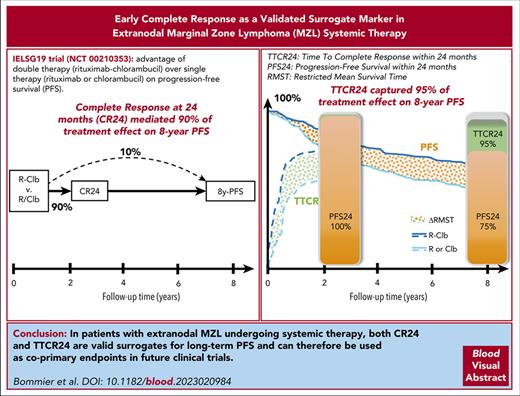
Surrogate markers provide early decision-making end points for regulatory agencies; they serve as substitutes for long-term end points.
In extranodal MZL, CR24 mediates 90% and TTCR24 captures 95% of treatment effect on 8-year PFS, which makes them valid early indicators.
Visual Abstract
Extranodal marginal zone lymphoma (EMZL) has a very indolent course, and the validation of surrogate markers could accelerate novel therapies. Although prognostic markers do exist, no surrogate markers have been validated in EMZL. We hypothesized that time to complete response within 24 months (TTCR24) and complete response (CR) at 24 months (CR24) could be valid surrogate markers of progression-free survival (PFS). The International Extranodal Lymphoma Study Group 19 phase 3 trial showed the advantage of double therapy (rituximab + chlorambucil) over single therapy (rituximab or chlorambucil) on PFS. We used 2 recently published single-trial approaches to assess whether TTCR24 and CR24 were good surrogate markers of 8-year PFS (8y-PFS). Among the 401 patients, 264 (66%) reached a CR in the first 24 months, of which 222 (84%) remained in CR at month 24. The cumulative incidence of CR over time was significantly higher in patients under double therapy (hazard ratio, 1.75; P < .001). The double therapy arm was associated with a higher CR24 rate, a shorter TTCR24, and a longer 8y-PFS. The estimated proportion of treatment effect on 8y-PFS explained by TTCR24 was 95% (95% confidence interval [CI], 0.27-1.87). CR24 was also a strong surrogate marker because it mediated 90% (95% CI, 0.51-2.22) of the treatment effect on PFS and its natural indirect effect was significant throughout the follow-up. We found that TTCR24 predicted 95% and that CR24 mediated 90% of the treatment effect on long-term PFS. Therefore, TTCR24 and CR24 could be used in clinical trials as informative and valid early indicators of treatment effect on PFS. This trial was registered at www.clinicaltrials.gov as #NCT00210353.
Introduction
Marginal zone lymphomas (MZL) involve 3 main subtypes, which present specific epidemiologic, molecular, and clinical features.1,2 Extranodal MZL (EMZL), also known as mucosa-associated lymphoid tissue lymphoma, represents the most common MZL subtype, accounting for ∼50% to 70% of MZLs and 5% to 8% of all B-cell lymphomas.3-5
Because of the natural indolent course of this disease, drug development for patients with MZL faces well-known issues. First, the higher the overall survival (OS) rate, the more difficult it is use as primary end point (because of the low expected number of events in each arm, and thus the very large number of required patients or follow-up duration), the higher the competing risk of noncancer deaths in the study population with needed long-term follow-up, as well as the associated higher risk of loss to follow-up. Second, the longer a trial takes, the higher the research costs, which discourages pharmaceutical companies of setting up randomized controlled trials; as an example, the International Extranodal Lymphoma Study Group (IELSG) 19 trial (hitherto they have only published a EMZL-specific phase 3 trial) started its accrual in 2003, published its 5-year interim analysis in 2013, and updated results in 2017.6 Third, the use of end points earlier than OS by regulatory agencies to grant accelerated approvals is, in many cancer subtypes, not supported by a statistical demonstration of surrogacy. Notably, in the cancer field, the US Food and Drug Administration (FDA) and the European Medicine Agency have considered that observed effects on progression-free survival (PFS), overall response rate, complete remission rate, event-free survival, disease-free survival, and time to progression as reasonably likely to predict clinical benefit in order to support accelerated and traditional approval, which, however, does not make them statistically validated surrogate markers in every type of malignancy.7,8
The general promise of a useful surrogate is that it provides information on how an experimental intervention would affect the primary outcome (called, true outcome) and thus potentially replace it, therefore shortening trial duration. The evaluation of a surrogate marker relies on the measure of the proportion of treatment effect on the true outcome that is explained by the surrogate marker,9 with extensive literature on the best means to evaluate putative surrogates now at investigators’ disposal.10 The gold standard for this evaluation is the meta-analytic approach, which uses multiple trials to evaluate surrogacy at 2 levels: the patient level and the trial level. This approach is based on the correlation between both treatment effects measured at the trial level on the surrogate marker and the true outcome.11 In follicular lymphoma, the Follicular Lymphoma Analysis of Surrogate Hypothesis group conducted a meta-analysis that validated complete response (CR) at 30 months after treatment start (ie, at end of maintenance therapy) as surrogate marker for PFS in first-line treatment.12 One of the drawbacks of the meta-analytical method is that it requires data from a large number of randomized controlled trials (that are not available in MZL), and it does not fully address confounding factors between trials, thus precluding causal interpretation.11,13-15 In the single-trial setting, several methods have been proposed to assess surrogacy in which the true outcome is a time-to-event outcome.16-18 Although it is still rarely used in practice, these methods assess the surrogate properties of time-to-event and binary end points at any time of interest.
Recently in the MZL field, 2 drugs have been approved by the FDA on the basis of early overall response rate and complete remission rate in phase 2 trials (ibrutinib and zanubrutinib), although CR is hitherto not a validated surrogate marker in MZL.19,20 Concomitantly, 2 studies reported that early progression of disease within 24 months was associated with OS across all MZL subtypes.21,22 Because a surrogate marker is necessarily prognostic, clinical markers encompassing the CR and PFS information up to 24 months became naturally candidate surrogate markers. In addition to CR at 24 months (CR24, binary end point), it was initially hypothesized that 24-month time to CR (TTCR24), as time-to-event end point, would capture more information than CR24, in particular regarding early CR achievement.
The objective of our study was to evaluate whether TTCR24 and CR24 could be valid surrogate markers for 8-year PFS, based on a single-trial approach.
Methods
Trial selection
The IELSG19 trial (#NCT00210353) is an open-label, randomized phase 3 trial, that was conducted at 78 centers from 6 countries with patients with EMZL to assess the benefit of the double therapy rituximab + chlorambucil (n = 132) over both single therapies rituximab (n = 138) and chlorambucil (n = 131). The investigators shared individual patient data from the trial in accordance with ethical standards. For this analysis, because we aimed to quantify how early markers captured the advantage of rituximab + chlorambucil over rituximab or chlorambucil, both single therapy arms were grouped, and comparison was made between double therapy vs single therapy.
True outcome and candidate surrogate markers
The true outcome was 8y-PFS, defined as the time from randomization to progression, relapse, or death from any cause, whichever occurs first, up to 8 years. PFS was chosen because it is the most used primary end point across MZL phase 3 trials.23 A specific time horizon was set because 1 of the methods defined the treatment effect as the difference in restricted mean survival time (RMST)24; this time horizon was set at 8 years based on data maturity (>95% of progressions had already occurred at this time point).
The analysis explored the surrogacy of 2 candidate markers, depicting the early achievement of CR, namely the TTCR24 (defined as the time from randomization to first CR, and censored at 24 months) and CR24 (defined as the CR rate at 24 months). In the IELSG19 trial, response assessment was performed according to the National Cancer Institute standardized response criteria for non-Hodgkin lymphoma25 at week 7 after treatment start, at end-of-therapy (week 25), then every 4 months for 2 years, then every 6 months for 3 years, and then annually.
Statistical analysis
All analyses, based on individual patient data, were performed on an intent-to-treat basis. PFS curves were estimated using the Kaplan-Meier method, and the log-rank test was used to compare the different treatment arms. CR cumulative incidence curves were estimated with progression/death as a competing event, and comparison between arms was performed using a Gray test.26
To summarize the 2 events of interest (CR and progression/death), a multistate model distinguishing no complete remission (no CR, initial state), CR, and progression/death was used to estimate over time the proportion of patients in CR and in progression within each arm (double therapy vs single therapy)27 and calculate mean time spent in each of these states.
To assess the surrogacy properties of the candidate markers, we used 2 validated single-trial methods.
The first approach was proposed by Parast et al17 to assess surrogacy when both surrogate and true outcomes are time-to-event end points. Surrogacy is defined as the proportion of treatment effect on the true outcome that is explained by the surrogate marker information. Importantly, surrogate marker information is defined as information on either surrogate (if the true outcome does not occur before the surrogate is assessed) or true outcome, if the latter occurs before the surrogate is assessed. The rationale is that if an individual can provide true outcome information before the surrogate assessment time, there is no need to consider the patient’s surrogate marker as we already know the true outcome. Using this method, we evaluated how much the treatment effect, that is the difference in RMST between arms, on TTCR24 would predict the treatment effect on 8y-PFS. As a reminder, RMST is the average survival from time 0 to a specified time point, and may be estimated as the area under the survival curve up to that point. The treatment effect can then be estimated using the difference of RMST between arms, thus reflecting the gain in 8-year PFS associated with the experimental therapy.28 Additionally, this method allows decomposition of the proportion of treatment effect explained by TTCR24 into both the proportion explained by the information on true outcome up to 24 months, that is PFS24, and the proportion specifically explained by TTCR24, namely its incremental value.
The second approach, developed by Vandenberghe et al,16 uses mediation analysis to decompose the treatment effect between an indirect effect via a binary surrogate and the remaining direct effect. Surrogacy is therefore the proportion of total treatment effect mediated through the surrogate. We used this method to assess the surrogate properties of CR24. Tellingly, this method was initially developed for an early binary marker, with a remaining concern that some patients may experience the true outcome before the mediator was assessed. Following considerations of Parast et al, we modified the technique to take into account the patients for which progression/death occurred within the first 24 months as information encapsulated in the surrogate. In this counterfactual framework, the direct effect captures the effect of double therapy on 8y-PFS as if allocation to double therapy rather than single therapy did not induce a change in CR24. The natural indirect effect captures the treatment effect on 8y-PFS as if we were to assign all participants to double therapy and if the distributions of CR24 were set to what it would have been with double therapy vs single therapy. The total effect provided a measure of the total causal effect of changing the treatment arm from single therapy to double therapy for each participant. Finally, the proportion of mediation yielded the proportion of the treatment effect that was genuinely mediated by CR24.
In both approaches, the closer to 1 the proportion of treatment effect that is explained by the surrogate, the better the surrogate. However, as in all single-trial approaches to date, the proportion may not always be bounded between 0 and 1. More details are provided in supplemental Methods available on the Blood website.
Results
The IELSG19 trial was a positive trial in regard with PFS and demonstrated the benefit for patients with EMZL of receiving double therapy instead of single therapy (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42-0.85; P = .003). A total of 160 events were observed throughout the follow-up (34% in double therapy arm vs 43% in single therapy arm), of which 78 (19%) and 153 (38%) occurred within 2 and 8 years after randomization, respectively.
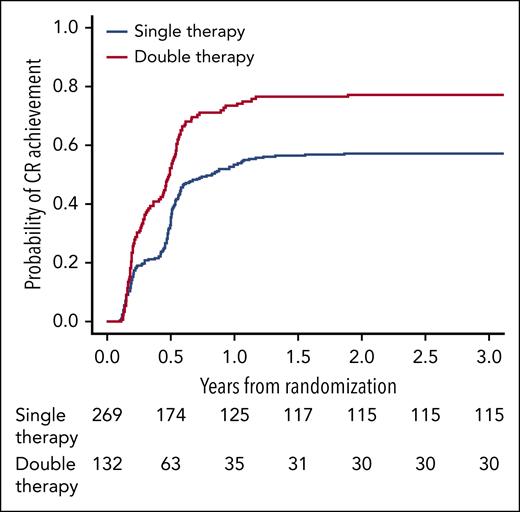
Among the 401 patients, 264 (66%) reached CR in the first 24 months, of which 222 (84%) remained in CR and 27 (10%) had progressed at month 24 (7 patients were lost to follow-up before month 24). The cumulative incidence of CR over time was significantly higher in patients under double therapy (HR, 1.75; 95% CI, 1.36-2.25; P < .001; Figure 1).
Estimated cumulative incidence of CR according to treatment arm in the IELSG19 trial, with progression/death being a competing event. Note: curves reached a plateau from 2 years after randomization to the end of the study.
Estimated cumulative incidence of CR according to treatment arm in the IELSG19 trial, with progression/death being a competing event. Note: curves reached a plateau from 2 years after randomization to the end of the study.
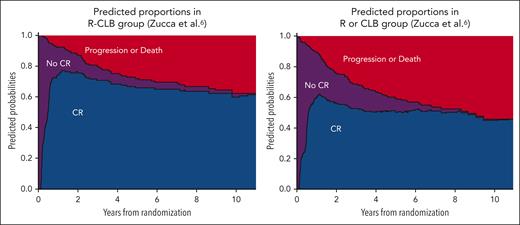
The relevance of early CR as candidate surrogate marker was also reinforced by the results of the multistate model. Between treatment start and 8 years of follow-up, patients under double therapy (mean, 6.90 years; standard error, 0.23) spent an average of 9.7 months longer in CR than those under single therapy (mean, 6.09 years; standard error, 0.24), P = .01. After adjustment on the mucosa-associated lymphoid tissue International Prognostic Index score, patients treated with double therapy had a shorter TTCR (median 0.48 vs 0.60 years; HR, 1.73; 95% CI, 1.35-2.23; P < .001) and a longer PFS (median not reached vs 8.35 years; HR, 0.54; 95% CI, 0.33-0.89; P = .02) than those in the single therapy arm (Table 1; Figure 2). In total, the double therapy arm was associated with a higher CR24 rate (72% vs 47%), a shorter TTCR24 by 3.8 months, and longer 8y-PFS by 11 months (estimations based on the RMST; see Table 2).
Multistate model for the IELSG19 trial accounting for time of complete remission occurrence
| . | Transition, no CR → CR . | Transition, no CR → progression/death . | Transition, CR → progression/death . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Double therapy vs single therapy, R-CLB vs R or CLB | 1.73 | 1.35-2.23 | <.001 | 1.03 | 0.63-1.67 | .91 | 0.54 | 0.33-0.89 | .02 |
| MALT-IPI score, high vs intermediate, or intermediate vs low | 0.65 | 0.54-0.78 | <.001 | 1.45 | 1.07-1.97 | .02 | 2.00 | 1.45-2.76 | <.001 |
| Time of transition to CR, y | — | — | — | — | — | — | 1.49 | 0.74-3.00 | .27 |
| . | Transition, no CR → CR . | Transition, no CR → progression/death . | Transition, CR → progression/death . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Double therapy vs single therapy, R-CLB vs R or CLB | 1.73 | 1.35-2.23 | <.001 | 1.03 | 0.63-1.67 | .91 | 0.54 | 0.33-0.89 | .02 |
| MALT-IPI score, high vs intermediate, or intermediate vs low | 0.65 | 0.54-0.78 | <.001 | 1.45 | 1.07-1.97 | .02 | 2.00 | 1.45-2.76 | <.001 |
| Time of transition to CR, y | — | — | — | — | — | — | 1.49 | 0.74-3.00 | .27 |
CLB, chlorambucil; R, rituximab; R-CLB, rituximab + chlorambucil; MALT-IPI: mucosa-associated lymphoid tissue International Prognostic Index.
Stacked probability plots based on the multistate model showing the proportion of patients in each state. Purple: from treatment start to experimenting either CR or progression/death. Blue: CR. Red: progression/death. R, rituximab; CLB, chlorambucil.
Stacked probability plots based on the multistate model showing the proportion of patients in each state. Purple: from treatment start to experimenting either CR or progression/death. Blue: CR. Red: progression/death. R, rituximab; CLB, chlorambucil.
Survival data regarding the true outcome and the surrogate marker in IELSG19 trial
| . | Double therapy . | Single therapy . | Difference between groups . | |
|---|---|---|---|---|
| Median PFS, y | Not reached | 8.35 | — | |
| 8y-PFS | RMST, y | 6.30 | 5.38 | 0.92 |
| Median TTCR, y | 0.49 | 0.83 | — | |
| TTCR24 | RMST, y | 1.03 | 0.71 | 0.32 |
| . | Double therapy . | Single therapy . | Difference between groups . | |
|---|---|---|---|---|
| Median PFS, y | Not reached | 8.35 | — | |
| 8y-PFS | RMST, y | 6.30 | 5.38 | 0.92 |
| Median TTCR, y | 0.49 | 0.83 | — | |
| TTCR24 | RMST, y | 1.03 | 0.71 | 0.32 |
Surrogacy analyses
Using the approach of Parast et al,17 the estimated that the proportion of treatment effect on 8y-PFS explained by TTCR24 was 95% (95% CI, 0.27-1.87) of which PFS24 explained 75% (95% CI, 0.31-1.27). Therefore, the incremental value offered by the use of TTCR24 rather than PFS24 was 20% (see Table 3).
Results of the evaluation of TTCR24 and CR24 as surrogate markers for PFS at 8 years after randomization
| . | Estimations . | Value (95% CI) . |
|---|---|---|
| Parast et al17 | ||
| TTCR24 | Proportion explained by PFS24 | 0.75 (0.31-1.27) |
| Incremental value of TTCR24 | 0.20 (−0.23 to 0.86) | |
| Total surrogate property | 0.95 (0.27-1.87) | |
| Vandenberghe et al16 | ||
| CR24 | Natural indirect effect (risk) | 1.26 (1.11-1.42) |
| Natural direct effect (risk) | 1.03 (0.85-1.21) | |
| Mediated proportion | 0.90 (0.51-2.22) |
| . | Estimations . | Value (95% CI) . |
|---|---|---|
| Parast et al17 | ||
| TTCR24 | Proportion explained by PFS24 | 0.75 (0.31-1.27) |
| Incremental value of TTCR24 | 0.20 (−0.23 to 0.86) | |
| Total surrogate property | 0.95 (0.27-1.87) | |
| Vandenberghe et al16 | ||
| CR24 | Natural indirect effect (risk) | 1.26 (1.11-1.42) |
| Natural direct effect (risk) | 1.03 (0.85-1.21) | |
| Mediated proportion | 0.90 (0.51-2.22) |
Bold type indicates the main result of each analysis.
Using the mediation analysis of Vandenberghe et al, CR24 mediated 90% (95% CI, 0.51-2.22) of the treatment effect on PFS. Notably, the natural indirect effect risk ratio (RR) was significant over time, that is, its lower bound was >1 throughout the follow-up (RR, 1.26; 95% CI, 1.11-1.42 at 8 years; details in supplemental Data).
Discussion
To our knowledge, this is the first time a surrogacy analysis has been performed in the MZL field. We found that TTCR24 and CR24 were good surrogate markers for PFS in EMZL, based on 2 validated single-trial approaches. In our study, the proportion explained by the surrogate was >80%, which is an accepted threshold regarding surrogacy validity. According to a recent systematic review, PFS is the most used primary end point across all phase 3 trials including patients with MZL.23 It is noteworthy that using TTCR24 or CR24 as a primary end point instead of PFS would drastically shorten the follow-up period needed for a clinical trial that included patients with EMZL (irrespective of the primary site). However, the 2 markers differ by the number of assessments they require. In practice, whereas CR24 only requires 1 response assessment at 24 months after treatment start, TTCR24 would require multiple early response assessments. More precisely, to demonstrate that a new regimen offers a shorter time to CR than rituximab + chlorambucil (median, 5.9 months, which corresponds to the time of end-of-treatment response assessment, ie, time of second assessment), it would be reasonable to plan at least 3 response assessments throughout the treatment course, which is not in accordance with the current guidelines.1 Nonetheless, if this new regimen was to substantially shorten the median time to CR (eg, down to 3 months), the new trial would require much less time and fewer patients to demonstrate superiority.
We found that the direction and magnitude of the treatment effect on TTCR24 enabled us to predict 95% of the direction and magnitude of the treatment effect on 8y-PFS. This striking result was supported by the causal mediation analysis results showing that CR24 mediated 90% of the treatment effect on 8y-PFS. Indeed, while a good surrogate marker is not mandatorily a mediator, a good mediator is necessarily a good surrogate marker.
It must be underlined that, for both methods, the surrogate marker involve not only information about CR but also information about progression and survival gathered until time point of surrogate marker assessment (here, 2 years). Taken together, these results suggest that (1) early achievement of CR reliably predicts a better 8y-PFS in EMZL, and (2) a large proportion of TTCR24 and CR24 surrogate properties rely on the time between treatment start and time of the surrogate marker assessment (additional results of CR12 and TTCR12 evaluations are provided in supplemental Data). Because early response assessments were consistently associated with OS across the 3 MZL subtypes,21,22,29 it is plausible that our results may also be generalized to the nodal MZL and splenic MZL (SMZL) subtypes, which will be investigated in the coming years.
In MZL, 1 issue is the heterogeneity of assessment: if progression is undoubtedly diagnosed with imaging in all subtypes, CR is either radiological for nodal MZL, SMZL, and nongastric EMZL; and biological in SMZL or histological in localized gastric EMZL.30-32 In this context, measuring progression of disease is routinely easier than complete remission. The reasons why we decided to evaluate early CR instead of early progression are that regulatory agencies such as the FDA historically prioritized the surrogate markers that translate into a deep response, and that the information on CR was reasonably thought to provide an incremental value to the information on progression (PFS24), which turned out to be the trend in our analysis (>20%; Table 3).
A first limitation in the interpretation of our results is that the CI of the incremental value of TTCR24 over PFS24 crossed 0, thus suggesting that, despite it being a valid surrogate, TTCR24 does not significantly improve the PFS24 capture of treatment effect on 8y-PFS. A second limitation is that CR24 and TTCR24 were evaluated in the setting of an immunochemotherapeutic regimen and a response assessment by computed tomography scan. Although future surrogacy analysis would need to confirm their surrogate properties in randomized trials using new targeted therapies (eg, zanubrutinib) and 18[F]fluoro-2-deoxy-d-glucose–positron emission tomography/computed tomography for response assessment, these 2 markers may reasonably be used as surrogates in the meantime.
Single-trial approaches also have their own limitations. First, the current measures used to evaluate surrogate markers can display wide CIs outside of (0,1), unless forced with a transformation. This is a known challenge in the use of the proportion of treatment effect explained quantity as a measure of surrogacy, and future work is needed to identify methods to improve interpretation. Second, single-trial approaches suffer from the lack of trial-level evidence. However, they have the advantage of requiring only 1 trial, and of providing information on potential surrogate markers that would be worth exploring through a meta-analytic approach. Because of the scarcity of randomized controlled trials in MZL, a meta-analytic approach was unrealistic at the time of this analysis but could be undertaken as more trials are completed. To that end, we established the MASH (MZL Assessment of Surrogacy Hypothesis) project, an international academic collaboration that aims at constructing a database integrating individual patient data from the latest MZL trials to allow the meta-analytic evaluation of CR24 as surrogate marker for PFS across all MZL subtypes.
In conclusion, CR24 and TTCR24 are validated surrogate markers for PFS in first-line EMZL. From now on, they can be used in randomized trials as valid coprimary end points with PFS. Because CR24 and TTCR24 can be relied upon to predict a clinical benefit, their use may expedite therapeutic development and justify the granting of accelerated approvals by regulatory agencies.
Acknowledgments
The authors sincerely thank Sjouke Vandenberghe and Stijn Vansteelandt (University of Ghent), Layla Parast (RAND Corporation), and Tianxi Cai (Harvard University) for the fruitful exchanges we had with them to explore their surrogacy single-trial approaches.
Authorship
Contribution: C.B., J.L., and C.T. conceived the presented idea, and set up the methodology; A.C. and E.Z. provided the individual patient data; C.B. and J.L. performed the statistical analysis; all authors are active contributors to the MZL Assessment of Surrogacy Hypothesis (MASH) project; and all authors participated in the writing of the draft manuscript and reviewed the final paper.
Conflict-of-interest disclosure: C.B., A.C., E.Z., G.N., and C.T. are part of the International Extranodal Lymphoma Study Group (IELSG), which carried out the IELSG19 trial. C.B. was supported by INSERM/AvieSan-Institut Thématique Multi-Organisme Cancer (ITMO) through a doctoral grant, and was awarded the Bertrand Coiffier Prize by Lymphoma Study Association (LYSA) /European Lymphoma Institute (ELI); and as mobility support, he also received funding from the Philippe Foundation and Institut Servier (CT0101951). The remaining authors declare no competing financial interests.
Correspondence: Côme Bommier, Department of Hemato-Oncology, Hôpital Saint-Louis, DMU DHI, 1 Ave Claude Vellefaux, 75010 Paris, France; email: come.bommier@aphp.fr.
References
Author notes
∗C.T. and J.L. contributed equally to this work.
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 December 2022 (Poster #1557).
The data that support the findings of this study are available from the International Extranodal Lymphoma Study Group. Restrictions apply to the availability of these data. Data are available on request from the authors Emanuele Zucca (emanuele.zucca@eoc.ch), Annarita Conconi (annarita.conconi@gmail.com), and Côme Bommier (come.bommier@gmail.com) with the permission of the International Extranodal Lymphoma Study Group.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal