen-carrying capacity are the major determinants of performance and that perfusion but not diffusion is limiting.
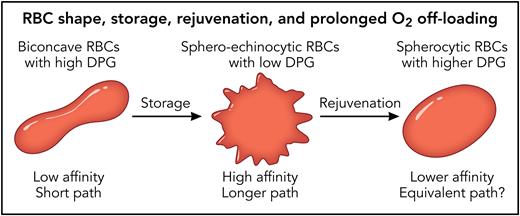
The Swietach group, who also conducted this study, has developed a mixing device, tied to computer imaging of the color change from oxy- to deoxyhemoglobin, for measuring the oxygen off-rate of individual RBCs.6 Their measured off-rate half-time for oxygen out of the RBC is of the order of a second, far longer than the hemoglobin off-rate, because the path of oxygen out of the cell involves many separate hemoglobin bindings and unbindings. The exact number and duration of these bindings and unbindings in an individual RBC is a function both of the oxygen affinity of the hemoglobin, modulated by 2,3-diphosphoglycerate (2,3-DPG), and the path length out of the cell, modulated by membrane loss and the evolution of spheroechinocytic shape (see figure). Stored cells from individual donors showed off-rate half-times that varied from 0.5 to over 2 seconds.
During storage, RBCs undergo changes known as storage lesions that include shape change and the loss of DPG. Several hours of warm rejuvenation with PIPA solution will increase RBC DPG and partially restore morphology but with significant loss of membrane. The data from Dumbill et al suggest that the lower hemoglobin affinity is more important than the shape change with its longer path for individual oxygen molecules out of the RBC. Professional illustration by Patrick Lane, ScEYEnce Studios.
During storage, RBCs undergo changes known as storage lesions that include shape change and the loss of DPG. Several hours of warm rejuvenation with PIPA solution will increase RBC DPG and partially restore morphology but with significant loss of membrane. The data from Dumbill et al suggest that the lower hemoglobin affinity is more important than the shape change with its longer path for individual oxygen molecules out of the RBC. Professional illustration by Patrick Lane, ScEYEnce Studios.
Stored RBCs develop metabolic slowing, oxidative injury, and shape change that accumulate as storage lesions in donor-dependent patterns.7 Some of these storage lesions are reversable with several hours of incubation of the RBCs with a rejuvenation solution of phosphate, inosine, pyruvate, and adenine (PIPA). Splitting units and rejuvenating half with PIPA allowed the Dumbill group to demonstrate the greater kidney oxygen consumption and energy-dependent tubular secretion when perfusing with the rejuvenated RBCs.8
The kidney has evolved to sense oxygen availability, whereas other tissues may have evolved to meet other priorities. Multiple large randomized clinical trials have failed to demonstrate a clinical impact of prolonged RBC storage, so it is important not to disrupt an already tenuous blood supply with demands for “fresher” RBCs on the basis of this report. In the Dumbill study, the perfused kidneys “corrected” the oxygen diffusion problem and recovered tubular secretion over several hours as perfusing RBC intracellular pH normalized. If faster corrections or reduced induction of the RBC storage lesions are needed, better RBC storage systems that limit membrane loss, pH decrease, and 2,3-DPG loss are available.
We now know that part of the kidney’s sensitivity to oxygen is due to diffusion-delayed delivery of oxygen to the hypoxia-inducible factor sensor in the juxtaglomerular apparatus as an intrinsic dysfunction of the perfusing RBC. We do not know why increased perfusion does not override this effect in the kidney as it appears to do in the rest of the body. And we know how to treat the resulting hypoxia, but we do not know if it is important.
Conflict-of-interest disclosure: J.R.H. is the inventor of the AS-7 RBC additive solution and a paid consultant of Hemerus Medical, LLC.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal