In this issue of Blood, Ellis et al1 report stunning observations on the role of glycoprotein Ibα (GPIbα) and filamin A (FlnA) in thrombopoiesis using cutting-edge microscopic techniques. Their results may impact the general view of thrombopoiesis, bringing the role of platelet size into focus.
Macrothrombocytopenia is a disease characterized by low platelet count but with enlarged platelet size. This disease has been associated with several mutations impacting the platelet receptor GPIbα, including glycosylation defects resulting in defective synthesis of GPIbα, and the cytoskeletal linker protein FlnA. Since the cytoplasmic domain of GPIbα binds directly to the domain 17 of FlnA,2 the interaction of GPIbα and FlnA should be essential to the generation of platelets, especially normal-sized platelets. The underlying mechanism of this interaction, however, has been elusive.
Platelets are generated from megakaryocytes (MKs) in bone marrows and, to some extent, other tissues such as lungs. MKs are differentiated from hematopoietic stem cells. Once differentiated, an MK matures into or generates hundreds of platelets in a very efficient manner in vivo, the detail of which is not clear. This maturation step has not been completely reproduced in vitro, despite remarkable progress in the area.3,4 Previous studies have demonstrated that missing GPIbα or FlnA impacts not the differentiation but the maturation step. Thus, further investigating the effect of the GPIbα-FlnA interaction on MK maturation in an animal model is warranted.
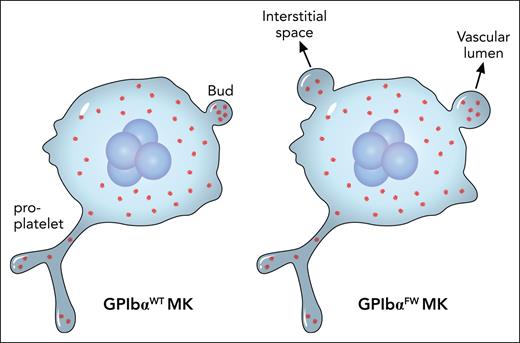
In their earlier studies5,6 Jackson and colleagues identified a double mutation termed FW in the cytoplasmic domain of GPIbα that specifically abolished its interaction with FlnA. In this study, they created a knock-in mouse strain that expressed the mutated human GPIbαFW and compared it with the wild-type counterpart. As expected, the GPIbαFW strain had macrothrombocytopenia. After carefully ruling out possibilities such as an effect on MK ploidy or platelet clearance, they made 2 major observations that could nicely explain the phenotype. First, there is irregular distribution of FlnA and abnormal formation of the demarcation membrane system in the GPIbαFW MK. Second, and surprisingly, no changes in proplatelet formation but significant changes in MK budding are present in the mutant (see figure). More specifically, neither the frequency of budding nor the number of buds in MKs are altered. Rather, the size of the bud in the MK and its misplacement into the interstitial compartment of the bone marrow are significantly increased.
The major difference between GPIbαWT and GPIbαFW MKs is not in the proplatelet formation therein, but in the size and location of the buds. This could nicely explain macrothrombocytopenia associated with the FW mutation that disrupts the GPIbα-FlnA interaction. Professional illustration by Patrick Lane, ScEYEnce Studios.
The major difference between GPIbαWT and GPIbαFW MKs is not in the proplatelet formation therein, but in the size and location of the buds. This could nicely explain macrothrombocytopenia associated with the FW mutation that disrupts the GPIbα-FlnA interaction. Professional illustration by Patrick Lane, ScEYEnce Studios.
Budding in MKs is a new and controversial concept in thrombopoiesis.7-9 The current paradigm of MK maturation centers around formation of proplatelets, which are long, branching membrane structures protruding from the MK surface, and subsequent release of preplatelets and platelets from proplatelets in the vascular lumen space.10 By observing abnormal budding but little change of proplatelets in MKs of the GPIbαFW mouse, Ellis et al provide additional evidence supporting the budding model. That mouse knockout models of GPIbα and FlnA, as well as GPIbαFW, largely recapitulate the human disease and add pathological and clinical relevance to the budding model of thrombopoiesis.
The finding of Ellis et al raises a few new questions. Only the transmembrane and cytoplasmic domains of GPIbα are needed to restore normal distribution of FlnA in the MK and ameliorate the macrothrombocytopenic phenotype.1 Presumably they mediate the attachment of FlnA to a specific membrane-proximal location. How does FlnA in this location regulate synthesis and assembly of lipids that make up the demarcation membrane system? Another related question is about the size of the bud on the MK surface: how is it determined or regulated? Although the finding of Ellis et al provides the first clues for the puzzle, there are still many missing pieces in the puzzle that connects the GPIbα-FlnA interaction with the formation of the marginal band, the circular microtubule structure that sets the platelet periphery, in a bud. Finally, how does the GPIbα-FlnA interaction inside the MK coordinate with the sinusoidal microenvironment in the bone marrow and determine the direction of budding on the MK surface?
It took Jackson and colleagues 18 years to place the FW mutation into appropriate animal models and produce sufficient evidence for the exciting and provocative connection between macrothrombocytopenia and dysregulated budding on the MK surface.1,6 One can only imagine the hurdles they needed to overcome to bring the study to a fruitful completion. We need to appreciate their dedication for answering the long-standing questions about platelet size and the exciting new venue of investigation into MK budding that they have now made possible.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal