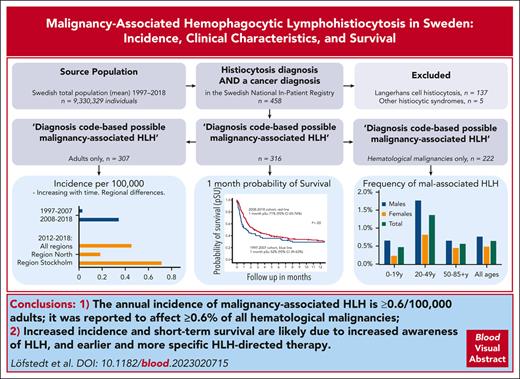
The annual incidence of malignancy-associated HLH is ≥0.62 per 100 000 adults, and it affects ≥0.6% of all hematological malignancies.
Increased incidence and short-term survival are likely due to increased awareness of HLH and earlier and more specific HLH-directed therapy.
Visual Abstract
We evaluated malignancy-associated hemophagocytic lymphohistiocytosis (mal-HLH) in Sweden regarding population-based incidence, clinical features, and survival. From 1997 to 2018, we identified 307 adults (≥18 years old) and 9 children (209 males, 107 females; P < .001) with both an HLH-related diagnosis and malignant disease, corresponding to 0.19 per 100 000 adults annually (0.15/100 000 for the entire population), increasing from 0.026 (1997-2007) to 0.34 (2008-2018) (P < .001). In the latest 7-year period (2012-2018), the annual incidence was 0.45 per 100 000 adults (n = 246). This incidence varied between the 6 health care regions in Sweden, from 0.18 to 0.71 (Region Stockholm) per 100 000 adults annually (P < .001), likely due to variable awareness. Mal-HLH was reported in 0.6% of all hematological malignancies, with the highest proportion (2.5%) in young males. Among the 316 patients, the 1-month probability of survival, likely representing the HLH episode, increased significantly from 52% (95% confidence interval [CI], 40-63) (1997-2007) to 71% (95% CI, 65-76) (2008-2018), whereas 2-year survival remained poor (25%; 95% CI, 20-30). Altogether, 52% were lymphomas, 29% leukemias, 8% other hematological malignancies, and 11% solid tumors. Males were more affected than females by mal-HLH, also taking the over-representation of males with hematological malignancies into account (P = .0012). Validation by medical-file reviews revealed 13% over-reporting of HLH. We conclude that the annual mal-HLH incidence has increased 10-fold and was at least 0.71 per 100 000 adults from 2012 to 2018, that is, 0.62 per 100 000 adults considering 13% estimated HLH over-reporting, and that early survival improved significantly, likely due to increased awareness and more HLH-directed therapy.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a severe and often fatal hyperinflammation caused by immune dysregulation and excessive activation of macrophages and lymphocytes,1 often referred to as a “hypercytokinemia” or a “cytokine storm.”2,3 The syndrome is characterized by unremitting fever, hyperferritinemia, cytopenia, coagulopathy, hepatosplenomegaly, and hemophagocytosis.4 HLH is divided into a primary (genetic/familial) form (pHLH/FHL), which is typically caused by aberrations in the genes involved in the cytotoxic pathway, and a secondary (acquired) form (sHLH). In sHLH, hyperinflammation is most commonly triggered by infections, malignancies, or autoimmune/autoinflammatory conditions.5,6
HLH diagnosis is often based on the fulfillment of 5 of the 8 HLH-2004 diagnostic criteria.4 An alternative in adults is HScore, in which a patient is defined as having HLH if HScore ≥169, reported to accurately classify 90% of the patients (sensitivity, 93%; specificity, 86%).7 Both the HLH-2004 criteria and the HScore have been proven to have good diagnostic accuracy when evaluated in critically ill adults.8 Awareness of sHLH has recently grown markedly, and consensus recommendations for the management of HLH in adults, HLH in malignancies, and for HLH in critically ill are now available.9-11 Moreover, a soluble CD25 (sCD25)/ferritin ratio >2.0 has been reported to have a positive predictive value of 85% in predicting that a patient with HLH has malignancy-associated HLH (mal-HLH).12 In addition, levels of sCD25 >3900 U/mL and ferritin >1000 μg/L have been reported to be predictive of mortality in patients with diverse hematological malignancies and HLH.13
Malignancy-associated HLH is, together with infection-associated HLH, the most common form of sHLH, and of 2197 adult patients with HLH, 1047 (48%) were associated with neoplasms.6 Others have reported that 70% of sHLH cases may be malignancy-associated.14 Mal-HLH has been associated with various hematological malignancies, most commonly T and NK-cell lymphomas (35%), B-cell lymphomas (32%), Hodgkin lymphomas (6%), and leukemia (6%), but rarely with solid malignancies (3%).6,15 The estimated overall incidence of mal-HLH has been reported to be ∼1% in patients with hematological malignancies,16 but as high as 9% in patients with acute myeloid leukemia (AML) undergoing intensive chemotherapy, with infections being the most frequent trigger.17 In hematological malignancies, and in AML in particular, several HLH criteria are often fulfilled by the consequences of the malignancy and its treatment per se, making correct diagnosis challenging. In 2015, a nomenclature dividing mal-HLH into either malignancy-triggered HLH (M-HLH), in which HLH typically is present before or concomitantly with the diagnosis of the malignancy, or HLH during chemotherapy (Ch-HLH), the latter often triggered by infections, was suggested to segregate the origin of the inflammatory response and to assist in treatment decisions.14
Mal-HLH is the subgroup of sHLH with the worst prognosis.6,18,19 Furthermore, the accurate incidence of mal-HLH is not fully known and may be underestimated. Here, taking advantage of population-based registers in Sweden, we aimed to evaluate the national population-based incidence of mal-HLH in Sweden and its prognosis and characterize its clinical and laboratory features.
Patients and methods
The entire Swedish population between 1997 and 2018 was studied, with an average of 9 330 329 individuals per year (7 363 505 adults ≥18 years old). Using the Swedish National Patient Registry (NPR), a population-based registry of inpatient care within the National Board of Health and Welfare in Sweden with complete data on all discharged care events in specialized care since 1987,20 we identified patients with HLH-associated diagnoses that were also reported to have malignancies according to the International Classification of Diseases (ICD). The ICD-10 diagnoses D76.0-D76.3 and C96.0 were used to identify patients with histiocytic disorders and those that also had a registered malignant diagnosis (C00-C97 in ICD-10) on 31 December, 2018, were included in the study. The assigned index date was when they first received a histiocytosis-related diagnosis. All the included histiocytosis-related diagnoses are presented in supplemental Table 1, available on the Blood website. Additional data on the subtype of cancer as well as the date and cause of death were retrieved from the Swedish Cancer Registry (SCR) and Cause of Death Registry (CoDR), respectively. Patients with histiocytic disorders identified as HLH that also had a malignant disease from 1997 to 2018 were labeled as having “diagnosis code-based possible mal-HLH,” the incidence of which was calculated based on available NPR data. All information regarding various cohorts in the Swedish population was retrieved from “Population statistics” in Statistics Sweden21 (Figure 1; Table 1). For more information on the NPR, SCR, and CoDR, refer to supplemental Methods.
Flowchart of all individuals studied from 1997 to 2018. ∗Histiocytosis-related disease = ICD-10 codes D76.0 to D76.3 and C96.0, and cancer diagnosis = ICD-10 codes C00.0 to C97.00. ∗∗The Langerhans cell histiocytosis = ICD-10 codes D76.0, C96.0, C96.5, C96.6, and the other specified histiocytic syndromes = ICD-10 codes D76.3A to E. ∗∗∗Probable malignancy-associated HLH (mal-HLH) is defined as HLH within −90 to +365 days in relation to the cancer diagnosis.
Flowchart of all individuals studied from 1997 to 2018. ∗Histiocytosis-related disease = ICD-10 codes D76.0 to D76.3 and C96.0, and cancer diagnosis = ICD-10 codes C00.0 to C97.00. ∗∗The Langerhans cell histiocytosis = ICD-10 codes D76.0, C96.0, C96.5, C96.6, and the other specified histiocytic syndromes = ICD-10 codes D76.3A to E. ∗∗∗Probable malignancy-associated HLH (mal-HLH) is defined as HLH within −90 to +365 days in relation to the cancer diagnosis.
Annual incidence of malignancy-associated HLH per health care region overall and in adults from 2012 to 2018 per 100 000 individuals in “diagnosis code-based possible malignancy-associated HLH” and “probable malignancy-associated HLH”
| Health care region . | Incidence . | Cases . | Mean population 31 December 2012-2018 . |
|---|---|---|---|
| ”Diagnosis code-based possible malignancy associated HLH” (n = 249) | |||
| Stockholm | |||
| Total | 0.55 | 89 | 2 292 250 |
| Adults | 0.71 | 89 | 1 795 693 |
| South East | |||
| Total | 0.39 | 28 | 1 035 005 |
| Adults | 0.48 | 28 | 824 810 |
| South (excluding Halland) | |||
| Total | 0.51 | 59 | 1 657 096 |
| Adults | 0.63 | 58 | 1 313 444 |
| West (including Halland) | |||
| Total | 0.27 | 37 | 1 968 503 |
| Adults | 0.32 | 35 | 1 562 747 |
| Mid Sweden | |||
| Total | 0.19 | 27 | 2 038 479 |
| Adults | 0.24 | 27 | 1 629 897 |
| North | |||
| Total | 0.15 | 9 | 886 482 |
| Adults | 0.18 | 9 | 716 320 |
| ”Probable malignancy-associated HLH” from −90 days to +365 days in relation to the cancer diagnosis (n = 146) | |||
| Stockholm | |||
| Total | 0.30 | 48 | 2 292 250 |
| Adults | 0.38 | 48 | 1 795 693 |
| South East | |||
| Total | 0.28 | 20 | 1 035 005 |
| Adults | 0.35 | 20 | 824 810 |
| South (excluding Halland) | |||
| Total | 0.28 | 32 | 1 657 096 |
| Adults | 0.35 | 31 | 1 313 444 |
| West (including Halland) | |||
| Total | 0.17 | 24 | 1 968 503 |
| Adults | 0.21 | 23 | 1 562 747 |
| Mid Sweden | |||
| Total | 0.11 | 15 | 2 038 479 |
| Adults | 0.14 | 15 | 1 629 897 |
| North | |||
| Total | 0.11 | 7 | 886 482 |
| Adults | 0.14 | 7 | 716 320 |
| Health care region . | Incidence . | Cases . | Mean population 31 December 2012-2018 . |
|---|---|---|---|
| ”Diagnosis code-based possible malignancy associated HLH” (n = 249) | |||
| Stockholm | |||
| Total | 0.55 | 89 | 2 292 250 |
| Adults | 0.71 | 89 | 1 795 693 |
| South East | |||
| Total | 0.39 | 28 | 1 035 005 |
| Adults | 0.48 | 28 | 824 810 |
| South (excluding Halland) | |||
| Total | 0.51 | 59 | 1 657 096 |
| Adults | 0.63 | 58 | 1 313 444 |
| West (including Halland) | |||
| Total | 0.27 | 37 | 1 968 503 |
| Adults | 0.32 | 35 | 1 562 747 |
| Mid Sweden | |||
| Total | 0.19 | 27 | 2 038 479 |
| Adults | 0.24 | 27 | 1 629 897 |
| North | |||
| Total | 0.15 | 9 | 886 482 |
| Adults | 0.18 | 9 | 716 320 |
| ”Probable malignancy-associated HLH” from −90 days to +365 days in relation to the cancer diagnosis (n = 146) | |||
| Stockholm | |||
| Total | 0.30 | 48 | 2 292 250 |
| Adults | 0.38 | 48 | 1 795 693 |
| South East | |||
| Total | 0.28 | 20 | 1 035 005 |
| Adults | 0.35 | 20 | 824 810 |
| South (excluding Halland) | |||
| Total | 0.28 | 32 | 1 657 096 |
| Adults | 0.35 | 31 | 1 313 444 |
| West (including Halland) | |||
| Total | 0.17 | 24 | 1 968 503 |
| Adults | 0.21 | 23 | 1 562 747 |
| Mid Sweden | |||
| Total | 0.11 | 15 | 2 038 479 |
| Adults | 0.14 | 15 | 1 629 897 |
| North | |||
| Total | 0.11 | 7 | 886 482 |
| Adults | 0.14 | 7 | 716 320 |
For individuals with “diagnosis code-based possible mal-HLH” from 1997 to 2011 (n = 67), the treating hospital and department were provided by the National Board of Health and Welfare in Sweden. Their medical files were requested by their treating hospitals via postal mail with reminders. Ultimately, files from 62 of 67 (93%) patients were available and used to validate the “diagnosis code-based possible mal-HLH,” retrieve clinical and laboratory data, and categorize them as M-HLH or Ch-HLH.14
For individuals with “diagnosis code-based possible mal-HLH” from 2012 to 2018 (n = 249), additional information on malignancy subtype, date of cancer diagnosis, date of death, and the region was retrieved from the SCR and CoDR. Patients with an index date of HLH from 90 days before the cancer diagnosis until 365 days thereafter were labeled as having “probable mal-HLH” (Figure 1). Data on individuals with hematological malignancies in Sweden from 2012 to 2018 were retrieved from the SCR using ICD-7 codes 200 to 207. We have no information on the genetic testing of HLH-causing mutations in most patients, and this is likely limited to younger patients.
In addition, PubMed-indexed articles on mal-HLH in Sweden until February 2023 were reviewed to compare the regional annual incidences.16,22,23
The studies were approved by the Ethics Committee at Karolinska Institutet (2008/571-31/3; supplementary approvals 2012/713-32 and 2019-03974). All authors had access to the primary data.
Statistical analysis
The incidence rate was calculated as the number of individuals diagnosed with mal-HLH per 100 000 individuals per year. To compare the annual incidence during the first 11-year period from 1997 to 2007 with the last 11-year period from 2008 to 2018, the Pearson χ2 test was used. This test was also used to compare the incidence of mal-HLH in different health regions in Sweden, and when comparing the proportions of men and women with hematological “diagnosis code-based possible malignancy-associated HLH” from 2012 to 2018 (n = 222) to all Swedish patients with hematological malignancies in this period. When comparing age at diagnosis in these patients with age at diagnosis in all hematological malignancies in Sweden during this period, Fisher exact test was used. To compare the differences in incidence between males and females, an exact binomial test was used. All tests were performed in R version 4.2.1 (R Core Team, 2022).24 The probability of survival was estimated using the Kaplan-Meier method for univariate tests using SPSS (SPSS for Windows; IBM Corp, Chicago, IL). For comparing the probability of survival during the first 11 years (1997-2007) with the last 11 years (2008-2018), the generalized Wilcoxon and log-rank tests were used.
Results
Incidence of “diagnosis code-based possible malignancy-associated HLH” over time
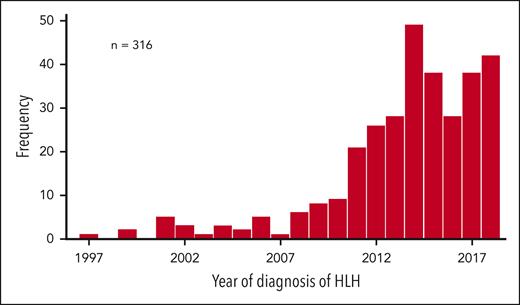
The 458 patients with histiocytic diagnoses of D76.0-D76.3 or C96.0, according to ICD-10, as well as a registered malignant diagnosis (C00-C97 in ICD-10) from 1997 to 2018 were included in the study. Patients with Langerhans cell histiocytosis (n = 137) or other specified histiocytic syndromes (n = 5) were then excluded because these diseases do not fulfill the hallmarks of cancer as described by Hanahan and Weinberg,25,26 leaving 316 patients (209 males, 107 females; P < .001) with “diagnosis code-based possible mal-HLH.” With a mean population of 9 330 329, this corresponds to 0.15 per 100 000 individuals per year, with 0.023 during the first 11-year period from 1997 to 2007 (n = 23, population 8 966 098) and 0.27 per 11-year period from 2008 to 2018 (n = 293, population 9 964 560) (P < .001) (Figure 2). For adults (n = 307, population 7 363 505), the incidence was 0.19 per 100 000 per year; 0.026 during 1997 to 2007 (n = 20, population 7 025 956), 0.34 during 2008 to 2018 (n = 287, population 7 701 054), (P < .001), and 0.49 during the last 5-year period from 2014 to 2018 (n = 192, population 7 842 911).
Frequency of malignancy-associated HLH over time. The frequency of patients with “diagnosis code-based possible malignancy-associated HLH” from 1997 to 2018 (n = 316) is shown (y-axis) relative to the year of HLH diagnosis (x-axis).
Frequency of malignancy-associated HLH over time. The frequency of patients with “diagnosis code-based possible malignancy-associated HLH” from 1997 to 2018 (n = 316) is shown (y-axis) relative to the year of HLH diagnosis (x-axis).
Males (57%) are more affected than females (43%) by hematological malignancies in general; however, men (68%) were more affected than females (32%) by “diagnosis code-based possible mal-HLH” as compared with all patients with hematological malignancies from 2012 to 2018 (P = .0012) (Figure 3A). Of all patients with hematological malignancies between 2012 and 2018, 0.6% had “diagnosis code-based possible mal-HLH” reported, with the highest proportion (2.5%) in males aged 25 to 29 years (Figure 3B).
Frequency and age at diagnosis of “diagnosis code-based possible malignancy-associated HLH” in patients with hematological malignancies (n = 222) and of all hematological malignancies in Sweden from 2012 to 2018 (n = 35 117). (A) Number of patients with “diagnosis code-based possible malignancy-associated HLH” from 2012 to 2018 with an underlying hematological malignancy (bar chart with y-axis to the left) in relation to the total number of hematological malignancies in Sweden from 2012 to 2018 (line chart with y-axis to the right). All individuals are divided into age groups of 5 years (x-axis); males are depicted as blue and females as orange. (B) Proportion of patients with hematological malignancies in Sweden from 2012 to 2018 that have “diagnosis code-based possible malignancy-associated HLH.” Individuals are divided into age groups of 5 years (x-axis); males are depicted with blue, females with orange, and both sexes with green bars.
Frequency and age at diagnosis of “diagnosis code-based possible malignancy-associated HLH” in patients with hematological malignancies (n = 222) and of all hematological malignancies in Sweden from 2012 to 2018 (n = 35 117). (A) Number of patients with “diagnosis code-based possible malignancy-associated HLH” from 2012 to 2018 with an underlying hematological malignancy (bar chart with y-axis to the left) in relation to the total number of hematological malignancies in Sweden from 2012 to 2018 (line chart with y-axis to the right). All individuals are divided into age groups of 5 years (x-axis); males are depicted as blue and females as orange. (B) Proportion of patients with hematological malignancies in Sweden from 2012 to 2018 that have “diagnosis code-based possible malignancy-associated HLH.” Individuals are divided into age groups of 5 years (x-axis); males are depicted with blue, females with orange, and both sexes with green bars.
Incidence of “probable malignancy-associated HLH”
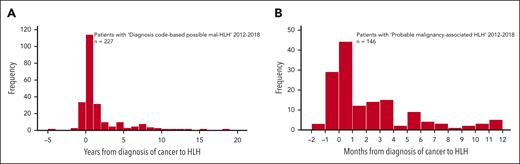
From 2012 to 2018, 249 patients were identified as having “diagnosis code-based possible mal-HLH.” Of these, 146 (94 males and 52 females; P < .001) were diagnosed with HLH from 90 days before to 365 days after cancer diagnosis. We labeled this group as “probable mal-HLH,” being aware that we may exclude patients for whom HLH and malignancy were actually associated and, but less likely, include patients for whom HLH and malignancy were not associated. Of the 103 nonselected patients, 4 had HLH >90 days before the cancer diagnosis and 77 had HLH >1 year thereafter (missing data = 22) (Figure 4A). The estimated incidence of “probable mal-HLH” was 0.21 per 100 000 per year (population 9 877 816). In adults (n = 144, population 7 842 911), the incidence was 0.26 per 100 000 per year.
Time relation between HLH and cancer diagnoses. (A) Frequency of patients with “diagnosis code-based possible malignancy-associated HLH” 2012 to 2018 with a date of cancer diagnosis in Swedish Cancer Register (n = 227) (y-axis) in relation to the time (years) of HLH diagnosis to the cancer diagnosis (x-axis, HLH diagnosis ranging from −4.6 years to +18.2 years from cancer diagnosis). (B) Frequency of patients with “probable malignancy-associated HLH” defined as HLH occurring within day −90 to day +365 from their cancer diagnosis between 2012 and 2018 (n = 146) in relation to the time (months) from HLH diagnosis to cancer diagnosis (x-axis). The time point zero is the diagnosis of cancer.
Time relation between HLH and cancer diagnoses. (A) Frequency of patients with “diagnosis code-based possible malignancy-associated HLH” 2012 to 2018 with a date of cancer diagnosis in Swedish Cancer Register (n = 227) (y-axis) in relation to the time (years) of HLH diagnosis to the cancer diagnosis (x-axis, HLH diagnosis ranging from −4.6 years to +18.2 years from cancer diagnosis). (B) Frequency of patients with “probable malignancy-associated HLH” defined as HLH occurring within day −90 to day +365 from their cancer diagnosis between 2012 and 2018 (n = 146) in relation to the time (months) from HLH diagnosis to cancer diagnosis (x-axis). The time point zero is the diagnosis of cancer.
Incidence of malignancy-associated HLH per health care region
Next, we investigated the possible regional differences within Sweden, which could indicate missed diagnoses in some regions. Sweden has 6 national health care regions: South, Southeast, West, Stockholm, Mid Sweden, and North. The incidence of “diagnosis code-based possible malignancy-associated HLH” from 2012 to 2018 among the 249 identified individuals varied between 0.55 per 100 000 per year in Region Stockholm and 0.15 per 100 000 per year in Region North, corresponding to a 3.7-fold difference (P < .001) (Table 1); for an illustrative map, refer to supplemental Figure 1. The corresponding figures among the 246 adults were 0.71 and 0.18 per 100 000 per year (P < .001), respectively. Pearson χ2 test confirmed a difference in incidence in adults across all regions (P < .001) (Table 1).
Similarly, the incidence of “probable mal-HLH” in this period among the 146 individuals identified varied between 0.30 per 100 000 per year in Stockholm and 0.11 per 100 000 per year in the North (Table 1). The corresponding figures among the 144 adults were 0.38 and 0.14 (P = .015), respectively, and there was a different incidence across all regions (P < .001) (Table 1). Figure 4B presents these 146 malignancies, classified as above, in relation to the number of days from the diagnosis of cancer to HLH diagnosis. Notably, almost half of these patients had their HLH diagnosis within 30 days before or after their cancer diagnosis.
Validation of the diagnosis code–based diagnosis of HLH
Of the 67 with “diagnosis code-based possible mal-HLH” from 1997 to 2011, medical files were received on 62 (93%). After review, 8 (13%) were excluded due to alternative diagnoses: misdiagnosed Langerhans cell histiocytosis (n = 3) or other diagnoses sharing features with HLH (lymphadenopathy [n = 1], thrombotic thrombocytopenic purpura [n = 1], peritoneal carcinoma [n = 1]), and for 2 patients with HLH mentioned in the medical files, no clinical parameters establishing the HLH diagnosis could be noted (rectal cancer [n = 1], T-cell lymphoma [n = 1]). One additional patient had primary HLH. Four patients had undergone allogeneic hematopoietic stem cell transplantation (HSCT) before the HLH episode and their hyperinflammation was considered HSCT-associated, leaving 49 patients for review of clinical characteristics and laboratory parameters.
Clinical characteristics and laboratory parameters of patients with malignancy-associated HLH
Detailed clinical characterization of these 49 patients, in addition to the 4 with HSCT-associated HLH, is presented in supplemental Table 2. Of these 49, 20 were categorized as M-HLH, 15 as Ch-HLH, and 14 had <4 diagnostic criteria fulfilled, largely because the criteria were not analyzed and they were not categorized into any group. All 35 patients with M-HLH or Ch-HLH had fever and elevated ferritin, and for all diagnostic criteria evaluated, most evaluated patients fulfilled the criteria. Of the 14 patients with <4 diagnostic criteria fulfilled, the majority fulfilled the criteria for fever, hemophagocytosis, bicytopenia, hypofibrinogenemia, elevated ferritin, and elevated sCD25, but not splenomegaly (supplemental Table 2).
Classification of HLH-associated malignancies
Lymphomas were the most common malignancies associated with mal-HLH, followed by leukemias. Among the 316 patients with “diagnosis code-based possible mal-HLH,” 30% had B-cell lymphoma, 16% had natural killer (NK)/T-cell lymphoma, 6% had Hodgkin lymphoma, 16% had myeloid leukemia, 13% had lymphocytic leukemia, and 9% had other hematological malignancies. Only 11% had solid tumors.
Among the 146 individuals with “probable mal-HLH” from 2012 to 2018, 34% had B-cell lymphomas, 15% had NK/T-cell lymphomas, 8% had Hodgkin lymphoma, 22% had myeloid leukemia, 8% had lymphocytic leukemia, and 8% had other malignant hematological conditions, whereas 6% had solid tumors. Of these 9 solid tumors, 2 were located in the prostate and 1 each in the esophagus, gastric cardia, transverse colon, peritoneum, vulva, breast, and skin (malignant melanoma). Four of these patients also had hematological malignancies but further away in time from their HLH.
Among the 35 patients with “confirmed mal-HLH’” from 1997 to 2011, the most common associated malignancy in the M-HLH group was by far NK/T-cell lymphomas (53%) whereas only 1 had lymphocytic leukemia and none had myeloid leukemia. In contrast, in Ch-HLH, the most common form was B-cell lymphoma (30%), followed by myeloid leukemia (20%), and NK/T-cell lymphoma (20%) (supplemental Table 3).
Age at diagnosis of malignancy-associated HLH
The median age at diagnosis was 65 years (interquartile range = Q3 − Q1 = 73 − 51 years) in the 249 patients with “diagnosis code-based possible mal-HLH” from 2012 to 2018. The 222 patients with hematological malignancies had a significantly earlier age of onset than all patients with hematological malignancies in Sweden during this period (P < .001) (Figure 3A).
Therapy and outcome
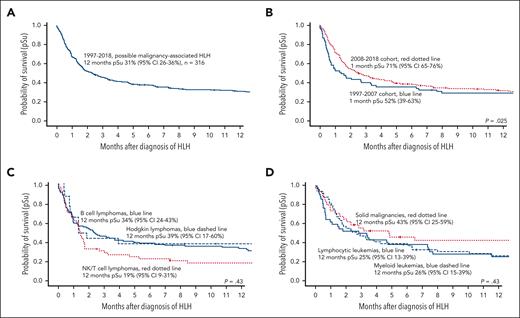
In “diagnosis code-based possible mal-HLH,” the 1-month probability of survival improved significantly from 52% (95% confidence interval [CI], 40-63) for patients diagnosed the first 11-year-period 1997 to 2007 to 71% (95% CI, 65-76) for the second 11-year-period 2008 to 2018 (Table 2, Figure 5A-B). The corresponding 2-month probability of survival was 45% (95% CI, 32-56) and 53% (95% CI, 47-60), respectively; that is, there was a nonsignificant trend toward better survival, whereas survival after 6-month and 12-month was not significantly different (Table 2). Nevertheless, the entire 1-year survival for patients diagnosed between 2008 and 2018 was significantly better than that for those diagnosed from 1997 to 2007 according to the generalized Wilcoxon test (P = .025), which gives more weight to deaths at early time points, whereas the log-rank test, which gives equal weight to all time points, showed no significance (P = .14) (Figure 5B).
Survival in all 316 patients with “diagnosis code-based possible malignancy-associated HLH” and a comparison between those diagnosed during the first 11 years of the study and those diagnosed during the remaining 11 years of the study
| Time period . | Probability of survival, % (95% CI) . | ||||
|---|---|---|---|---|---|
| 1 mo . | 2 mo . | 6 mo . | 12 mo . | 24 mo . | |
| 1997-2007 | 52 (40-63)∗ | 45 (32-56) | 35 (24-47) | 29 (19-40) | 21 (12-32) |
| 2008-2018 | 71 (65-76)∗ | 53 (47-60) | 38 (32-44) | 31 (25-37) | 26 (20-31) |
| 1997-2018 | 67 (62-72) | 52 (46-57) | 37 (32-43) | 31 (25-36) | 25 (20-30) |
| Time period . | Probability of survival, % (95% CI) . | ||||
|---|---|---|---|---|---|
| 1 mo . | 2 mo . | 6 mo . | 12 mo . | 24 mo . | |
| 1997-2007 | 52 (40-63)∗ | 45 (32-56) | 35 (24-47) | 29 (19-40) | 21 (12-32) |
| 2008-2018 | 71 (65-76)∗ | 53 (47-60) | 38 (32-44) | 31 (25-37) | 26 (20-31) |
| 1997-2018 | 67 (62-72) | 52 (46-57) | 37 (32-43) | 31 (25-36) | 25 (20-30) |
There is a significant difference between the probability of survival at 1 month after diagnosis between patients diagnosed from 1997 to 2007 and those diagnosed from 2008 to 2018, as the CIs do not overlap.
Probability of survival according to Kaplan-Meier estimates in “diagnosis code-based possible malignancy-associated HLH.” (A) Overall probability of survival in all 316 patients. (B) Overall probability of survival in all 316 patients shown separately for the time periods 1997 to 2007 and 2008 to 2018. (C) Overall probability of survival in patients diagnosed with HLH and B-cell lymphoma (n = 94), NK/T-cell lymphoma (n = 52), or Hodgkin lymphoma (n = 18) from 1997 to 2018. (D) Overall probability of survival in patients diagnosed with HLH and myeloid leukemia (n = 49), lymphocytic leukemia (n = 42), or solid tumors (n = 34) from 1997 to 2018. The generalized Wilcoxon test was used to compare the probability of survival.
Probability of survival according to Kaplan-Meier estimates in “diagnosis code-based possible malignancy-associated HLH.” (A) Overall probability of survival in all 316 patients. (B) Overall probability of survival in all 316 patients shown separately for the time periods 1997 to 2007 and 2008 to 2018. (C) Overall probability of survival in patients diagnosed with HLH and B-cell lymphoma (n = 94), NK/T-cell lymphoma (n = 52), or Hodgkin lymphoma (n = 18) from 1997 to 2018. (D) Overall probability of survival in patients diagnosed with HLH and myeloid leukemia (n = 49), lymphocytic leukemia (n = 42), or solid tumors (n = 34) from 1997 to 2018. The generalized Wilcoxon test was used to compare the probability of survival.
Long-term survival in mal-HLH was dismal (Figure 5C-D). The overall 2-year survival in all 316 patients with “diagnosis code-based possible mal-HLH,” was 25% (95% CI, 20-30), with 26% (95% CI, 19-37) in B-cell lymphomas, 20% (95% CI, 10-34) in lymphocytic leukemias, 16% (8-28) in NK/T-cell lymphomas, and 13% (95% CI, 5-24) in myeloid leukemia.
No differences in survival were observed between the M-HLH and Ch-HLH groups. In total, 6 of 15 patients (40%) with M-HLH survived for 28 days and 4 of 14 (29%) survived for 56 days. In patients with Ch-HLH, 8 of 20 (40%) survived for 28 days, and 4 of 20 (20%) survived for 56 days. Information on treatment was only available on patients with “confirmed mal-HLH.” In total, 11 of 15 patients (73%) with M-HLH received HLH-targeted therapy, consisting of immunoglobulins, corticosteroids, and/or cyclosporine A, and 7 of 15 patients (47%) received etoposide. Of the patients with Ch-HLH, 17 of 20 (85%) received HLH-targeted therapy and 11 of 20 (55%) received etoposide. Patients who received etoposide were more affected by HLH, with higher mean ferritin levels and significantly higher sCD25 levels (P = .02), and a nonsignificant trend toward shorter survival (P = .06).
Children with “diagnosis code-based possible malignancy-associated HLH”
The whole cohort from 1997 to 2018 (n = 316) included 9 children (<18 years old); 5 boys and 4 girls, with a median age of 8 years (range, 4 to 17 years). Five patients had acute lymphocytic leukemia, 1 had AML, 2 had B-cell lymphoma, and 1 had NK/T-cell lymphoma. The probability of survival at 1 and 2 months was 89% (95% CI, 43-98) and at 1 and 2 years it was 64% (95% CI, 24-87); the median follow-up was 25 months (range, 1-203).
Discussion
In this 22-year national population-based study of malignancy-associated HLH, the reported incidence increased significantly over the last 2 decades (P < .001). Although there may be medical factors partly contributing to the rapid growth in HLH diagnosis, such as increased incidence of hematological malignancies and the expanded use of immunomodulating/immunosuppressive therapies, we cannot see medical reasons explaining most of the increase, such as the doubled incidence from 2010 to 2011. It is also unlikely that the revised diagnostic criteria for HLH since 2004 markedly contributed to this increase, which occurred several years later.4 Instead, intensive educational efforts by HLH-interested pediatric and adult hemato-oncologists in Stockholm, which raised awareness locally and to a somewhat lesser degree nationally, is a more likely explanation.16,27-30 An increasing incidence has also been reported in England but with a lower overall incidence.31
The nationwide reported annual incidence of “probable mal-HLH” from 2012 to 2018 in adults was estimated to be 0.26 per 100 000 individuals. Notably, large regional differences were found, with the highest regional incidence being 0.38 per 100 000 per year, in Stockholm, and the lowest being 0.14 per 100 000 per year (Table 1). We observed no medical reasons for these substantial regional differences because there is no tradition of transferring patients across regions for the treatment of HLH. Instead, there is likely underreporting in many regions. Moreover, our strict limitation to define “probable mal-HLH” as HLH occurring at most 365 days after the cancer diagnosis in order to have a conservative estimate in this cohort likely results in an underestimated incidence because Ch-HLH per definition develops during or after chemotherapy, and such therapy may extend beyond 365 days. Thus, the true annual incidence for adults may be close to the “diagnosis code-based possible mal-HLH” in Stockholm; that is, 0.71 per 100 000 per year, which, minus the 13% estimated overdiagnosis in this study among “confirmed mal-HLH,” results in an estimated incidence of 0.62 per 100 000 per year (Table 1). It may be higher if there still are cases not recognized or reported.
There have been 3 previously published regional studies on mal-HLH in Sweden. They estimated the regional annual incidences to be 0.36, 0.38, and 0.42 per 100 000 adults, respectively, that is, internally very similar results.16,22,23 The first study focused on 8 adults with hematological malignancies between 1996 and 2009 in southern Sweden.16 The second and largest study reported on 51 adults from 2009 to 2016 referred to the Hematology Center Karolinska, Stockholm, with hematological malignancies and HLH, defined as having ≥5 HLH-2004 criteria.23 The third study reported on 5 adults with HLH-2004-verified mal-HLH treated between 2010 and 2015 at the Department of Hematology, Uppsala University Hospital.22 Notably, the 2 studies with the lowest incidences investigated only hematological malignancies, and, moreover, awareness may have increased further since these studies were performed. For comparison, the reported incidence rate of mal-HLH in England between 2003 and 2018 was 0.064 per 100 000 per year, and that of hematological malignancies was 0.055 per 100 000 per year.32 In Japan, the incidence of all forms of HLH from 2001 to 2005 was estimated to be 0.12 per 100 000 per year.15
Of all reported patients with hematological malignancies between 2012 and 2018, 0.6% had “diagnosis code-based possible mal-HLH,” which is somewhat lower than the 0.9% reported by Machaczka et al.16 Patients with acute leukemia or certain types of lymphoma may fulfill several criteria—even up to 5—as a consequence of their disease and subsequent treatment. Therefore, the standard criteria in this setting may have a lower positive predictive value for HLH, and careful clinical evaluation is required for a correct diagnosis.
The survival in mal-HLH depends on both the survival of the HLH episode, which is often limited to 1 to 2 months, and the underlying malignancy. Notably, the 1-month probability of survival increased significantly during the study period, presumably because of the increased awareness of mal-HLH and, as a result, intensified HLH-directed therapy. However, long-term survival in mal-HLH is poor and likely predominantly related to underlying malignancy and other medical conditions. This dismal prognosis is in line with previous studies, in which mal-HLH had the poorest survival among patients with sHLH.15
We want to highlight the international recommendation for a thorough work-up for possible underlying malignancy in individuals without known triggers of HLH, with a special focus on lymphomas, including positron emission tomography–guided imaging and repetitive tissue sampling.9 Even splenectomy may be considered to detect lymphomas hidden in the spleen or perisplenic tissue.9
Regarding treatment, in 1997 to 2011 HLH-targeted therapy was already administered to most of the patients diagnosed with HLH; and approximately half had received etoposide. The current consensus on therapy for mal-HLH is to first administer HLH-directed therapy, including corticosteroids, immunoglobulin, and etoposide, in case of HLH-causing organ dysfunction, and start malignancy-directed therapy once organ function is restored or acceptable.9,10 Importantly, patients with mal-HLH should not be administered the full HLH-94/HLH-2004 protocol, but rather a shorter duration and lower intensity; an etoposide dose of 50 to 100 mg/m2 (age-dependent) intravenously once weekly is recommended, as well as weekly considerations of stopping therapy.9 Notably, in patients with lymphoma-associated HLH, the addition of etoposide to the initial treatment has been reported to improve 2-month survival (79.8% vs 46.8%, P = .035) and overall survival (median survival 25.8 vs 7.8 weeks, P = .048).33 In recent years, promising efficacy has been seen using targeted inhibitors of inflammatory cytokines or signaling, including interleukin-1 (anakinra), interleukin-6 (tociluzumab), and JAK1/2 (ruxolitinib).9-11
Hematological malignancies are generally more common in males and, overall, males are at greater risk and have worse prognosis than females for most cancers.34,35 In our study, men were more affected than females by “diagnosis code-based possible mal-HLH,” also as compared with the known male predominance in all patients with hematological malignancies (P = .0012), for as yet unknown reasons (Figure 3A). Moreover, the age at diagnosis of patients with “diagnosis code-based possible mal-HLH” was significantly lower than that of all hematological malignancies in Sweden (P < .001) (Figure 3A). That sex and age of onset show an association with the probability of HLH may reflect differences in both endogenous factors (predisposing genetic factors)36,37 and exogenous factors (more potent antitumoral treatment in younger patients, including HSCT and exogenous triggers). Such different factors are superimposed until a certain threshold point is reached, beyond which inflammation is no longer controlled, and fulminant HLH develops.38 A further explanation could be that the peak incidence of different hematological malignancies differs between age groups; for example, chronic lymphocytic leukemia, which has a higher incidence in the elderly, is typically associated with low treatment intensity and less Ch-HLH. Notably, many individuals with HLH were not genetically tested for HLH-causing mutations.
We observed a difference regarding the type of associated malignancies between M-HLH and Ch-HLH in the 35 patients with “confirmed mal-HLH,” with NK/T-cell lymphomas (53%) being the most common type in M-HLH, where only 1 was leukemia, whereas B-cell lymphomas (30%), myeloid leukemias (20%), and NK/T-cell lymphomas (20%) were most common in Ch-HLH (supplemental Table 3). These findings are in line with those of Lehmberg et al in 29 pediatric and adolescent patients.39 Moreover, a higher incidence of Ch-HLH in patients with AML than in those with acute lymphocytic leukemia has been reported, suggesting that the risk of Ch-HLH increases with the intensity of chemotherapy and the extent of myeloablation.40 It has also been suggested that certain cytotoxic agents, such as cytosine arabinoside, may be more prone to induce HLH.41 In M-HLH, it has been hypothesized that cytokines produced by malignant cells trigger the HLH episode.42 Among 146 individuals with “probable mal-HLH” from 2012 to 2018, B-cell lymphoma (34%), myeloid leukemia (22%), and NK/T-cell lymphoma (15%) were the most frequent. This is partly in contrast to the large review by Ramos-Casals et al in which 35% had NK/T-cell lymphomas, 32% had B-cell lymphomas, and only 6% had leukemia.6 One possible explanation for this discrepancy could be that there is an over-representation in the literature of patients with M-HLH because the association between HLH and malignancy is more obvious here. Another possible explanation can be underlying genetic predisposition affecting the incidence of various forms of mal-HLH, similar to that of mal-HLH is more frequent in East Asia and Latin America than in Europe.6
The main limitation of this study is that we cannot retrospectively identify all affected patients with mal-HLH with certainty. With the diagnosis code-based approach, we will obviously miss patients who de facto were affected by mal-HLH but were not diagnosed and registered. In contrast, 8 of 62 (13%) patients registered as having HLH actually had other diagnoses. However, even making an effort to retrospectively study the medical files of all patients with suspected mal-HLH will not be sufficient to establish the diagnosis of mal-HLH for certain, because a lot of relevant information was not studied during the care period, as in the 14 patients with <4 fulfilled the HLH-2004 criteria in the 1997 to 2011 cohort. However, based on the use of different approaches to evaluate the incidence, including comparing various health regions, relating to previous local studies, and the review of medical files (n = 62), and that HLH has been well known in some national health regions during the last decade, it is likely that our reported incidence of mal-HLH is reasonably relevant, albeit it may still be an underestimation.
In conclusion, the annual incidence of mal-HLH in Sweden increased substantially from 1997 to 2018 and was during the period 2012 to 2018 estimated to 0.62 per 100 000 adults. Moreover, short-term survival, which we presume to be the most HLH-related outcome, significantly improved during the study period. Major challenges remain regarding mal-HLH, including raising awareness of the syndrome, improved diagnostics, a better understanding of the pathophysiology, and optimized treatment of affected patients.
Acknowledgments
The authors thank Julia Eriksson, Division of Biostatistics, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden, for excellent statistical assistance, and Indranil Sinha, Division of Pediatric Oncology and Surgery, Department of Women’s and Children’s Health, Karolinska Institutet, for assistance with data management.
This work was supported by grants to J.-I.H. from the Swedish Children's Cancer Fund (KP2021-0006), Swedish Cancer Society (Dnr 19 0362 Pj), Region Stockholm (ALF-project; FoUI-960717), and Cancer and Allergy Foundation of Sweden (Reference number 10118). M.M. received grants from the Swedish Children's Cancer Fund (ST2020-0008). A.L. received grants from the Mary Béve Foundation and was supported by the Clinical Scientist Training PhD Program at Karolinska Institutet and a combined Research and Clinical training path scholarship at the Karolinska Institutet and Karolinska University Hospital.
Authorship
Contribution: J.-I.H. and M.M. designed the study, with contributions by A.L.; A.L. generated data; A.L., M.M., and J.-I.H. analyzed data; M.J. contributed with specific knowledge; and A.L. wrote the first draft of the manuscript, which was edited by J.-I.H. and the other authors.
Conflict-of-interest disclosure: J.-I.H. is a consultant for Sobi. The remaining authors declare no competing financial interests.
Correspondence: Jan-Inge Henter, Department of Women’s and Children’s Health, Karolinska Institutet, Tomtebodavägen 18A, SE-171 77 Stockholm, Sweden; email: jan-inge.henter@ki.se.
References
Author notes
∗M.M. and J.-I.H. contributed equally to this work.
Data cannot be shared because this was not applied for in the ethical applications.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal