In this issue of Blood, Kassim et al present the results of a large, multicenter, international phase 2 trial of a nonmyeloablative haploidentical bone marrow transplant (BMT) for pediatric and adult patients with sickle cell disease (SCD).1
Forty years ago, a patient with SCD was cured by human leukocyte antigen (HLA)–identical BMT. This patient had concurrent acute myeloid leukemia, and the BMT was used to cure both diseases.2 In 1988, Vermylen et al described 5 children treated successfully with HLA-identical BMT specifically to cure their SCD.3 Since then, there have been a multitude of reports of successful transplants for SCD. So, why are there so few patients treated with BMT given the potential number of patients with SCD who could benefit, given the significant morbidity the disease causes. First, there is the issue of perception among patients and parents. Roth et al described in 2012 the results of a survey showing that less than half of adolescents with SCD and their parents would consider undergoing BMT, and most believed their disease would get better and not affect their longevity.4 Thankfully, in 2020, we learned from a survey reported by Bakshi et al that, although most patients with SCD face a serious dilemma comparing potential palliative vs curative therapies, most wanted to learn more about their options.5 Therefore, it appears that patients and families are becoming more open to the idea of BMT over time, likely because of better outcomes, improved education, and greater awareness of therapies available. There are also data showing that among pediatric hematologists-oncologists, there remains significant concern about transplant-related mortality (TRM) and graft-versus-host disease (GVHD), leading to underreferral of patients who may benefit from this approach. Providers specializing in SCD are more likely to refer patients with SCD for BMT,6 likely because of greater awareness of the increasing safety and low TRM from BMT for SCD today. Patients deserve to be cared for by specialists who have the most up-to-date information on available therapies and who will work with patients and their families to access that therapy.
Another issue impacting the relatively low number of transplants is donor availability and donor access. We have shown that only ≈20% of patients with SCD without an HLA-identical sibling have a fully matched unrelated donor available.7 Patients in many developing countries have limited to no access to BMT, much less to unrelated donor registries.8
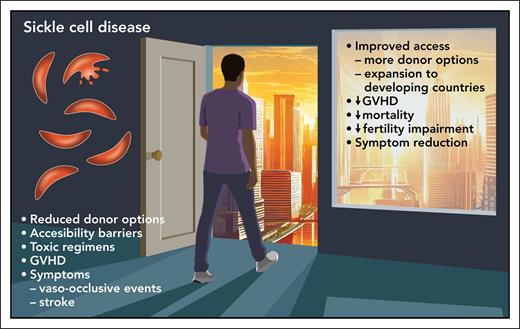
Here, Kassim et al report a 1-year overall survival rate of 95.7% in an international trial of children and adults receiving nonablative, haploidentical BMT for SCA. The authors have confirmed results from smaller studies, that haplotransplants using posttransplant cyclophosphamide (PTCy) for GVHD prophylaxis have leveled the playing field (see figure). Although survival was high, graft rejection episodes did occur in 11.4% of the patients, all of whom were aged <18 years and all had autologous recovery. Was this high rate of graft failure related to a more robust immune system in children? At this point, the answer is unknown and will require further study.
Another important factor is the economic issue. BMT is incredibly complex and expensive, given the tremendous number of resources required to successfully support a patient through BMT. Although the results of haploidentical BMT using graft manipulation techniques are outstanding,9 the PTCy approach, which was used here, is much simpler, is less expensive, and does not require the immense resources needed for graft manipulation from a good manufacturing practices laboratory. And until prices come down and the technical availability increases for SCD gene therapy, it will not be an option in middle-income and developing countries.
The results presented by Kassim et al are outstanding and encouraging. Some of us may think that 40 years is too long from the first BMT for SCD to where we are today. But this article shows that new strategies can adapt therapy to be expanded to countries with limited resources. The authors of this article are to be congratulated for pushing the boundaries of SCA care and should encourage all to make curative care available to all patients with this disease, regardless of their location.
The result of 95.7% 1-year survival in a large, multicenter, international phase 2 trial of a nonmyeloablative haploidentical bone marrow transplant (BMT) with posttransplant cyclophosphamide for GVHD prophylaxis for pediatric and adult patients with sickle cell disease has opened a new door of opportunity for curative therapy. The approach uses a less expensive and labor-intensive approach that expands its availability to countries outside of well-resourced areas of the world. Professional illustration by Patrick Lane, ScEYEnce Studios.
The result of 95.7% 1-year survival in a large, multicenter, international phase 2 trial of a nonmyeloablative haploidentical bone marrow transplant (BMT) with posttransplant cyclophosphamide for GVHD prophylaxis for pediatric and adult patients with sickle cell disease has opened a new door of opportunity for curative therapy. The approach uses a less expensive and labor-intensive approach that expands its availability to countries outside of well-resourced areas of the world. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: D.A.J. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal