In this issue of Blood, Duell et al1 describe their effort to trace complex evolutionary trajectories of both aggressive and indolent B-cell lymphomas as these tumors escape elimination by CD19-directed chimeric antigen receptor-armed T cells (CART-19) and bispecific CD20xCD3 T-cell engagers. The authors observed considerable spatial and temporal heterogeneity in target antigen expression during relapses. Notably, in 1 patient CD20+ tumor cells disappeared from the main lesion, only to reemerge later in some, but not all, distant lymph nodes. This striking example of tumor evolution evokes the steady unraveling of seemingly conflicting scenario in the short story “The Garden of Forking Paths” by Jorge Luis Borges. Its protagonist believes that “this web of time—the strands of which approach one another, bifurcate, intersect or ignore each other through the centuries—embraces every possibility ... in some you exist and not I, while in others I do, and you do not.” This quote aptly describes the natural history of many relapsing or primarily refractory blood cancers treated with potent immunotherapeutics.
In the mid-2010s, harnessing the power of autologous T lymphocytes to kill cancer cells proved to be a game changer in the field of hematologic malignancies. At the time, CART-19 cells and bispecific CD19xCD3 T-cell engagers (such as blinatumomab) were among the few effective treatments available to patients with relapsed or refractory B-cell leukemias and lymphomas. Although these therapies have generally improved overall survival, treatment failure is frequently observed in patients with leukemia as well as in those with lymphoma, often due to poor CAR T-cell persistence and autochthonous T-cell exhaustion. However, a significant percentage of relapsing neoplasms exhibit loss of cognate epitopes. This can occur by mechanisms ranging from deleterious gene mutations to messenger RNA (mRNA) splicing aberrations to epigenetic reprogramming causing transdifferentiation of infant leukemia with KMT2A gene rearrangements into myeloid lineages2 and mantle cell lymphomas into sarcomas.3
Fortunately, besides CD19, there are other highly specific B-cell lineage markers. CD19− relapses can now be routinely treated with immunotherapies directed against other cluster of differentiation (CD) antigens, such as CD22, CD79B, and CD20. In fact, the oldest form of lymphoma immunotherapy is the anti-CD20 monoclonal antibody rituximab. Beyond rituximab and several next-generation CD20-directed monoclonal antibodies, the antilymphoma armamentarium now includes CD20-directed CAR T cells and bispecific CD20xCD3 T-cell engagers, such as glofitamab and mosunetuzumab. The latter bispecific was granted accelerated approval by the Food and Drug Administration in December 2022 for relapsed or refractory follicular lymphoma based on the results of a large multicenter clinical trial in which the overall response rate approached 80% and complete response rate approached 60%.4 However, recent data still suggest that resistance due to epitope loss remains an ongoing problem, with up to 25% of patients relapsing with CD20− disease.5 What drives postmosunetuzumab relapses remained an open question, motivating the study by Duell et al.
The samples available for this study included sequential biopsies from 7 patients with relapsed B-cell neoplasms (4 diffuse large B-cell, 2 follicular, and 1 transformed follicular lymphomas). These relapses came in 2 varieties: CD20− (with epitope loss) and CD20+ (with epitope retention). The 4 that were CD20− (as judged by flow cytometry and immunohistochemistry) arose due to various deleterious events affecting the MS4A1 gene, which in humans encodes the CD20 protein. These events included deep deletions, frameshift mutations, splice sites alterations, and even an apparent chromosomal translocation. Their functional impact was easy to interpret, although 1 of the longitudinal samples exhibited the loss of MS4A1 mRNA without any corresponding alterations in the gene structure. All this was to be expected because similar events were previously found to drive CD19− and CD22-dim relapses in pediatric patients with B-cell acute lymphoblastic leukemia.6
In contrast, the 3 CD20+ relapses appeared to rely on nongenetic, non–tumor cell autonomous mechanisms, such as T-cell exhaustion. Specifically, the authors observed that the exhaustion scores were significantly higher in CD8 effector T cells from 2 CD20+ relapses than from 1 CD20− relapse. Despite the very small sample size, this observation raises interesting questions about evolutionary trajectories of lymphoma cells facing the selective pressures of T cell–based immunotherapies. Is it more effective for a cancer cell to lose its cognate epitope or to persist unaffected despite the onslaught of CD8+ killer cells, to the point at which these T lymphocytes simply exhaust themselves? The answer likely depends both on the cell of origin and on the nature of targeted antigen: although CD19− and CD20− relapses are fairly common, inactivating mutations in the CD22 gene are not routinely observed, at least not in childhood leukemia.
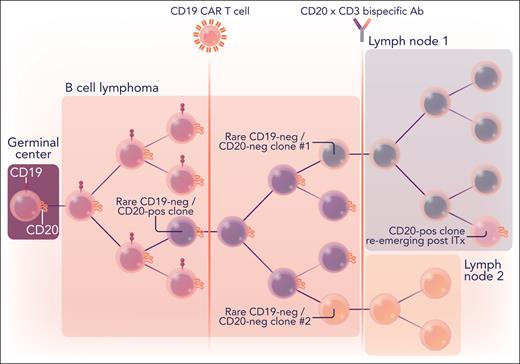
This epitope-positive vs epitope-negative dichotomy, although useful, needs to be approached with care. In 1 patient treated with CART-19 before enrollment in the Duell et al study, lymphoma cells were uniformly CD19−/CD20+. After treatment with mosunetuzumab and eventual relapse, all cells appeared to be double-negative by flow cytometry. However, after 8 weeks of salvage chemotherapy, 7% of cancer cells were CD20+, according to single-cell RNA sequencing (RNA-seq) and immunohistochemistry (see figure). In another case, the clonal evolution was even more complex. That patient initially relapsed on CD20-directed bispecific antibodies with CD20+ disease, then received salvage therapy with a CART-19 product, and then relapsed again with CD19+ disease but now with a small subset of CD20− clones. These convoluted trajectories highlight the complex evolutionary pressures applied on lymphoma cells by T cell–based immunotherapeutics.
Branching evolution of B-cell lymphomas surviving successive rounds of CD19- and CD20-directed immunotherapies. B-cell lymphomas originate in germinal centers of the secondary lymphoid organs and are originally CD19/CD20 double-positive. Upon immunotherapy (ITx) with CD19-directed CAR T cells and/or bispecific CD20xCD3 T-cell engagers (represented by vertical lines), they routinely lose 1 or both antigens. However, CD20+ clones can reemerge in some of the distant lymph nodes upon discontinuation of ITx. The dendrogram illustrates one of the cases described by Duell et al. Ab, antibody; neg, negative; pos, positive. Professional illustration by Somersault18:24.
Branching evolution of B-cell lymphomas surviving successive rounds of CD19- and CD20-directed immunotherapies. B-cell lymphomas originate in germinal centers of the secondary lymphoid organs and are originally CD19/CD20 double-positive. Upon immunotherapy (ITx) with CD19-directed CAR T cells and/or bispecific CD20xCD3 T-cell engagers (represented by vertical lines), they routinely lose 1 or both antigens. However, CD20+ clones can reemerge in some of the distant lymph nodes upon discontinuation of ITx. The dendrogram illustrates one of the cases described by Duell et al. Ab, antibody; neg, negative; pos, positive. Professional illustration by Somersault18:24.
Several practical questions arise from this and similar studies. For example, how should apparently epitope-negative relapses be treated? Are clinical flow cytometry and immunohistochemistry assays sensitive enough to detect meaningful immunotherapy-responsive levels of expression of cognate antigens? As Duell et al point out, in both ZUMA-1 and JULIET trials of 2 distinct CART products, objective responses were observed in patients deemed CD19− at baseline.7,8 With respect to CD20 as a target, we reported in a recent issue of Blood that lymphoma cells expressing alternatively spliced, translation-deficient MS4A1 mRNA isoforms were resistant to killing by rituximab and mosunetuzumab. Strikingly, they were still sensitive to killing by CD20-directed CAR T cells.9 As sequential losses of B-cell lineage markers become commonplace occurrences,10 there is a need to incorporate into clinical practice successive biopsies aided by high-resolution immunohistochemical, flow cytometrical, and single-cell RNA-seq analyses. These analyses could inform the selection of appropriate immunotherapeutics and thereby improve outcomes.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal