Histologic transformation of Waldenström macroglobulinemia (HT-WM) carries a poor prognosis with standard treatments. Here, we report the first series of HT-WM treated with chimeric antigen receptor T cells showing a high efficacy and no unexpected toxicity.
TO THE EDITOR:
Histologic transformation (HT) of Waldenström macroglobulinemia (WM)/lymphoplasmacytic lymphoma represents a rare but severe event occurring in <5% of cases. Patients usually present with adverse prognostic features, including extranodal involvement and elevated lactate dehydrogenase levels.1-3 These patients carry a poor prognosis after standard treatments with frequent refractory disease, short duration of response, and/or central nervous system (CNS) relapses, with a median overall survival of 1.5 to 2.7 years.4,5 Chimeric antigen receptor T cells (CAR-T) have been approved for the treatment of aggressive B-cell lymphomas, including de novo large B-cell lymphoma (LBCL) and transformed follicular lymphoma.6 However, little is known about the efficacy of CAR-T in patients with transformed WM. Only a few cases have been reported in the literature, including 2 cases from the TRANSCEND study, 1 reaching a partial response (PR),7 and 1 case reported by Bansal et al,8 who achieved a complete response (CR) for 12 months at the time of publication.
Here, we conducted a retrospective collaborative study to evaluate the efficacy and safety of CAR-T in patients with HT-WM. Patients from the French Dispositif d'Enregistrement et de Suivi des Patients Traités par CAR-T cells (DESCAR-T) registry (NCT04328298; n = 19 patients) and from 2 US centers (n = 4 patients) were included if they met all of the following criteria: aged >18 years, had refractory/relapsed HT-WM, had biopsy-proven LBCL, and had received treatment with a commercial CAR-T. Patients received CAR-T as standard of care, and all provided informed consent before the treatment. The primary end point was to evaluate the best CR rate, according to the Lugano 2014 classification.9 Secondary end points included overall response rate, progression-free survival (PFS), overall survival (OS), and safety, including cytokine-release syndromes (CRSs), immune effector cell–associated neurotoxicity syndrome (ICANS), infections, and hematological toxicity. CRS and ICANS were graded according to the American Society for Transplantation and Cellular Therapy grading system. Other adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Patient characteristics are provided in Table 1. A total of 23 patients were treated between June 2017 and October 2023. The median age at WM diagnosis was 58 years (range, 35-72 years). MYD88L265P and CXCR4 mutations were detected in 86% (12/14) and 25% (2/8) of patients with available data, respectively. The median time from WM to HT diagnosis was 4.5 years (interquartile range [IQR], 0.3-16.7 years), including 5 cases (22%) with concurrent diagnosis of WM and LBCL. Patients received a median of 1 line of treatment (range, 0-4) for WM and 2 lines (range, 1-4) for HT before CAR-T infusion, and 8 patients (35%) had undergone prior autologous stem cell transplantation (2 as part of WM treatment and 6 for HT) and 1 allogeneic stem cell transplantation after HT (supplemental Table 1, available on the Blood website). CNS involvement due to LBCL was present in 3 patients at HT and 2 at the time of CAR-T therapy. The median time from HT diagnosis to CAR-T infusion was 26 months (IQR, 8-40 months). Median age at the CAR-T infusion time was 66 years (range, 41-82 years). Nearly half of the patients (48%) were refractory to the last therapy before CAR-T. Seventeen patients (74%) received a bridging therapy, of whom only 6 (35%) responded (CR or PR) (supplemental Table 2). Lymphodepletion consisted of fludarabine/cyclophosphamide for 20 patients and bendamustine for 3 patients (due to renal failure). Patients received axicabtagene-cileucel (n = 14) or tisagenlecleucel (n = 9).
Patient characteristics and outcome
| Variable . | Patients (n = 23) . |
|---|---|
| Sex: male/female (ratio) | 12/11 (1.1) |
| WM characteristics | |
| Age at WM diagnosis, median (range), y | 58 (35-72) |
| MYD88L265P mutation | 12/14 (86) |
| CXCR4 mutation | 2/8 (25) |
| Number of lines for WM, median (range) | 1 (0-4) |
| HT characteristics | |
| Age at HT, median (range), y | 65 (41-81) |
| Histology | |
| DLBCL, not otherwise specified | 22 (96) |
| HGBL (MYC and BCL6 translocations) | 1 (4) |
| Hans algorithm | |
| Non-GC | 18 (86) |
| GC | 3 (14) |
| Extranodal involvement | 19 (83) |
| CNS involvement | 3 (13) |
| Serum IgM level, median (range), g/L | 6.1 (0-31.1) |
| Ann Arbor stage III-IV | 22 (96) |
| IPI ≥3 | 10/20 (50) |
| Number of lines for HT, median (range) | 2 (1-4) |
| Characteristics at lymphodepletion | |
| Age at lymphodepletion, median (range), y | 66 (41-82) |
| >70 y | 8 (35) |
| Refractory to last therapy | 11 (48) |
| ECOG performance status ≥2 | 5/22 (23) |
| Elevated LDH | 11 (48) |
| Ann Arbor stage III-IV | 12/15 (80) |
| CNS involvement | 2/23 (9) |
| Bridging therapy | 17 (74) |
| Response to bridging therapy (n = 17) | |
| CR | 3 (18) |
| PR | 3 (18) |
| SD | 5 (29) |
| PD | 6 (35) |
| Lymphodepleting chemotherapy | |
| Flu/Cy | 20 (87) |
| Bendamustine | 3 (13) |
| CAR T-cell product | |
| Axicabtagene-ciloleucel | 14 (61) |
| Tisagenlecleucel | 9 (39) |
| Outcome after CAR-T infusion | |
| Best OR | 22 (96) |
| Best CR | 20 (87) |
| 1-y PFS (95% CI), % | 73.4 (50.2-87.1) |
| 1-y OS (95% CI), % | 80.5 (50.6-89.2) |
| Grade ≥3 CRS | 2 (9) |
| Grade ≥3 ICANS | 2 (9) |
| Variable . | Patients (n = 23) . |
|---|---|
| Sex: male/female (ratio) | 12/11 (1.1) |
| WM characteristics | |
| Age at WM diagnosis, median (range), y | 58 (35-72) |
| MYD88L265P mutation | 12/14 (86) |
| CXCR4 mutation | 2/8 (25) |
| Number of lines for WM, median (range) | 1 (0-4) |
| HT characteristics | |
| Age at HT, median (range), y | 65 (41-81) |
| Histology | |
| DLBCL, not otherwise specified | 22 (96) |
| HGBL (MYC and BCL6 translocations) | 1 (4) |
| Hans algorithm | |
| Non-GC | 18 (86) |
| GC | 3 (14) |
| Extranodal involvement | 19 (83) |
| CNS involvement | 3 (13) |
| Serum IgM level, median (range), g/L | 6.1 (0-31.1) |
| Ann Arbor stage III-IV | 22 (96) |
| IPI ≥3 | 10/20 (50) |
| Number of lines for HT, median (range) | 2 (1-4) |
| Characteristics at lymphodepletion | |
| Age at lymphodepletion, median (range), y | 66 (41-82) |
| >70 y | 8 (35) |
| Refractory to last therapy | 11 (48) |
| ECOG performance status ≥2 | 5/22 (23) |
| Elevated LDH | 11 (48) |
| Ann Arbor stage III-IV | 12/15 (80) |
| CNS involvement | 2/23 (9) |
| Bridging therapy | 17 (74) |
| Response to bridging therapy (n = 17) | |
| CR | 3 (18) |
| PR | 3 (18) |
| SD | 5 (29) |
| PD | 6 (35) |
| Lymphodepleting chemotherapy | |
| Flu/Cy | 20 (87) |
| Bendamustine | 3 (13) |
| CAR T-cell product | |
| Axicabtagene-ciloleucel | 14 (61) |
| Tisagenlecleucel | 9 (39) |
| Outcome after CAR-T infusion | |
| Best OR | 22 (96) |
| Best CR | 20 (87) |
| 1-y PFS (95% CI), % | 73.4 (50.2-87.1) |
| 1-y OS (95% CI), % | 80.5 (50.6-89.2) |
| Grade ≥3 CRS | 2 (9) |
| Grade ≥3 ICANS | 2 (9) |
Data are given as number/total (percentage) or number (percentage) unless otherwise indicated.
CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; Fly/Cy, fludarabine cyclophosphamide; GC, germinal center; HGBL, high-grade B-cell lymphoma; IPI, International Prognostic Index; LDH, lactate dehydrogenase; OR, overall response; PD, progressive disease; SD, stable disease.
The best overall response rate was 96% (95% confidence interval [CI], 78%-100%), including 87% CR (95% CI, 66%-97%) (Table 1; supplemental Figure 1; supplemental Table 3). Of 4 patients with a PR at 1 month, 2 converted to a CR at 3 and 6 months without additional treatment. At 6 months, 73% of the patients remained in CR. The 2 patients with CNS involvement achieved a CR at 1 month: 1 relapsed at 3 months, and 1 remained in CR at 12 months. Regarding WM disease evaluation, serum IgM levels before and after CAR-T were available for 18 patients (supplemental Figure 2). IgM peak at CAR-T infusion was undetectable in 8 patients. Among the 10 remaining patients, the mean IgM level before CAR-T was 6.1 g/L (IQR, 4-10 g/L). All 5 patients with IgM level >5 g/L before CAR-T achieved a PR after CAR-T. Low serum IgM level is a common feature in HT-WM.1,4
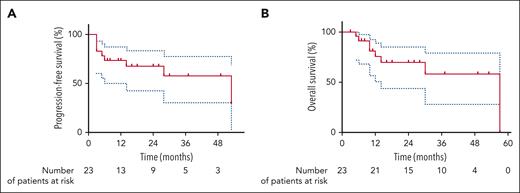
After a median follow-up of 28 months (range, 3-57 months), the estimated PFS rates at 1 and 2 years were 73.4% (95% CI, 50.2%-87.1%) and 67.5% (95% CI, 42.9%-83.2%), respectively (Figure 1A). The estimated OS rates at 1 and 2 years were 80.5% (95% CI, 50.6%-89.2%) and 69.5% (95% CI, 44.6%-85.3%), respectively (Figure 1B). Eight patients experienced relapse/progression during follow-up. Among them, 6 occurred within the first 6 months following CAR-T infusion and were related to LBCL relapse. One patient presented with a late relapse (4.5 years) of LBCL. Only 1 patient relapsed with nontransformed WM at 28 months. Among the 7 patients who relapsed with LBCL after CAR-T cells, salvage therapy consisted of lenalidomide (+/− rituximab or tafasitamab) in 4 patients, glofitamab in 1 patient, epigenetic agent in 1 patient, and ibrutinib in 1 patient with CNS relapse. Only 1 patient treated with lenalidomide obtained a sustained response for 2 years. At the time of data cutoff, 8 patients have died, 7 from lymphoma progression (LBCL) and 1 from COVID-19 infection.
Outcomes after CAR T-cell infusion. Kaplan-Meier estimates of PFS (A) and OS (B) from CAR T-cell infusion.
Outcomes after CAR T-cell infusion. Kaplan-Meier estimates of PFS (A) and OS (B) from CAR T-cell infusion.
There was no unexpected toxicity with 74% CRS (9% grade 3-4) and 39% ICANS (9% grade 3-4) (supplemental Table 4). The median time to onset of CRS was 2 days (IQR, 1-4 days) after CAR-T infusion, and the median duration was 5 days (IQR, 3-8 days). The median time to onset of ICANS was 5 days (IQR, 4-11 days) after CAR-T infusion, and the median duration was 7 days (IQR, 4-13 days). Four patients (17%) were admitted to the intensive care unit because of CAR-T toxicities. Twelve patients experienced infections, including 4 grade 3 to 4 events. Between 22% and 35% of the patients developed grade ≥3 prolonged cytopenias (ie, not resolved within 30 days postinfusion).
Although cross-trial comparison cannot be formally performed, these data compare favorably with the results of the ZUMA-110,11 and the JULIET trials12 with axicabtagene-cileucel and tisagenlecleucel, respectively. The CR rate was 58% in ZUMA-1 and 40% in JULIET, vs 87% in our study. The 1-year PFS and OS rates were 44% and 59% in ZUMA-1, respectively, and unknown (83% among responders at 3 months) and 49% in JULIET, respectively, vs 73.4% and 80.5%, respectively, in our study.13 The good efficacy of CAR-T in HT-WM seems to be in line with the results in transformed follicular lymphoma14-16 but contrasts with the poor efficacy of CAR-T in Richter transformation.17-19 This suggests that CAR-T efficacy may not be influenced by the transformation itself but rather by the underlying disease and/or the host immune system or microenvironment.
Overall, CAR-T therapy is associated with high efficacy in HT-WM, especially given the poor outcome of these patients with standard treatments. Furthermore, CAR-T therapy seems to be well tolerated in these patients, with no unexpected toxicity. Although these results need to be confirmed in a larger cohort, they support the use of CAR-T cells in this population. Extended follow-up is needed to confirm the long-term efficacy of CAR-T in HT-WM.
Acknowledgments
MPIYP (MC Béné; Paris, France) provided assistance with medical writing for this manuscript. The authors acknowledge Florian Slimano (Reims, France) and Lukshe Kanagaratnam (Reims, France) for statistical assistance.
Authorship
Contribution: E.D. and R.H. analyzed and interpreted data and wrote the manuscript; E.D., D.R.-W., A.C., J.D., R.D.B., T.G., H.B., M.C., C.J., S.G., F.-X.G., E.B., A.C., P.C.-M., S.P.T., A.D., R.R., S.L.G., J.J.C., and R.H. collected data; and all authors agreed to the final version of the manuscript.
Conflict-of-interest disclosure: D.R.-W. reports funds and/or consulting fees from AbbVie, AstraZeneca, BeiGene, and Janssen. R.D.B. reports funds and/or consulting fees from Novartis, Kite/Gilead, Janssen, Pfizer, Bristol Myers Squibb, AbbVie, and Incyte. M.C. reports honoraria from Amgen; research funds from AbbVie and Innate Pharma; and travel accommodations from AstraZeneca. F.-X.G. reports honoraria from Janssen, Kite/Gilead, Novartis, Bristol Myers Squibb, and Milteny. E.B. reports honoraria from Kite/Gilead, Roche, Bristol Myers Squibb, Novartis, Incyte, Takeda, and Pfizer; and research funds from Amgen. S.P.T. reports research funds and/or consulting fees from AbbVie, Janssen, BeiGene, Eli Lilly, and Bristol Myers Squibb. R.R. reports consulting or advisory roles with Allogene, Gilead Sciences, Incyte, TScan, Synthekine, Orca Bio, Quell Biotherapeutics, Autolus, and Capstan; expert witness role with Bayer; and research funding from Atara Biotherapeutics, Incyte, Sanofi, Immatics, AbbVie, TCR2, Takeda, Gilead Sciences, CareDx, TScan, Synthekine, Bristol Myers Squibb, Johnson & Johnson, Genentech, and Precision Biosciences. J.J.C. reports research funds and/or consulting fees from AbbVie, AstraZeneca, BeiGene, Cellectar, Janssen, Kite, LOXO, Mustang Bio, and Pharmacyclics. R.H. reports honoraria from Kite/Gilead, Novartis, Incyte, Janssen, Merck Sharp & Dohme, Takeda, and Roche; and consultancy at Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, ADC Therapeutics, Incyte, and Miltenyi. The remaining authors declare no competing financial interests.
Correspondence: Eric Durot, Service d’Hématologie, Hôpital Robert Debré, CHU de Reims, Rue du Général Koenig, 51100 Reims, France; email: edurot@chu-reims.fr.
References
Author notes
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 10 December 2023.
For original data, please contact edurot@chu-reims.fr.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal